

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052327 (6-hydroxy-15-isopropenyl-4,13,17-trimethyl-5-oxo-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052320 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052323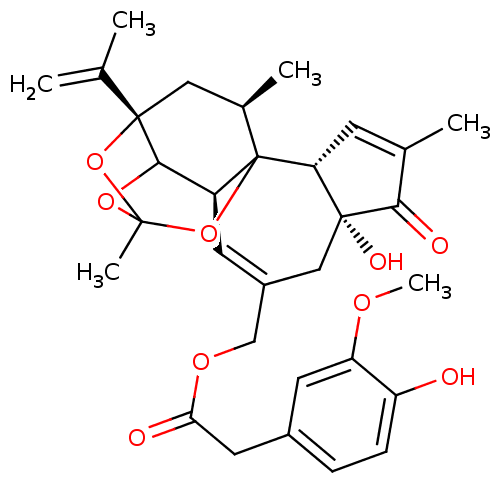 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid 4a,7b-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052333 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052328 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052330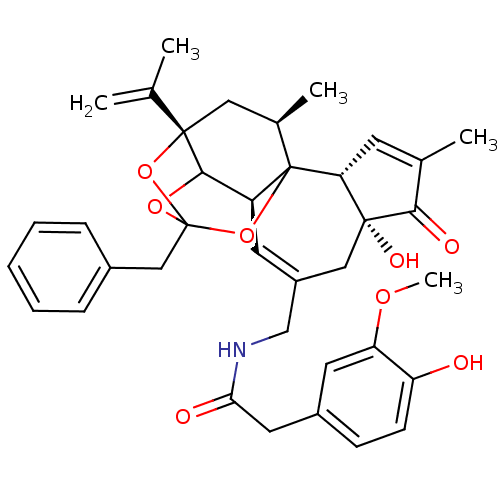 (13-benzyl-5,6-dihydroxy-15-isopropenyl-4,17-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23232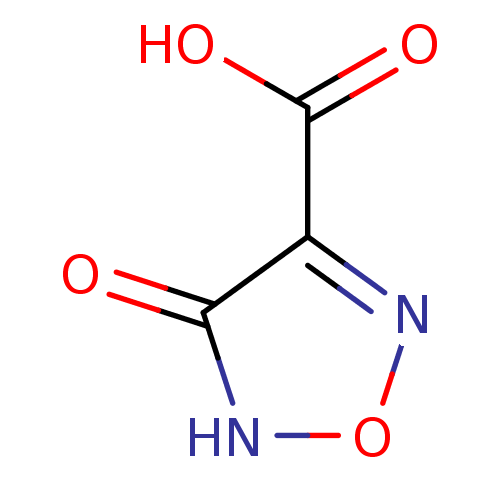 (1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23251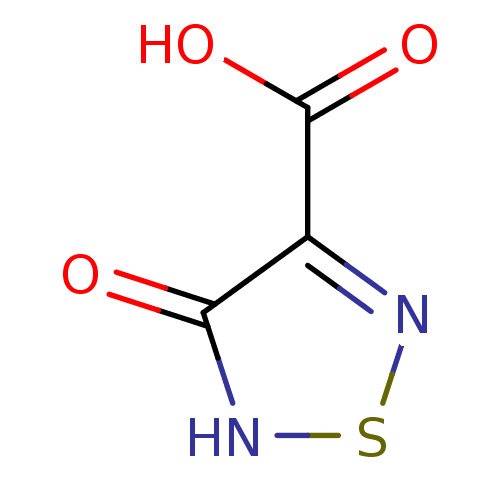 (1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50385474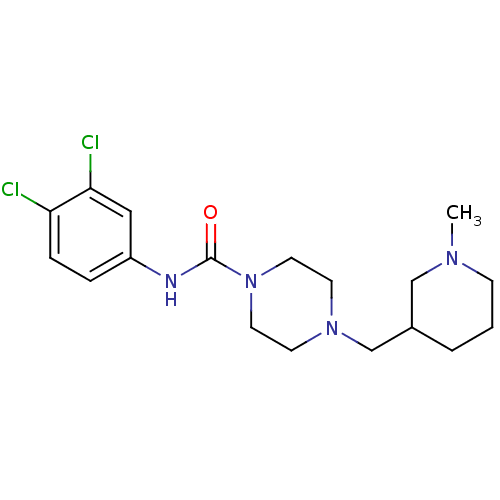 (CHEMBL2036748) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of 125I-MCP1 from human CCR2 receptor expressed in human HEK cell membrane | Bioorg Med Chem Lett 22: 3895-9 (2012) Article DOI: 10.1016/j.bmcl.2012.04.118 BindingDB Entry DOI: 10.7270/Q2PG1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23242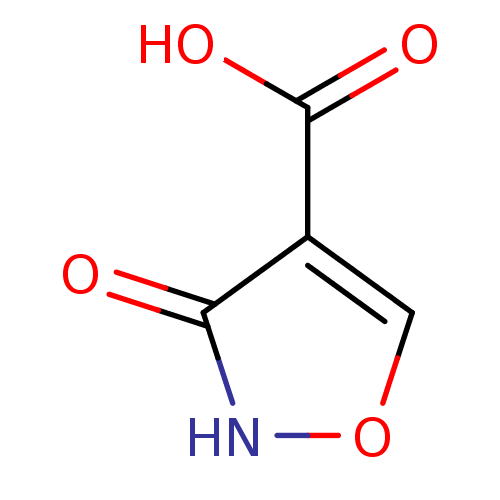 (1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052321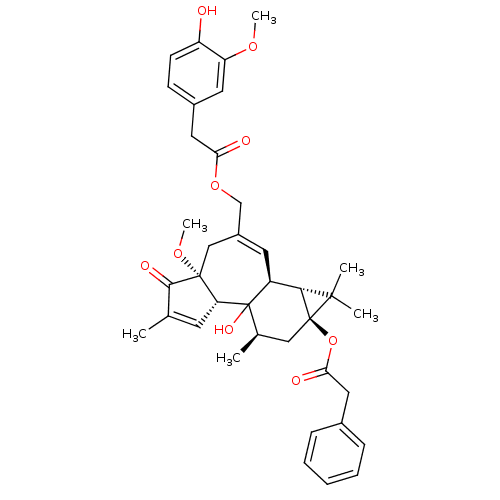 (CHEMBL98634 | Phenyl-acetic acid (1aR,1bS,4aR,7aS,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20461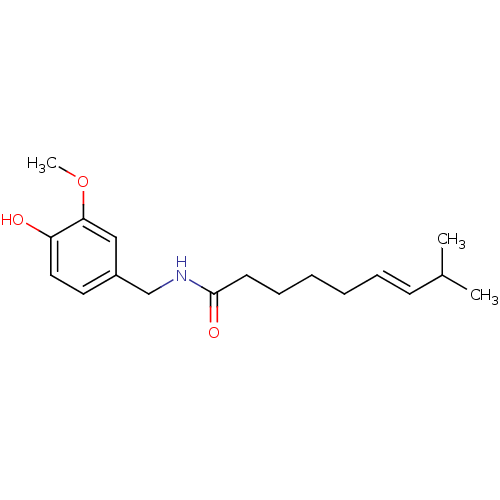 