 Found 265 hits with Last Name = 'weinstock' and Initial = 'j'
Found 265 hits with Last Name = 'weinstock' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(RAT) | BDBM60917
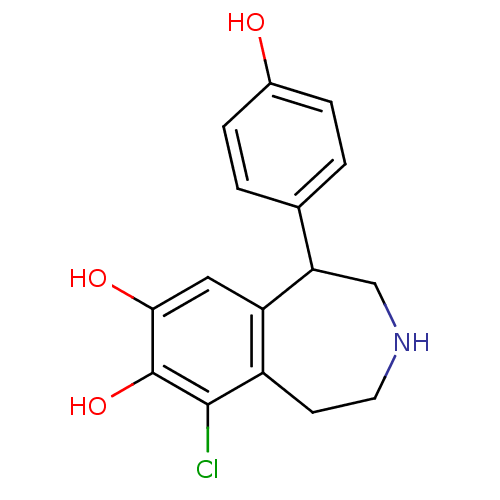
(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(RAT) | BDBM50025202

(9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...)Show InChI InChI=1S/C14H13NO3/c15-6-11-9-3-4-12(17)14(18)13(9)8-2-1-7(16)5-10(8)11/h1-5,11,16-18H,6,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(RAT) | BDBM50025206
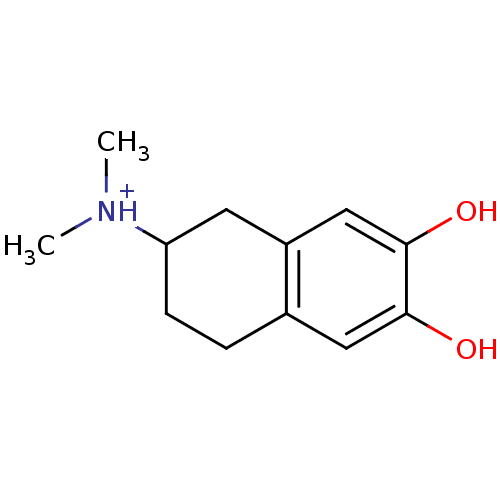
((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C12H17NO2/c1-13(2)10-4-3-8-6-11(14)12(15)7-9(8)5-10/h6-7,10,14-15H,3-5H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(BOVINE) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50025206

((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C12H17NO2/c1-13(2)10-4-3-8-6-11(14)12(15)7-9(8)5-10/h6-7,10,14-15H,3-5H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025201
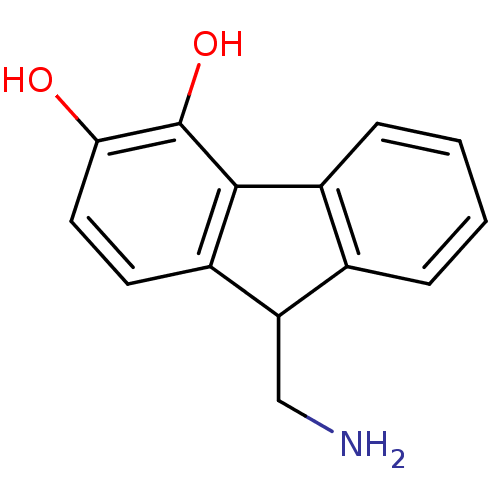
(9-Aminomethyl-9H-fluorene-3,4-diol | CHEMBL55693)Show InChI InChI=1S/C14H13NO2/c15-7-11-8-3-1-2-4-9(8)13-10(11)5-6-12(16)14(13)17/h1-6,11,16-17H,7,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025208

(6-Chloro-9-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-14-12-7-9-18-8-6-11(12)13(15(19)16(14)20)10-4-2-1-3-5-10/h1-5,18-20H,6-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50004821

