 Found 3377 hits with Last Name = 'vacca' and Initial = 'j'
Found 3377 hits with Last Name = 'vacca' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50292202
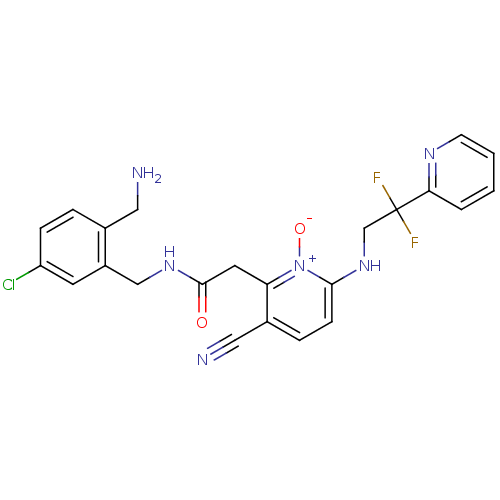
(CHEMBL382542 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cc1c(ccc(NCC(F)(F)c2ccccn2)[n+]1[O-])C#N Show InChI InChI=1S/C23H21ClF2N6O2/c24-18-6-4-15(11-27)17(9-18)13-30-22(33)10-19-16(12-28)5-7-21(32(19)34)31-14-23(25,26)20-3-1-2-8-29-20/h1-9,31H,10-11,13-14,27H2,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818
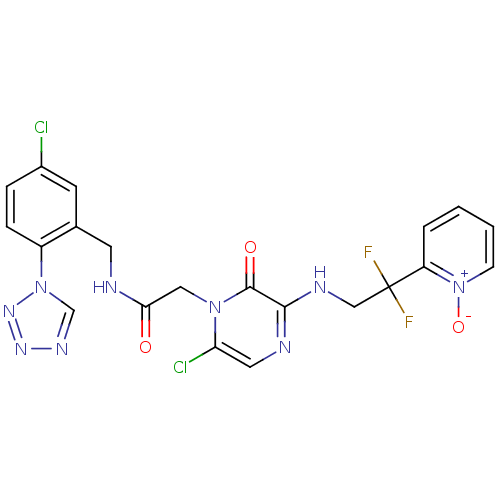
((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824
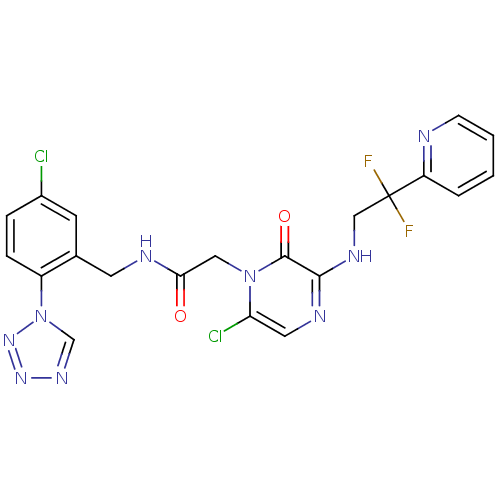
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292203
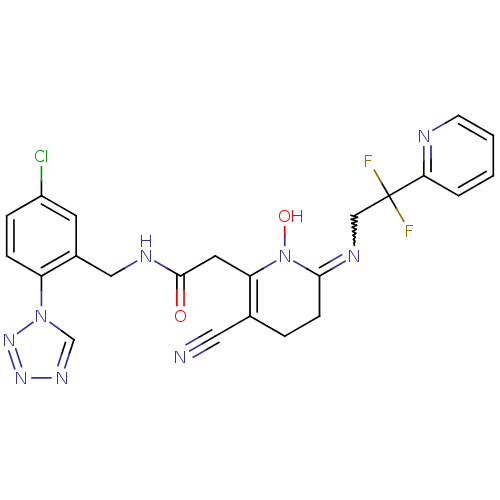
(CHEMBL196030 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES ON1C(CCC(C#N)=C1CC(=O)NCc1cc(Cl)ccc1-n1cnnn1)=NCC(F)(F)c1ccccn1 |w:26.29,c:7| Show InChI InChI=1S/C23H20ClF2N9O2/c24-17-5-6-18(34-14-31-32-33-34)16(9-17)12-29-22(36)10-19-15(11-27)4-7-21(35(19)37)30-13-23(25,26)20-3-1-2-8-28-20/h1-3,5-6,8-9,14,37H,4,7,10,12-13H2,(H,29,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133524
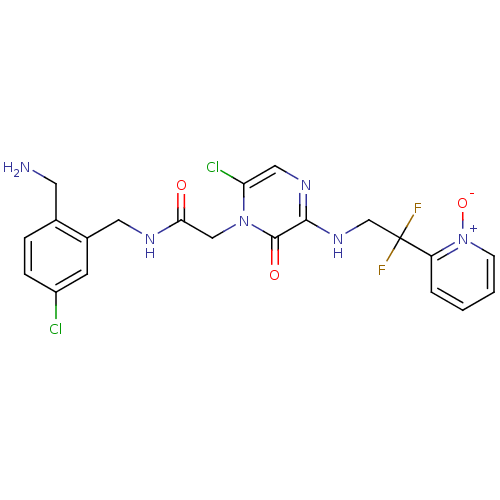
(CHEMBL419773 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cn1c(Cl)cnc(NCC(F)(F)c2cccc[n+]2[O-])c1=O Show InChI InChI=1S/C21H20Cl2F2N6O3/c22-15-5-4-13(8-26)14(7-15)9-27-18(32)11-30-17(23)10-28-19(20(30)33)29-12-21(24,25)16-3-1-2-6-31(16)34/h1-7,10H,8-9,11-12,26H2,(H,27,32)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50184069
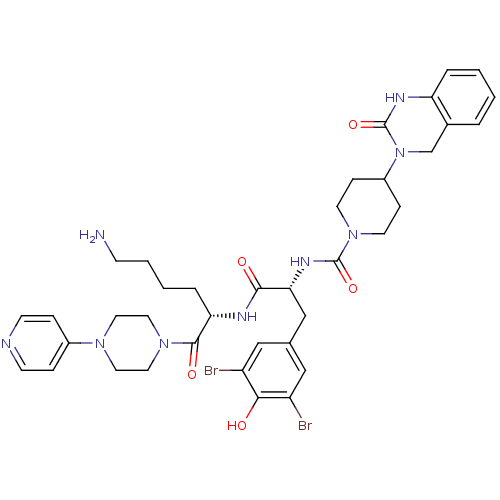
(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human cloned CLR/RAMP1 receptor expressed in E10 cells |
Bioorg Med Chem Lett 16: 2595-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.051
BindingDB Entry DOI: 10.7270/Q2HT2NX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356281
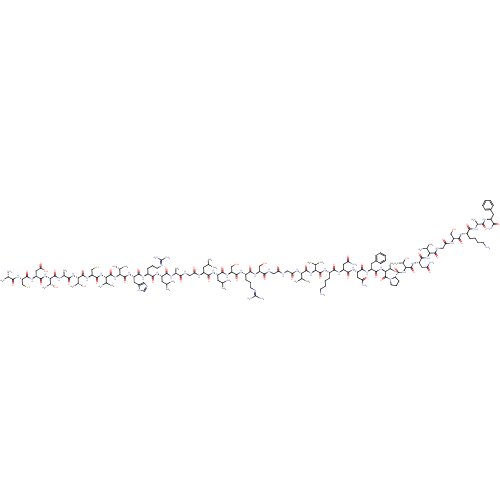
(CHEMBL1910953)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C163H269N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,62,72-88,92-111,120-128,215-221,264-265H,31-37,42-61,63-71,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,175,182)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177)/t81-,82-,83-,84-,85+,86+,87+,88+,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-,124-,125-,126-,127-,128-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356281