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052324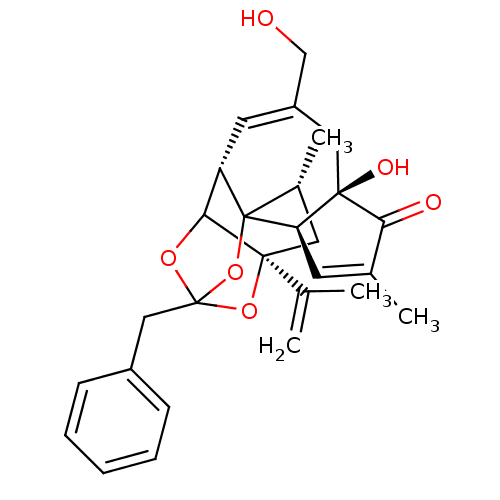 (13-benzyl-6-hydroxy-8-hydroxymethyl-15-isopropenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50563635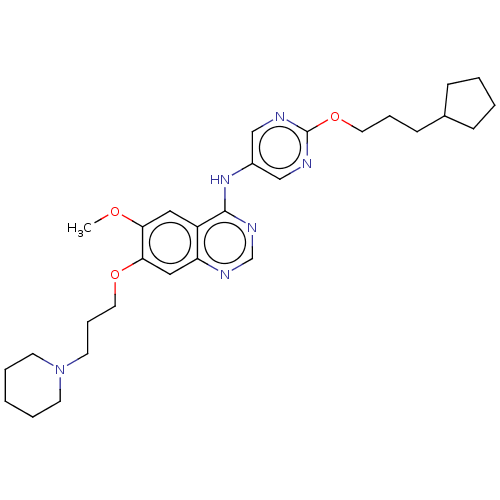 (CHEMBL4777640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50573136 (CHEMBL4875486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MER (unknown origin) by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00920 BindingDB Entry DOI: 10.7270/Q2S18686 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50172077 (CHEMBL3810063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50173389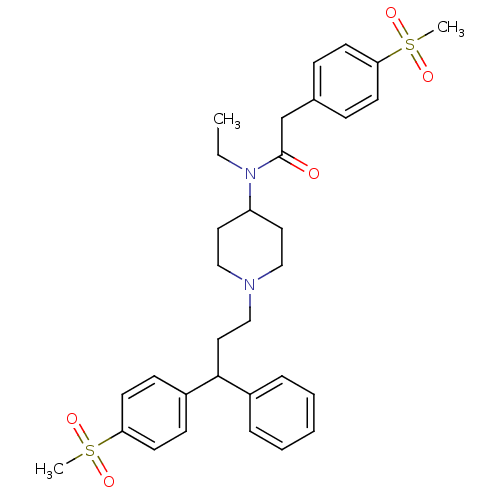 (CHEMBL198150 | N-Ethyl-2-(4-methanesulfonyl-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed in CHO cells | Bioorg Med Chem Lett 15: 5012-5 (2005) Article DOI: 10.1016/j.bmcl.2005.08.014 BindingDB Entry DOI: 10.7270/Q23R0SFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50573135 (CHEMBL4873217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MER (unknown origin) by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00920 BindingDB Entry DOI: 10.7270/Q2S18686 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50173388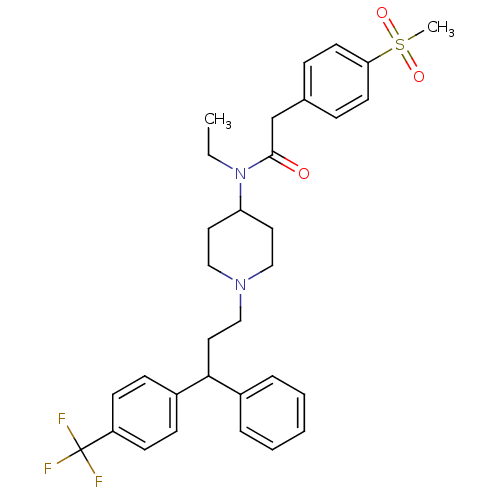 (CHEMBL197641 | N-Ethyl-2-(4-methanesulfonyl-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed in CHO cells | Bioorg Med Chem Lett 15: 5012-5 (2005) Article DOI: 10.1016/j.bmcl.2005.08.014 