(2,3,4,5-Tetrahydro-1H-benzo[d]azepine-7,8-diol | C...)Show InChI InChI=1S/C10H13NO2/c12-9-5-7-1-3-11-4-2-8(7)6-10(9)13/h5-6,11-13H,1-4H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against dDopamine receptor D1 using [3H]fenoldopam as a radioligand |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025204
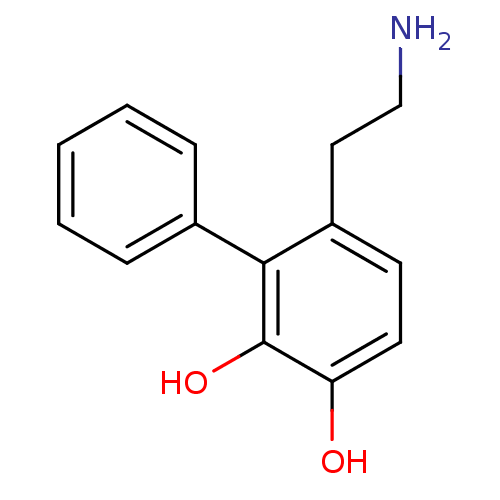
(6-(2-Amino-ethyl)-biphenyl-2,3-diol | CHEMBL299511)Show InChI InChI=1S/C14H15NO2/c15-9-8-11-6-7-12(16)14(17)13(11)10-4-2-1-3-5-10/h1-7,16-17H,8-9,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025209
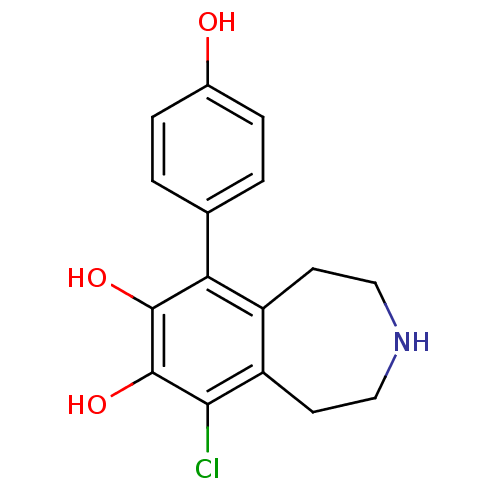
(6-Chloro-9-(4-hydroxy-phenyl)-2,3,4,5-tetrahydro-1...)Show InChI InChI=1S/C16H16ClNO3/c17-14-12-6-8-18-7-5-11(12)13(15(20)16(14)21)9-1-3-10(19)4-2-9/h1-4,18-21H,5-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025207
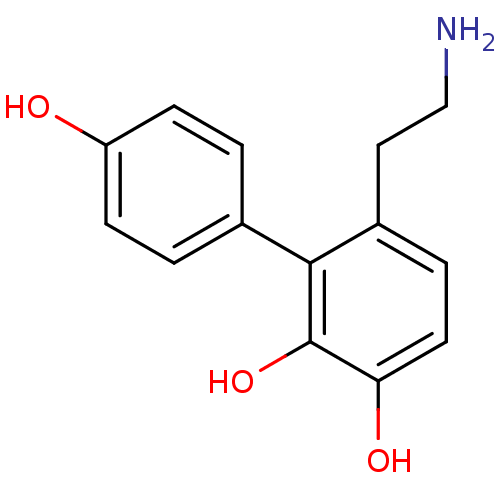
(6-(2-Amino-ethyl)-biphenyl-2,3,4'-triol | CHEMBL57...)Show InChI InChI=1S/C14H15NO3/c15-8-7-10-3-6-12(17)14(18)13(10)9-1-4-11(16)5-2-9/h1-6,16-18H,7-8,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025203
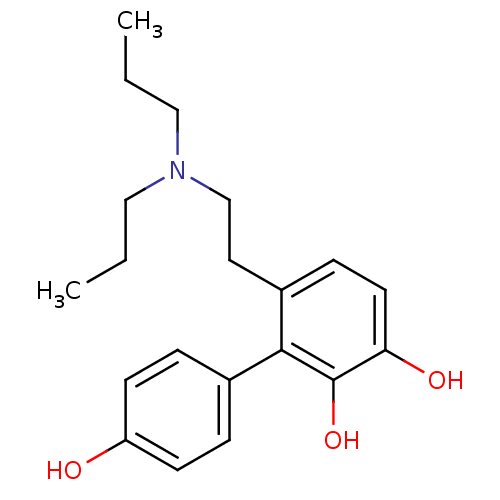
(6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...)Show InChI InChI=1S/C20H27NO3/c1-3-12-21(13-4-2)14-11-16-7-10-18(23)20(24)19(16)15-5-8-17(22)9-6-15/h5-10,22-24H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025210