(CHEMBL1910953)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C163H269N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,62,72-88,92-111,120-128,215-221,264-265H,31-37,42-61,63-71,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,175,182)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177)/t81-,82-,83-,84-,85+,86+,87+,88+,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-,124-,125-,126-,127-,128-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay in the absence of MgCl2 |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147793
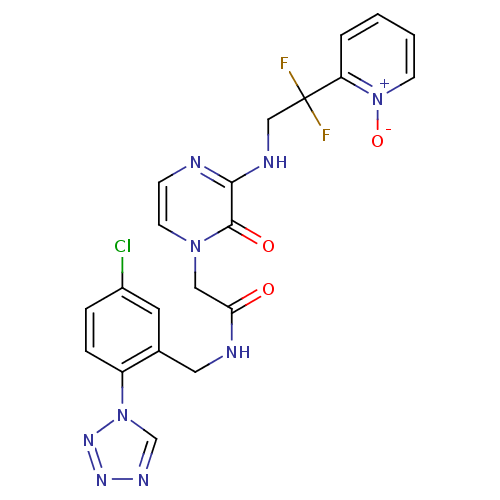
(CHEMBL323583 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1nccn(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H18ClF2N9O3/c22-15-4-5-16(32-13-28-29-30-32)14(9-15)10-26-18(34)11-31-8-6-25-19(20(31)35)27-12-21(23,24)17-3-1-2-7-33(17)36/h1-9,13H,10-12H2,(H,25,27)(H,26,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50184069

(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385312
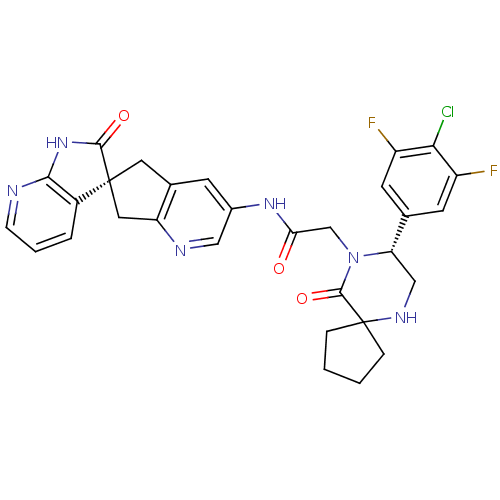
(CHEMBL2035984)Show SMILES Fc1cc(cc(F)c1Cl)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C30H27ClF2N6O3/c31-25-20(32)9-16(10-21(25)33)23-14-36-30(5-1-2-6-30)28(42)39(23)15-24(40)37-18-8-17-11-29(12-22(17)35-13-18)19-4-3-7-34-26(19)38-27(29)41/h3-4,7-10,13,23,36H,1-2,5-6,11-12,14-15H2,(H,37,40)(H,34,38,41)/t23-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50184069

(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385311
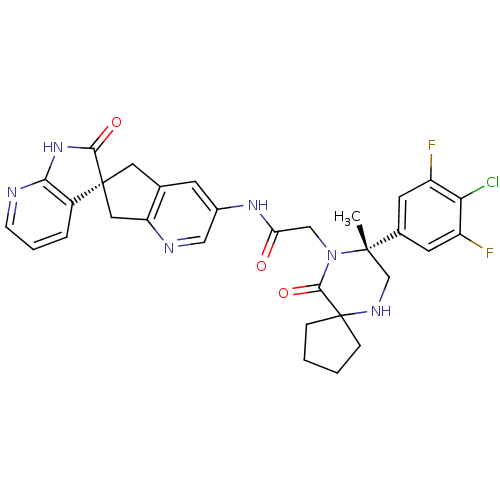
(CHEMBL2035985)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)c(Cl)c(F)c1 |r| Show InChI InChI=1S/C31H29ClF2N6O3/c1-29(18-10-21(33)25(32)22(34)11-18)16-37-31(6-2-3-7-31)28(43)40(29)15-24(41)38-19-9-17-12-30(13-23(17)36-14-19)20-5-4-8-35-26(20)39-27(30)42/h4-5,8-11,14,37H,2-3,6-7,12-13,15-16H2,1H3,(H,38,41)(H,35,39,42)/t29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147788