BindingDB Entry DOI: 10.7270/Q23R0SFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50573128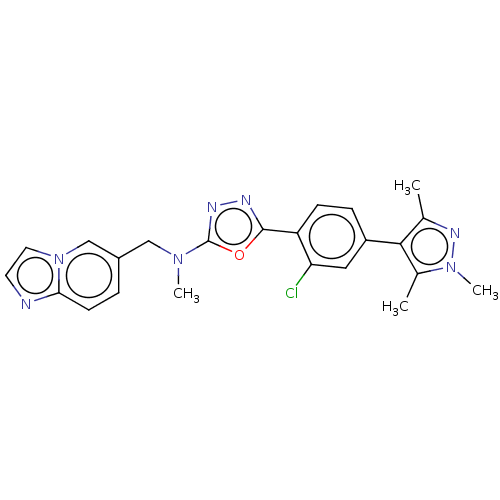 (CHEMBL4865752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MER (unknown origin) by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00920 BindingDB Entry DOI: 10.7270/Q2S18686 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (Homo sapiens (Human)) | BDBM50082118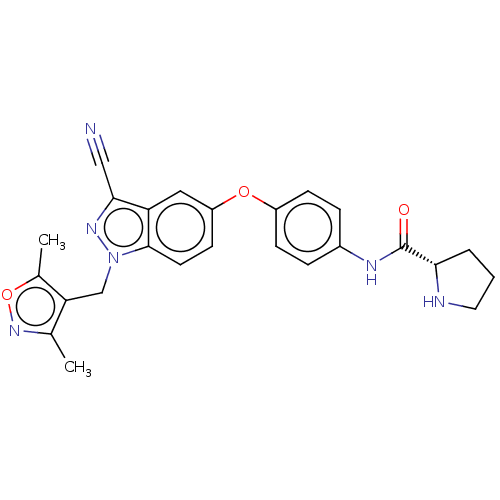 (CHEMBL3422678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay | J Med Chem 58: 3611-25 (2015) Article DOI: 10.1021/acs.jmedchem.5b00352 BindingDB Entry DOI: 10.7270/Q2571DQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264231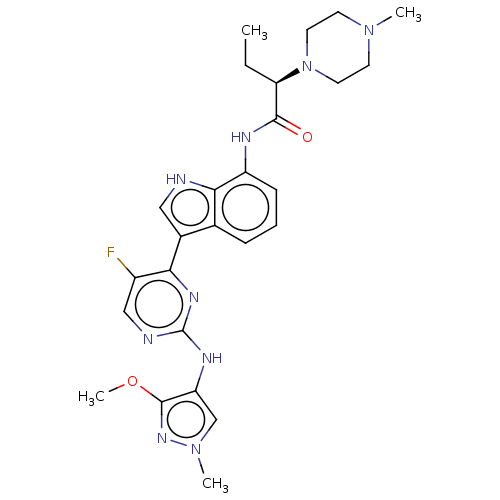 ((2R)-N-(3-{5-fluoro- 2-[(3-methoxy-1- methyl-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264233 ((2R)-N-(3-{2- [(1,3-dimethyl-1H- pyrazol-4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264225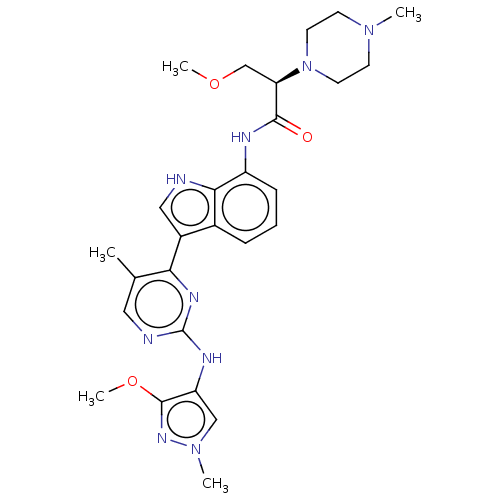 ((2R)-3-methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264233 ((2R)-N-(3-{2- [(1,3-dimethyl-1H- pyrazol-4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264225 ((2R)-3-methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264225 ((2R)-3-methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10167276 (2019) BindingDB Entry DOI: 10.7270/Q20R9RG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264231 ((2R)-N-(3-{5-fluoro- 2-[(3-methoxy-1- methyl-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10167276 (2019) BindingDB Entry DOI: 10.7270/Q20R9RG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264233 ((2R)-N-(3-{2- [(1,3-dimethyl-1H- pyrazol-4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10167276 (2019) BindingDB Entry DOI: 10.7270/Q20R9RG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264231 ((2R)-N-(3-{5-fluoro- 2-[(3-methoxy-1- methyl-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50172077 (CHEMBL3810063) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged AXL (unknown origin) (464 to 485 residues) using Axltide (CKKSRGDYMTMQJG-acid) peptide as substrate preincubated for 30 mins... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50563640 (CHEMBL4746916) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6/TEV fused-GST-tagged Flt3 (unknown origin) (H564 to S993 residues) using Axltide (CKKSRGDYMTMQJ-acid) peptide as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50573130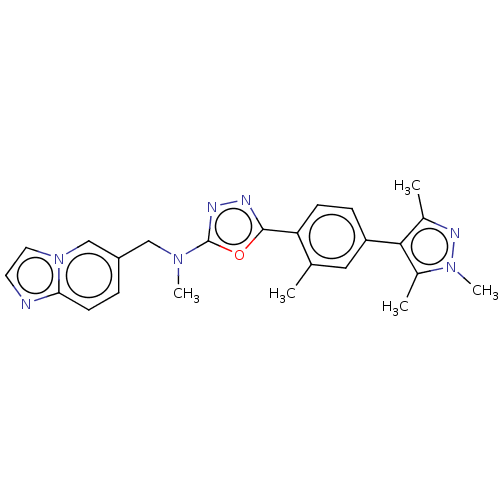 (CHEMBL4855381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MER (unknown origin) by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00920 BindingDB Entry DOI: 10.7270/Q2S18686 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50385450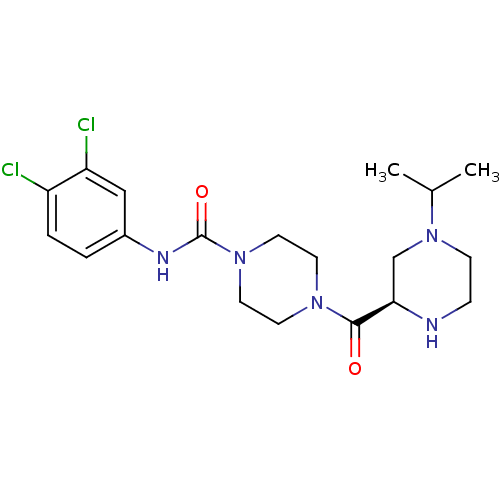 (CHEMBL2036782) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of 125I-MCP1 from human CCR2 receptor expressed in human THP-1 cell membrane by SPA assay | Bioorg Med Chem Lett 22: 3895-9 (2012) Article