(6-Phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8...)Show InChI InChI=1S/C16H17NO2/c18-14-10-12-6-8-17-9-7-13(12)15(16(14)19)11-4-2-1-3-5-11/h1-5,10,17-19H,6-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50025203

(6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...)Show InChI InChI=1S/C20H27NO3/c1-3-12-21(13-4-2)14-11-16-7-10-18(23)20(24)19(16)15-5-8-17(22)9-6-15/h5-10,22-24H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025205

(6-(2-Dipropylamino-ethyl)-biphenyl-2,3-diol | CHEM...)Show InChI InChI=1S/C20H27NO2/c1-3-13-21(14-4-2)15-12-17-10-11-18(22)20(23)19(17)16-8-6-5-7-9-16/h5-11,22-23H,3-4,12-15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025211

(6-(4-Hydroxy-phenyl)-2,3,4,5-tetrahydro-1H-benzo[d...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)15-13-6-8-17-7-5-11(13)9-14(19)16(15)20/h1-4,9,17-20H,5-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50025202

(9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...)Show InChI InChI=1S/C14H13NO3/c15-6-11-9-3-4-12(17)14(18)13(9)8-2-1-7(16)5-10(8)11/h1-5,11,16-18H,6,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011972

(CHEMBL385189 | Sar-Arg-Val-Tyr-Ile-His-Pro-Thi-OH)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cccs1)C(O)=O Show InChI InChI=1S/C47H69N13O10S/c1-6-27(4)39(44(67)56-34(21-29-23-51-25-53-29)45(68)60-18-8-12-36(60)42(65)57-35(46(69)70)22-31-10-9-19-71-31)59-41(64)33(20-28-13-15-30(61)16-14-28)55-43(66)38(26(2)3)58-40(63)32(54-37(62)24-50-5)11-7-17-52-47(48)49/h9-10,13-16,19,23,25-27,32-36,38-39,50,61H,6-8,11-12,17-18,20-22,24H2,1-5H3,(H,51,53)(H,54,62)(H,55,66)(H,56,67)(H,57,65)(H,58,63)(H,59,64)(H,69,70)(H4,48,49,52)/t27-,32-,33-,34-,35-,36-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230790

(CHEMBL292892)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C23H23N3O6S/c1-2-3-6-21-24-13-18(10-17(23(29)30)11-19-5-4-9-33-19)25(21)14-16-8-7-15(22(27)28)12-20(16)26(31)32/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282363
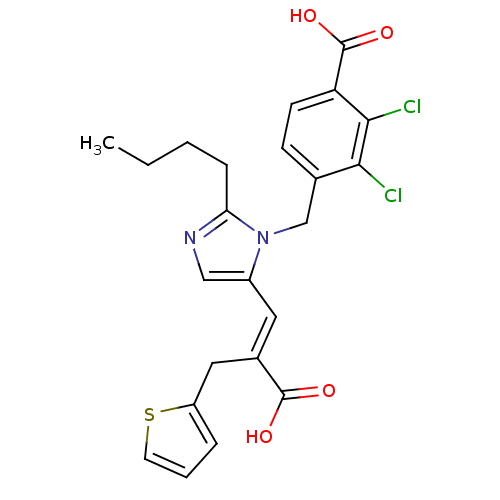
(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1Cl Show InChI InChI=1S/C23H22Cl2N2O4S/c1-2-3-6-19-26-12-16(10-15(22(28)29)11-17-5-4-9-32-17)27(19)13-14-7-8-18(23(30)31)21(25)20(14)24/h4-5,7-10,12H,2-3,6,11,13H2,1H3,(H,28,29)(H,30,31)/b15-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230779