(CHEMBL103874 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1-n1cnnn1 Show InChI InChI=1S/C23H22ClN7O4S/c1-16-7-9-20(27-36(34,35)14-17-5-3-2-4-6-17)23(33)30(16)13-22(32)25-12-18-11-19(24)8-10-21(18)31-15-26-28-29-31/h2-11,15,27H,12-14H2,1H3,(H,25,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131480
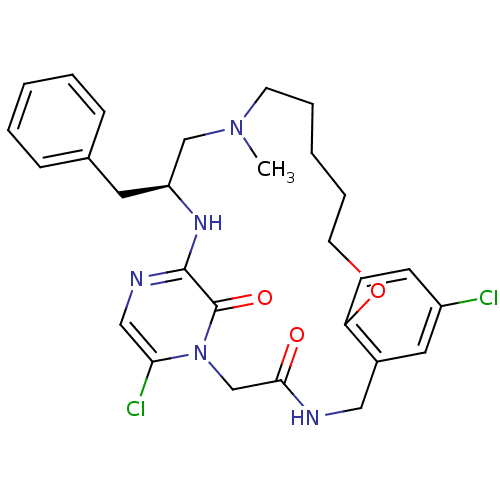
((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...)Show SMILES CN1CCCCCOc2ccc(Cl)cc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)C1)c2=O Show InChI InChI=1S/C28H33Cl2N5O3/c1-34-12-6-3-7-13-38-24-11-10-22(29)15-21(24)16-31-26(36)19-35-25(30)17-32-27(28(35)37)33-23(18-34)14-20-8-4-2-5-9-20/h2,4-5,8-11,15,17,23H,3,6-7,12-14,16,18-19H2,1H3,(H,31,36)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50356282
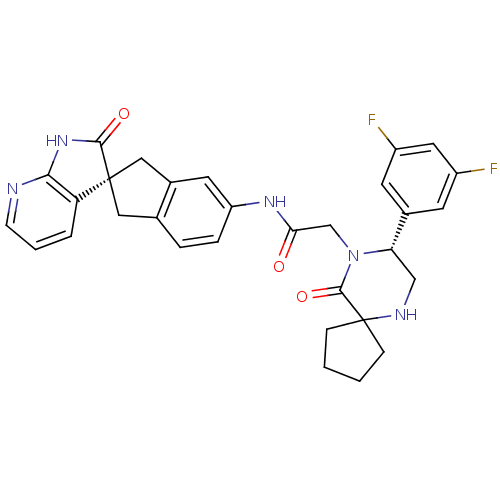
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50315401
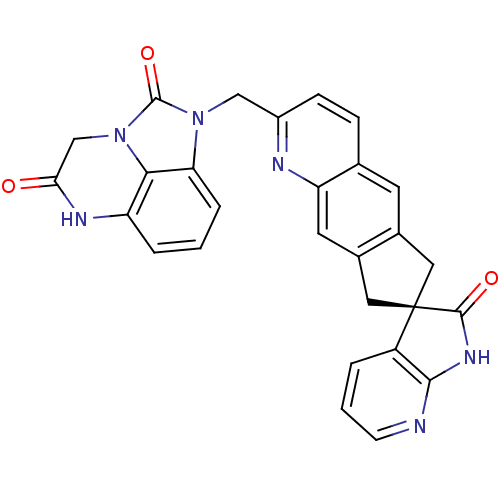
((R)-1-((2'-oxo-1',2',6,8-tetrahydrospiro[cyclopent...)Show SMILES O=C1Nc2ncccc2[C@]11Cc2cc3ccc(Cn4c5cccc6NC(=O)Cn(c56)c4=O)nc3cc2C1 |r| Show InChI InChI=1S/C28H20N6O3/c35-23-14-34-24-20(31-23)4-1-5-22(24)33(27(34)37)13-18-7-6-15-9-16-11-28(12-17(16)10-21(15)30-18)19-3-2-8-29-25(19)32-26(28)36/h1-10H,11-14H2,(H,31,35)(H,29,32,36)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 20: 2572-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.086
BindingDB Entry DOI: 10.7270/Q2765FFZ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356282

(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385313

(CHEMBL2035982)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C30H28F2N6O3/c31-19-8-17(9-20(32)11-19)24-15-35-30(5-1-2-6-30)28(41)38(24)16-25(39)36-21-10-18-12-29(13-23(18)34-14-21)22-4-3-7-33-26(22)37-27(29)40/h3-4,7-11,14,24,35H,1-2,5-6,12-13,15-16H2,(H,36,39)(H,33,37,40)/t24-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147809
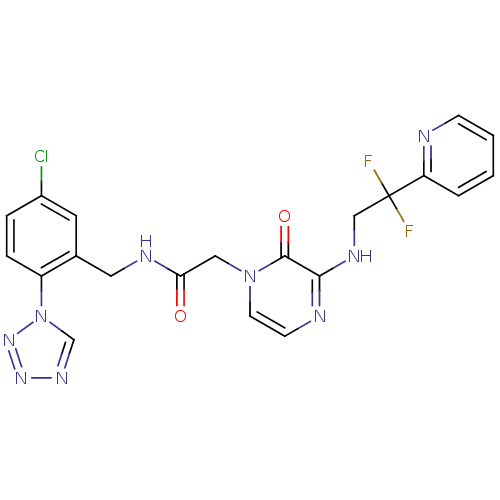
(CHEMBL103342 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES FC(F)(CNc1nccn(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H18ClF2N9O2/c22-15-4-5-16(33-13-29-30-31-33)14(9-15)10-27-18(34)11-32-8-7-26-19(20(32)35)28-12-21(23,24)17-3-1-2-6-25-17/h1-9,13H,10-12H2,(H,26,28)(H,27,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50301971