(CHEMBL294686)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)c2nn[nH]n2)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(29(21)15-16-7-9-17(10-8-16)23(30)31)12-18(22-25-27-28-26-22)13-20-5-4-11-32-20/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230811
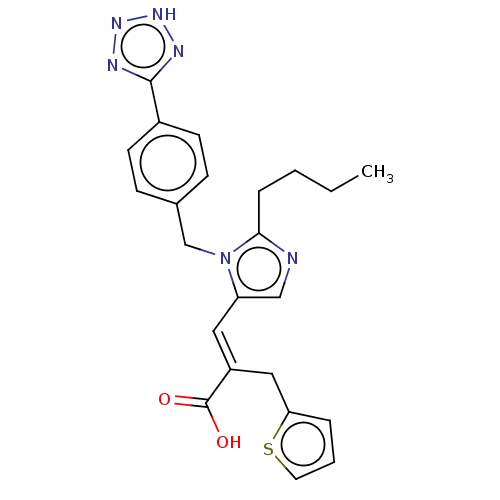
(CHEMBL293091)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1nn[nH]n1 Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(12-18(23(30)31)13-20-5-4-11-32-20)29(21)15-16-7-9-17(10-8-16)22-25-27-28-26-22/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230778
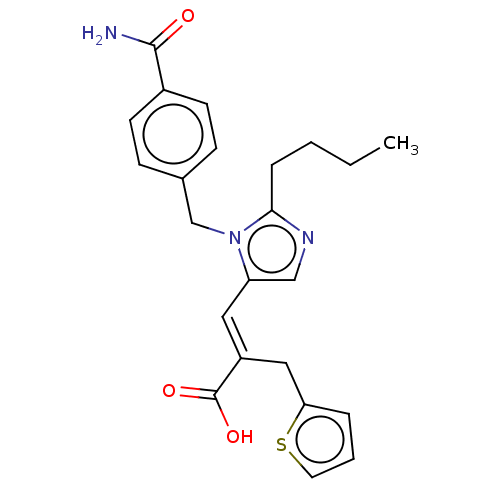
(CHEMBL56211)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(N)=O Show InChI InChI=1S/C23H25N3O3S/c1-2-3-6-21-25-14-19(12-18(23(28)29)13-20-5-4-11-30-20)26(21)15-16-7-9-17(10-8-16)22(24)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H2,24,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230771
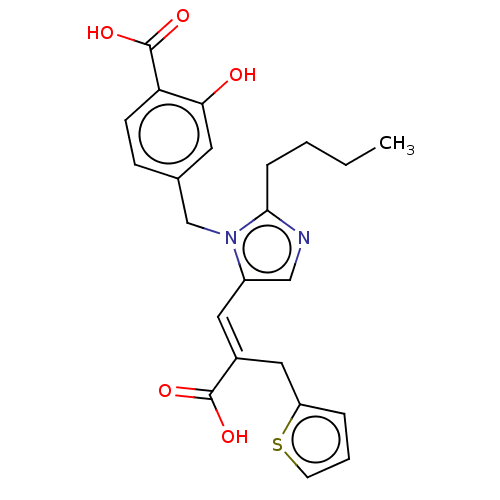
(CHEMBL55510)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(O)c1 Show InChI InChI=1S/C23H24N2O5S/c1-2-3-6-21-24-13-17(11-16(22(27)28)12-18-5-4-9-31-18)25(21)14-15-7-8-19(23(29)30)20(26)10-15/h4-5,7-11,13,26H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230806

(CHEMBL294415)Show SMILES CCCCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H28N2O4S/c1-2-3-4-5-8-23-26-16-21(14-20(25(30)31)15-22-7-6-13-32-22)27(23)17-18-9-11-19(12-10-18)24(28)29/h6-7,9-14,16H,2-5,8,15,17H2,1H3,(H,28,29)(H,30,31)/b20-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011977
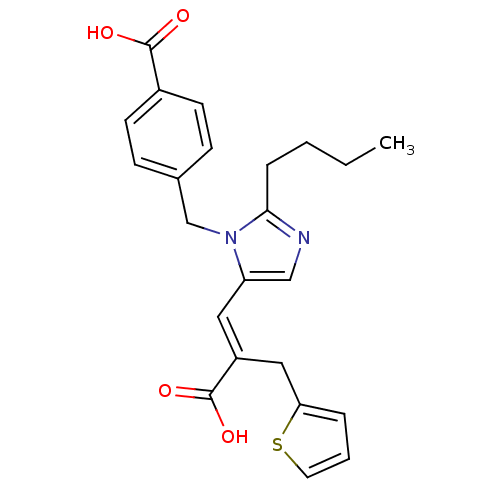
((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards Angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50048078