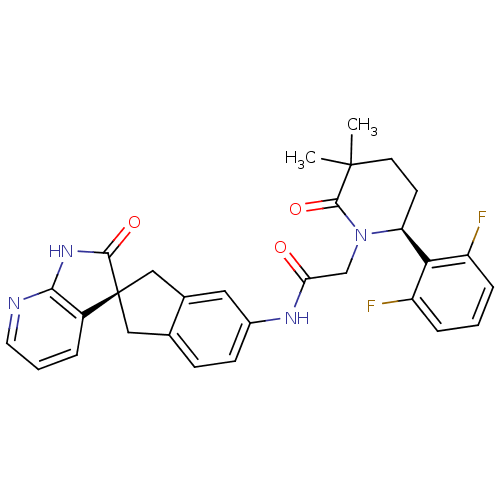
(2-((S)-6-(2,6-difluorophenyl)-3,3-dimethyl-2-oxopi...)Show SMILES CC1(C)CC[C@H](N(CC(=O)Nc2ccc3C[C@@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1c(F)cccc1F |r| Show InChI InChI=1S/C30H28F2N4O3/c1-29(2)11-10-23(25-21(31)6-3-7-22(25)32)36(28(29)39)16-24(37)34-19-9-8-17-14-30(15-18(17)13-19)20-5-4-12-33-26(20)35-27(30)38/h3-9,12-13,23H,10-11,14-16H2,1-2H3,(H,34,37)(H,33,35,38)/t23-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCGRP from human cloned CGRP receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 5787-90 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.134
BindingDB Entry DOI: 10.7270/Q2PG1RTW |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342639
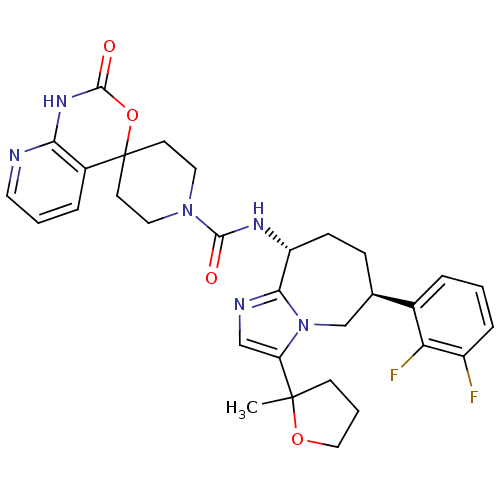
(CHEMBL1770729 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC1(CCCO1)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H34F2N6O4/c1-30(10-4-16-42-30)24-17-35-27-23(9-8-19(18-39(24)27)20-5-2-7-22(32)25(20)33)36-28(40)38-14-11-31(12-15-38)21-6-3-13-34-26(21)37-29(41)43-31/h2-3,5-7,13,17,19,23H,4,8-12,14-16,18H2,1H3,(H,36,40)(H,34,37,41)/t19-,23-,30?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50329174

(CHEMBL1269644 | N-(((R)-2,3-dihydro-1H-inden-1-yl)...)Show SMILES CC(C)(C)C(=O)N(C[C@@H]1CCc2ccccc12)Cc1cnc2cc3C[C@]4(Cc3cc2c1)C(=O)Nc1ncccc41 |r| Show InChI InChI=1S/C34H34N4O2/c1-33(2,3)32(40)38(20-23-11-10-22-7-4-5-8-27(22)23)19-21-13-24-14-25-16-34(17-26(25)15-29(24)36-18-21)28-9-6-12-35-30(28)37-31(34)39/h4-9,12-15,18,23H,10-11,16-17,19-20H2,1-3H3,(H,35,37,39)/t23-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCGRP from human cloned CGRP receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6827-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.105
BindingDB Entry DOI: 10.7270/Q2Z89CNJ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445
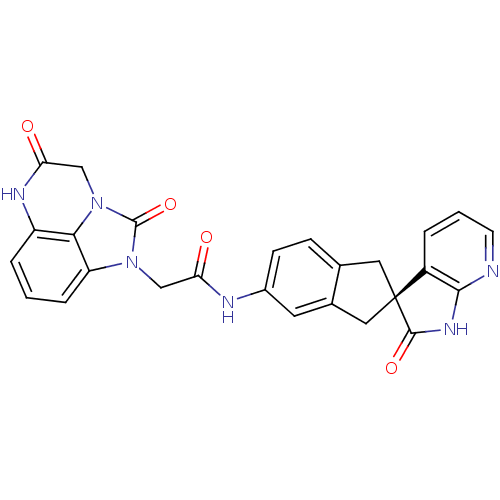
((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342638
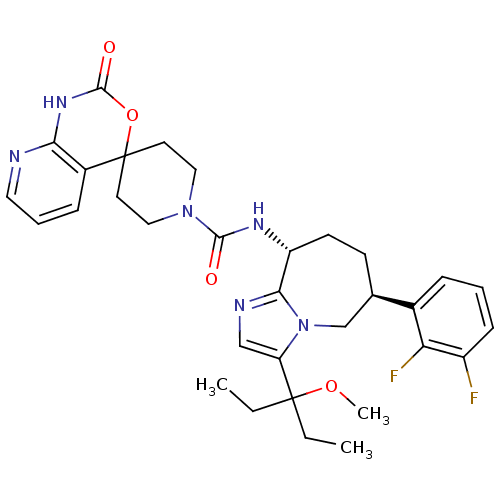