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-31-18)26(21)14-15-7-8-19(23(29)30)20(24)10-15/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50011977

((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50048078

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-31-18)26(21)14-15-7-8-19(23(29)30)20(24)10-15/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50282362
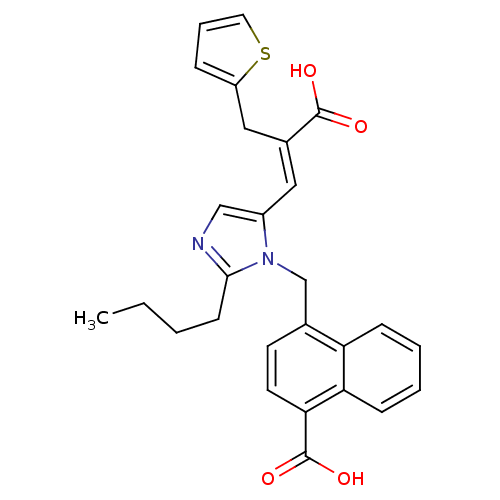
(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c2ccccc12 Show InChI InChI=1S/C27H26N2O4S/c1-2-3-10-25-28-16-20(14-19(26(30)31)15-21-7-6-13-34-21)29(25)17-18-11-12-24(27(32)33)23-9-5-4-8-22(18)23/h4-9,11-14,16H,2-3,10,15,17H2,1H3,(H,30,31)(H,32,33)/b19-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for Angiotensin II receptor, type 1 affinity in the absence of BSA |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011973
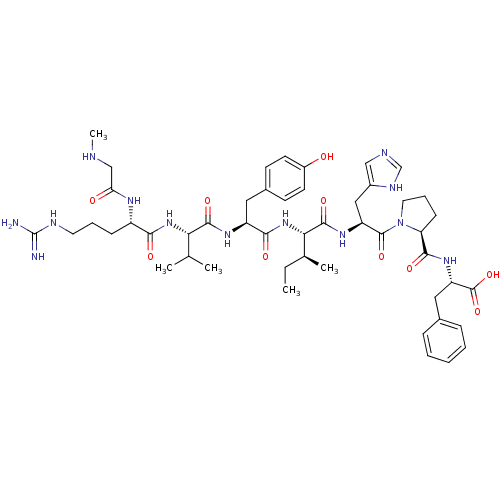
(CHEMBL216061 | Sar-Arg-Val-Tyr-Ile-His-Pro-Phe | S...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards Angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50011977

((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for Angiotensin II receptor, type 1 affinity in the absence of bovine serum albumin (BSA) |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230787

(CHEMBL54421)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)-c1ccccc1 Show InChI InChI=1S/C29H28N2O4S/c1-2-3-11-27-30-18-23(16-22(28(32)33)17-24-10-7-14-36-24)31(27)19-20-12-13-25(29(34)35)26(15-20)21-8-5-4-6-9-21/h4-10,12-16,18H,2-3,11,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50284297
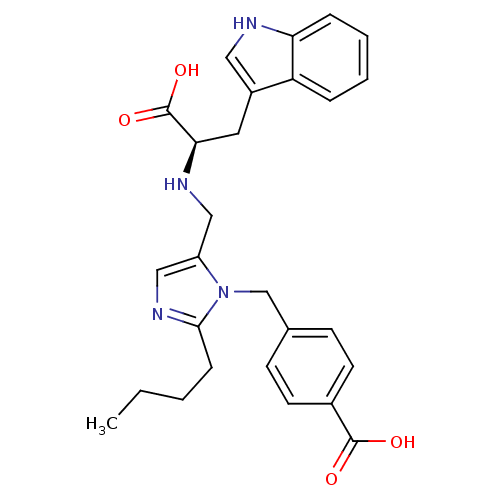
(4-(2-Butyl-5-{[(R)-1-carboxy-2-(1H-indol-3-yl)-eth...)Show SMILES CCCCc1ncc(CN[C@H](Cc2c[nH]c3ccccc23)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C27H30N4O4/c1-2-3-8-25-30-16-21(31(25)17-18-9-11-19(12-10-18)26(32)33)15-29-24(27(34)35)13-20-14-28-23-7-5-4-6-22(20)23/h4-7,9-12,14,16,24,28-29H,2-3,8,13,15,17H2,1H3,(H,32,33)(H,34,35)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of Angiotensin II specific binding to Angiotensin II receptor, type 1 in the recombinant human AT-1 receptor expressed in LhAT-... |
Bioorg Med Chem Lett 5: 19-24 (1995)
Article DOI: 10.1016/0960-894X(94)00456-P
BindingDB Entry DOI: 10.7270/Q2GF0TF6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230799
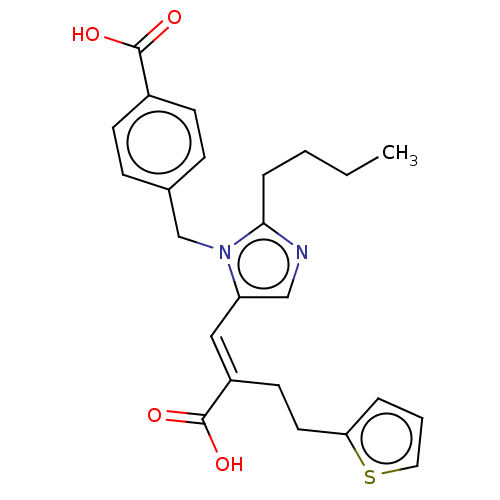
(CHEMBL56333)Show SMILES CCCCc1ncc(\C=C(/CCc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-2-3-6-22-25-15-20(14-19(24(29)30)11-12-21-5-4-13-31-21)26(22)16-17-7-9-18(10-8-17)23(27)28/h4-5,7-10,13-15H,2-3,6,11-12,16H2,1H3,(H,27,28)(H,29,30)/b19-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230792
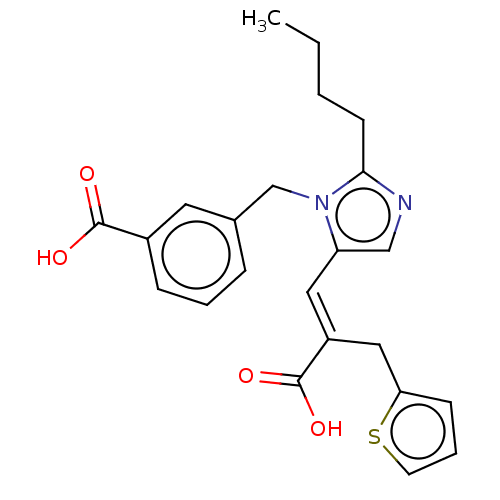
(CHEMBL56497)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1cccc(c1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-9-21-24-14-19(12-18(23(28)29)13-20-8-5-10-30-20)25(21)15-16-6-4-7-17(11-16)22(26)27/h4-8,10-12,14H,2-3,9,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230774
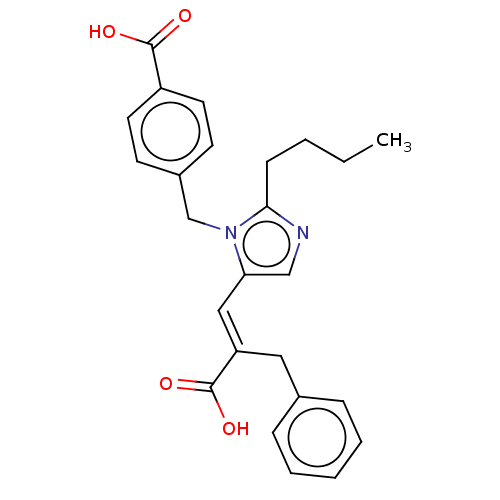
(CHEMBL291955)Show SMILES CCCCc1ncc(\C=C(/Cc2ccccc2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H26N2O4/c1-2-3-9-23-26-16-22(15-21(25(30)31)14-18-7-5-4-6-8-18)27(23)17-19-10-12-20(13-11-19)24(28)29/h4-8,10-13,15-16H,2-3,9,14,17H2,1H3,(H,28,29)(H,30,31)/b21-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230762