(CHEMBL1770728 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CCC(CC)(OC)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C32H38F2N6O4/c1-4-31(5-2,43-3)25-18-36-28-24(12-11-20(19-40(25)28)21-8-6-10-23(33)26(21)34)37-29(41)39-16-13-32(14-17-39)22-9-7-15-35-27(22)38-30(42)44-32/h6-10,15,18,20,24H,4-5,11-14,16-17,19H2,1-3H3,(H,37,41)(H,35,38,42)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445

((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCGRP from human CGRP receptor expressed in HEK293 cells coexpressing RAMP1 |
Bioorg Med Chem Lett 19: 214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.106
BindingDB Entry DOI: 10.7270/Q21C1XSK |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385314
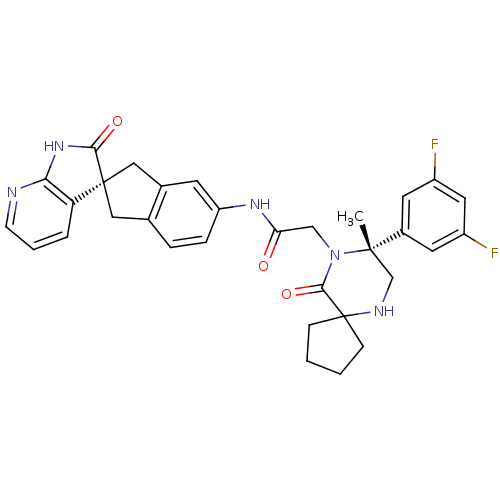
(CHEMBL2035981)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N5O3/c1-30(21-12-22(33)14-23(34)13-21)18-36-32(8-2-3-9-32)29(42)39(30)17-26(40)37-24-7-6-19-15-31(16-20(19)11-24)25-5-4-10-35-27(25)38-28(31)41/h4-7,10-14,36H,2-3,8-9,15-18H2,1H3,(H,37,40)(H,35,38,41)/t30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172826
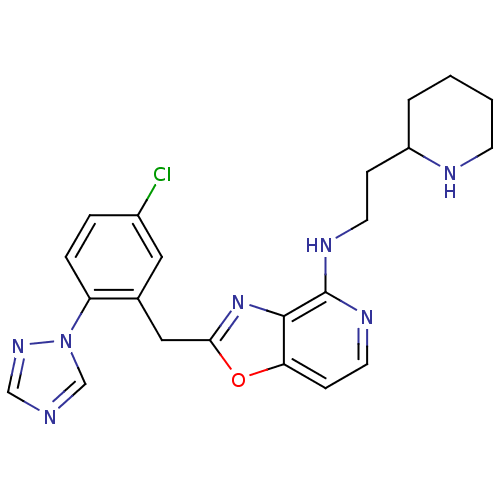
(CHEMBL426101 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES Clc1ccc(c(Cc2nc3c(NCCC4CCCCN4)nccc3o2)c1)-n1cncn1 Show InChI InChI=1S/C22H24ClN7O/c23-16-4-5-18(30-14-24-13-28-30)15(11-16)12-20-29-21-19(31-20)7-10-27-22(21)26-9-6-17-3-1-2-8-25-17/h4-5,7,10-11,13-14,17,25H,1-3,6,8-9,12H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172842
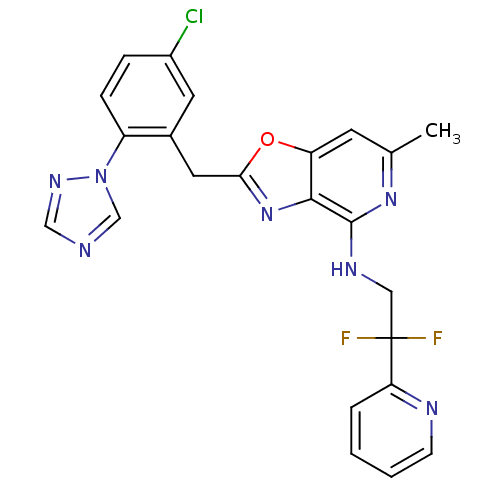
(CHEMBL198820 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES Cc1cc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc2c(NCC(F)(F)c2ccccn2)n1 Show InChI InChI=1S/C23H18ClF2N7O/c1-14-8-18-21(22(31-14)29-11-23(25,26)19-4-2-3-7-28-19)32-20(34-18)10-15-9-16(24)5-6-17(15)33-13-27-12-30-33/h2-9,12-13H,10-11H2,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292196
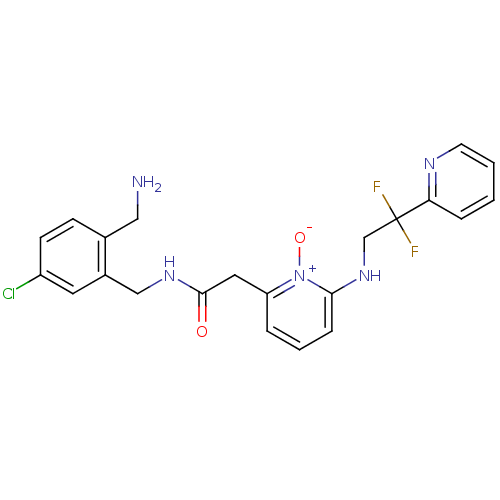
(CHEMBL195366 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cc1cccc(NCC(F)(F)c2ccccn2)[n+]1[O-] Show InChI InChI=1S/C22H22ClF2N5O2/c23-17-8-7-15(12-26)16(10-17)13-28-21(31)11-18-4-3-6-20(30(18)32)29-14-22(24,25)19-5-1-2-9-27-19/h1-10,29H,11-14,26H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445

((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 20: 2572-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.086
BindingDB Entry DOI: 10.7270/Q2765FFZ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342625
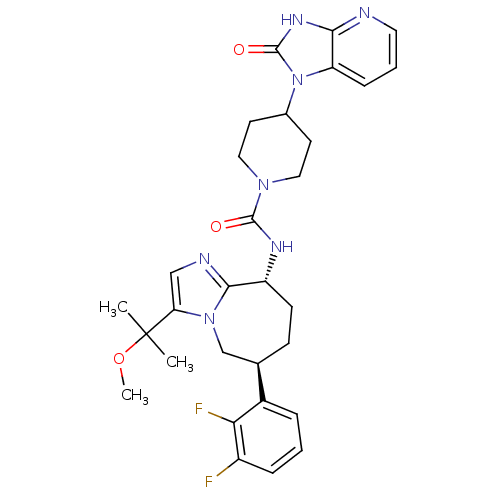
(CHEMBL1770715 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C30H35F2N7O3/c1-30(2,42-3)24-16-34-27-22(10-9-18(17-38(24)27)20-6-4-7-21(31)25(20)32)35-28(40)37-14-11-19(12-15-37)39-23-8-5-13-33-26(23)36-29(39)41/h4-8,13,16,18-19,22H,9-12,14-15,17H2,1-3H3,(H,35,40)(H,33,36,41)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
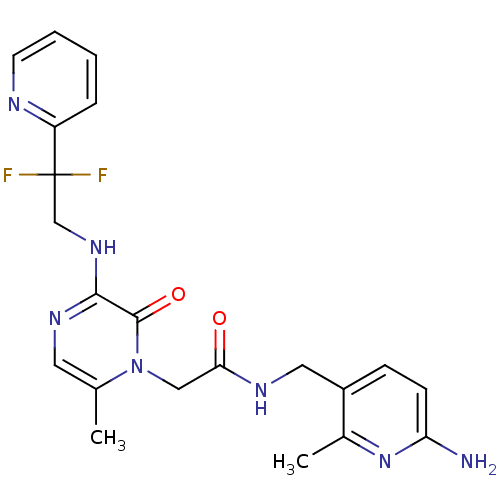
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133529
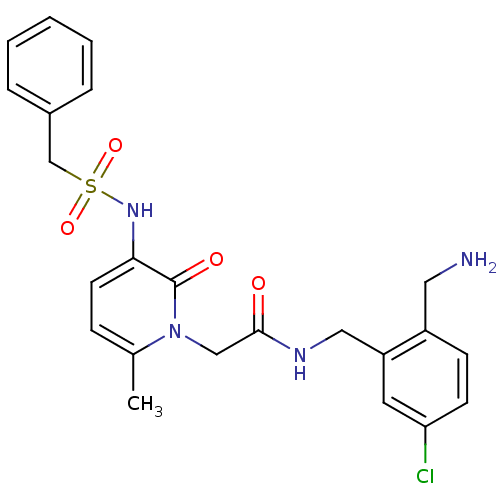
(CHEMBL420682 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C23H25ClN4O4S/c1-16-7-10-21(27-33(31,32)15-17-5-3-2-4-6-17)23(30)28(16)14-22(29)26-13-19-11-20(24)9-8-18(19)12-25/h2-11,27H,12-15,25H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385309
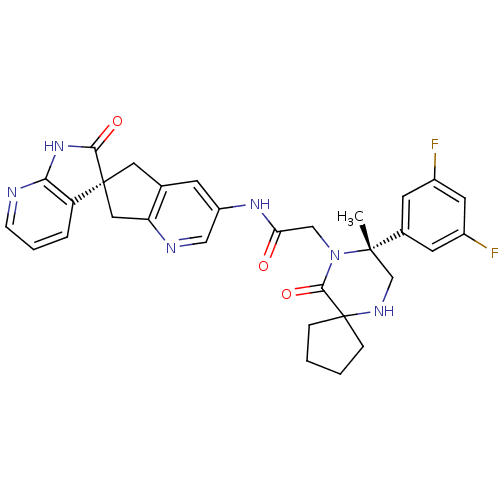
(CHEMBL2035983)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H30F2N6O3/c1-29(19-10-20(32)12-21(33)11-19)17-36-31(6-2-3-7-31)28(42)39(29)16-25(40)37-22-9-18-13-30(14-24(18)35-15-22)23-5-4-8-34-26(23)38-27(30)41/h4-5,8-12,15,36H,2-3,6-7,13-14,16-17H2,1H3,(H,37,40)(H,34,38,41)/t29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131464
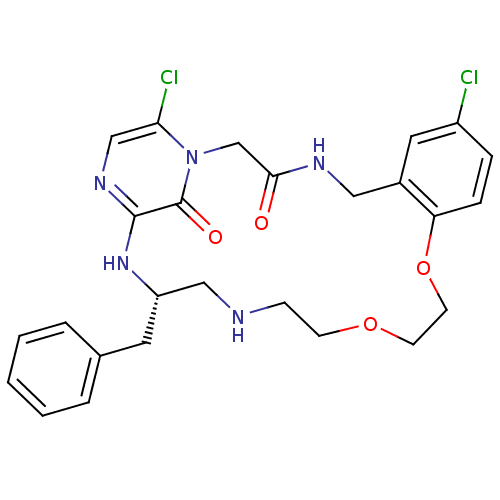
((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...)Show SMILES Clc1ccc2OCCOCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O4/c27-20-6-7-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-4-2-1-3-5-18)15-29-8-9-36-10-11-37-22/h1-7,13,16,21,29H,8-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485494

(CHEMBL2063089)Show SMILES [H][C@@]12C[C@@]1([H])OC(=O)N[C@H](C(=O)N1C[C@@]([H])(C[C@H]1C(=O)N[C@@]1(C[C@@]1([H])C=C)C(=O)NS(=O)(=O)C1CC1)Oc1cc3cc(OC)ccc3nc1CCCCC2)C(C)(C)C |r| Show InChI InChI=1S/C39H51N5O9S/c1-6-24-20-39(24,36(47)43-54(49,50)27-13-14-27)42-34(45)30-19-26-21-44(30)35(46)33(38(2,3)4)41-37(48)53-31-17-22(31)10-8-7-9-11-29-32(52-26)18-23-16-25(51-5)12-15-28(23)40-29/h6,12,15-16,18,22,24,26-27,30-31,33H,1,7-11,13-14,17,19-21H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t22-,24-,26-,30+,31-,33-,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342636