(CHEMBL56160)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H25N3O4S2/c1-2-3-6-21-24-14-18(12-17(22(26)27)13-19-5-4-11-30-19)25(21)15-16-7-9-20(10-8-16)31(23,28)29/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H2,23,28,29)/b17-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230810
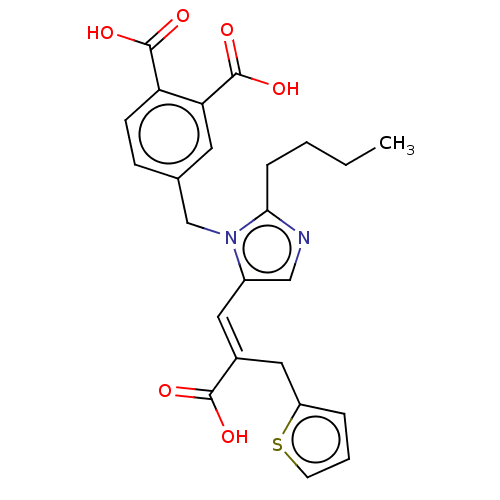
(CHEMBL299064)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C24H24N2O6S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-33-18)26(21)14-15-7-8-19(23(29)30)20(10-15)24(31)32/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)(H,31,32)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230764
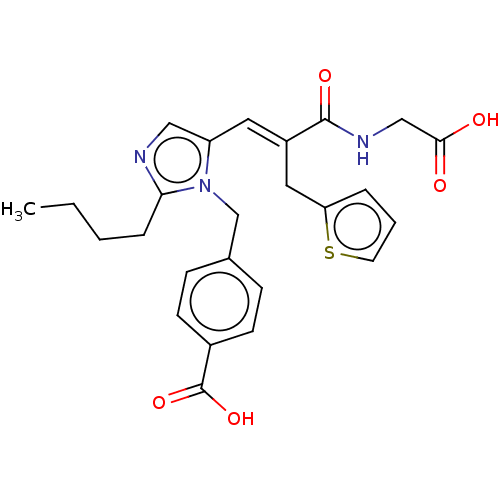
(CHEMBL299954)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(=O)NCC(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27N3O5S/c1-2-3-6-22-26-14-20(28(22)16-17-7-9-18(10-8-17)25(32)33)12-19(13-21-5-4-11-34-21)24(31)27-15-23(29)30/h4-5,7-12,14H,2-3,6,13,15-16H2,1H3,(H,27,31)(H,29,30)(H,32,33)/b19-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230767
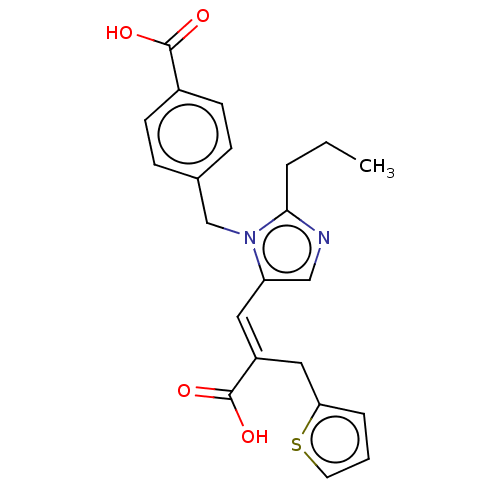
(CHEMBL56522)Show SMILES CCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H22N2O4S/c1-2-4-20-23-13-18(11-17(22(27)28)12-19-5-3-10-29-19)24(20)14-15-6-8-16(9-7-15)21(25)26/h3,5-11,13H,2,4,12,14H2,1H3,(H,25,26)(H,27,28)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230796

(CHEMBL56690)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N2O4S/c1-2-3-10-27-30-18-23(16-22(28(32)33)17-24-7-6-15-36-24)31(27)19-20-11-13-21(14-12-20)25-8-4-5-9-26(25)29(34)35/h4-9,11-16,18H,2-3,10,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50011977

((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for Angiotensin II receptor, type 1 affinity in the presence of 0.25% bovine serum albumin (BSA) |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data