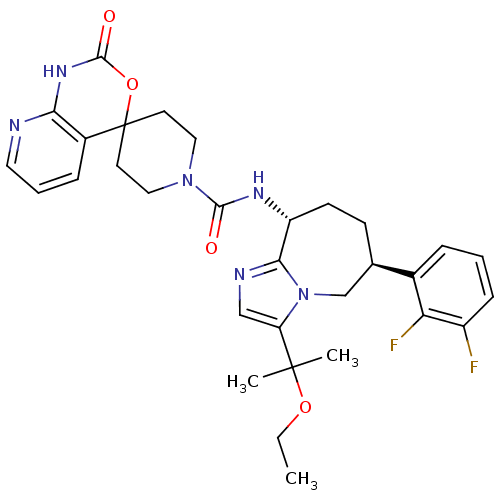
(CHEMBL1770726 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CCOC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H36F2N6O4/c1-4-42-30(2,3)24-17-35-27-23(11-10-19(18-39(24)27)20-7-5-9-22(32)25(20)33)36-28(40)38-15-12-31(13-16-38)21-8-6-14-34-26(21)37-29(41)43-31/h5-9,14,17,19,23H,4,10-13,15-16,18H2,1-3H3,(H,36,40)(H,34,37,41)/t19-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133521
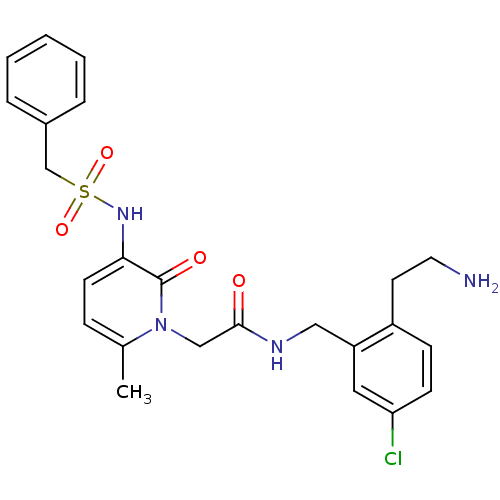
(CHEMBL116202 | N-[2-(2-Amino-ethyl)-5-chloro-benzy...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1CCN Show InChI InChI=1S/C24H27ClN4O4S/c1-17-7-10-22(28-34(32,33)16-18-5-3-2-4-6-18)24(31)29(17)15-23(30)27-14-20-13-21(25)9-8-19(20)11-12-26/h2-10,13,28H,11-12,14-16,26H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292200
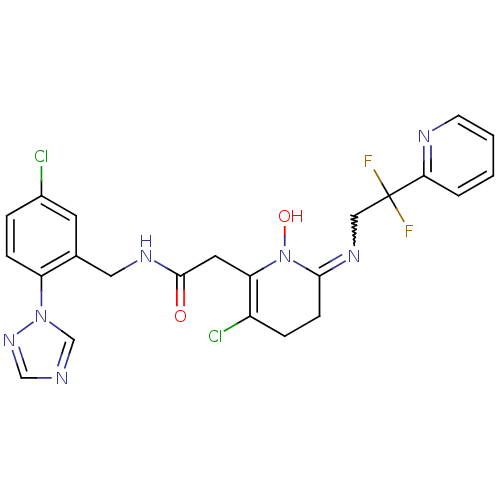
(2-[3-Chloro-6-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES ON1C(CCC(Cl)=C1CC(=O)NCc1cc(Cl)ccc1-n1cncn1)=NCC(F)(F)c1ccccn1 |w:25.28,c:6| Show InChI InChI=1S/C23H21Cl2F2N7O2/c24-16-4-6-18(33-14-28-13-32-33)15(9-16)11-30-22(35)10-19-17(25)5-7-21(34(19)36)31-12-23(26,27)20-3-1-2-8-29-20/h1-4,6,8-9,13-14,36H,5,7,10-12H2,(H,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131460
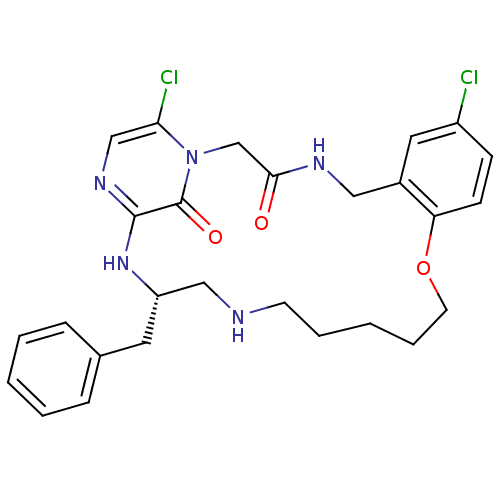
((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...)Show SMILES Clc1ccc2OCCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-9-10-23-20(14-21)15-31-25(35)18-34-24(29)17-32-26(27(34)36)33-22(13-19-7-3-1-4-8-19)16-30-11-5-2-6-12-37-23/h1,3-4,7-10,14,17,22,30H,2,5-6,11-13,15-16,18H2,(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147812
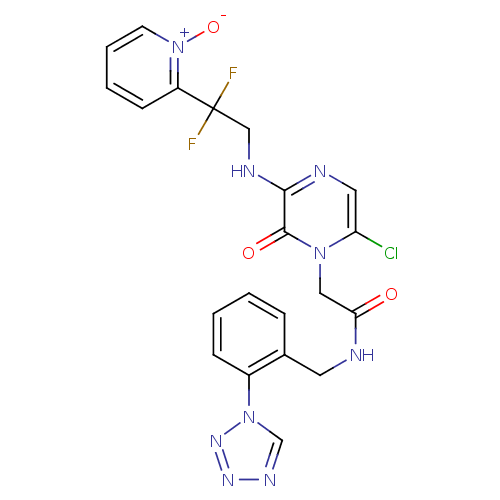
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2ccccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H18ClF2N9O3/c22-17-10-26-19(27-12-21(23,24)16-7-3-4-8-33(16)36)20(35)31(17)11-18(34)25-9-14-5-1-2-6-15(14)32-13-28-29-30-32/h1-8,10,13H,9,11-12H2,(H,25,34)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342634
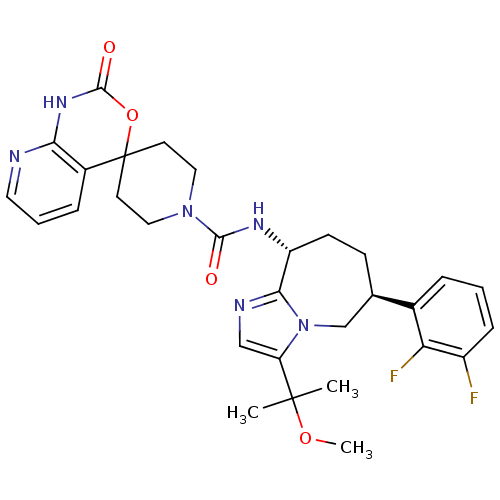
(CHEMBL1770724 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C30H34F2N6O4/c1-29(2,41-3)23-16-34-26-22(10-9-18(17-38(23)26)19-6-4-8-21(31)24(19)32)35-27(39)37-14-11-30(12-15-37)20-7-5-13-33-25(20)36-28(40)42-30/h4-8,13,16,18,22H,9-12,14-15,17H2,1-3H3,(H,35,39)(H,33,36,40)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50385309

(CHEMBL2035983)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H30F2N6O3/c1-29(19-10-20(32)12-21(33)11-19)17-36-31(6-2-3-7-31)28(42)39(29)16-25(40)37-22-9-18-13-30(14-24(18)35-15-22)23-5-4-8-34-26(23)38-27(30)41/h4-5,8-12,15,36H,2-3,6-7,13-14,16-17H2,1H3,(H,37,40)(H,34,38,41)/t29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,V571I]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0520 | -59.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172829
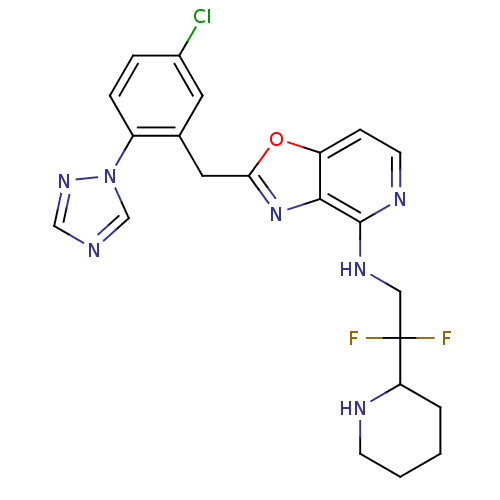
(CHEMBL198735 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES FC(F)(CNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12)C1CCCCN1 Show InChI InChI=1S/C22H22ClF2N7O/c23-15-4-5-16(32-13-26-12-30-32)14(9-15)10-19-31-20-17(33-19)6-8-28-21(20)29-11-22(24,25)18-3-1-2-7-27-18/h4-6,8-9,12-13,18,27H,1-3,7,10-11H2,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131471
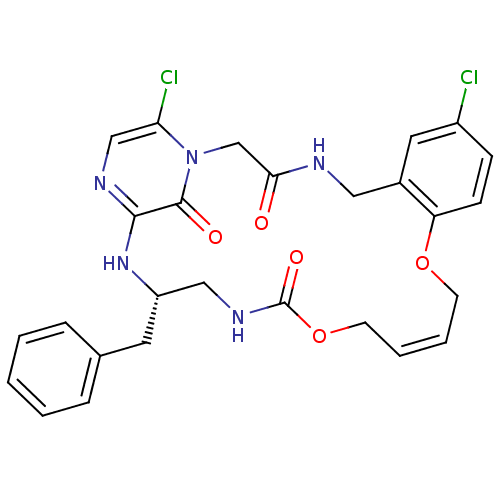
((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C/COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |c:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4-/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485491

(CHEMBL2063088)Show SMILES [H][C@@]12C[C@H](N(C1)C(=O)[C@@H](NC(=O)O[C@]1([H])CCC[C@@]1([H])CCCCCc1nc3ccc(OC)cc3cc1O2)C(C)(C)C)C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C41H55N5O9S/c1-6-26-22-41(26,38(49)45-56(51,52)29-16-17-29)44-36(47)32-21-28-23-46(32)37(48)35(40(2,3)4)43-39(50)55-33-14-10-12-24(33)11-8-7-9-13-31-34(54-28)20-25-19-27(53-5)15-18-30(25)42-31/h6,15,18-20,24,26,28-29,32-33,35H,1,7-14,16-17,21-23H2,2-5H3,(H,43,50)(H,44,47)(H,45,49)/t24-,26-,28-,32+,33-,35-,41-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of Wild-type protease |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data