DOI: 10.1016/j.bmcl.2012.04.118 BindingDB Entry DOI: 10.7270/Q2PG1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (Homo sapiens (Human)) | BDBM50082117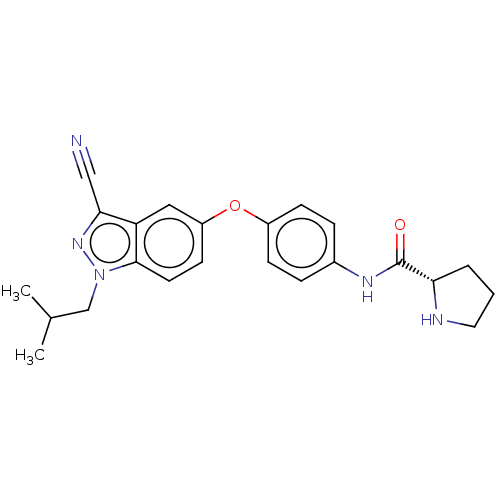 (CHEMBL3422677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay | J Med Chem 58: 3611-25 (2015) Article DOI: 10.1021/acs.jmedchem.5b00352 BindingDB Entry DOI: 10.7270/Q2571DQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264224 ((2R)-N-(3-{2-[(3- Methoxy-1-methyl- 1H-pyrazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264229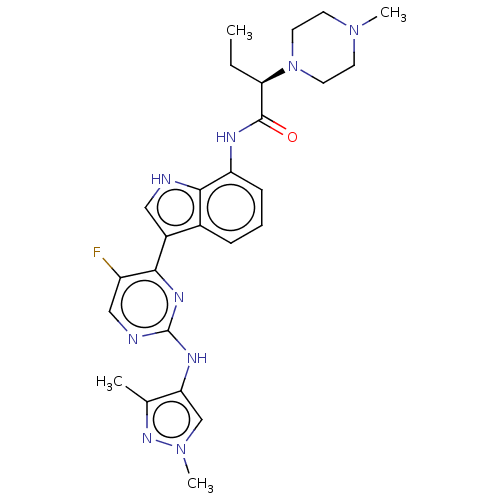 ((2R)-N-(3-{2-[(1,3- dimethyl-1H-pyrazol- 4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264229 ((2R)-N-(3-{2-[(1,3- dimethyl-1H-pyrazol- 4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10167276 (2019) BindingDB Entry DOI: 10.7270/Q20R9RG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264240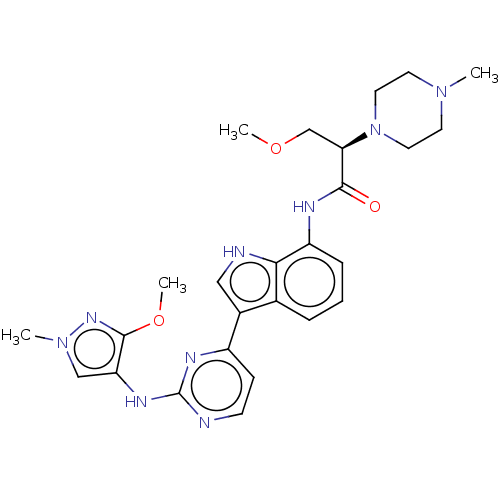 ((2R)-3-Methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10167276 (2019) BindingDB Entry DOI: 10.7270/Q20R9RG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264240 ((2R)-3-Methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50563644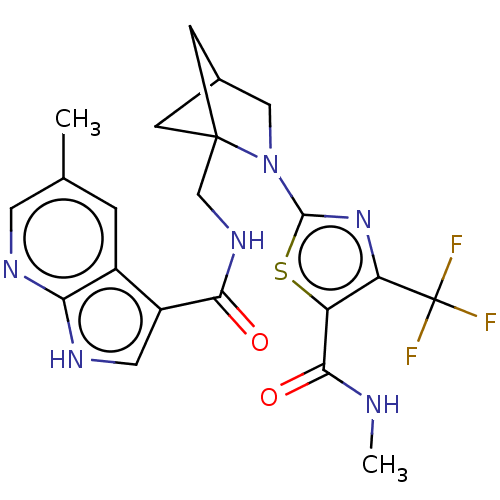 (CHEMBL4782810) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged AXL (unknown origin) (464 to 485 residues) using Axltide (CKKSRGDYMTMQJG-acid) peptide as substrate preincubated for 30 mins... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527916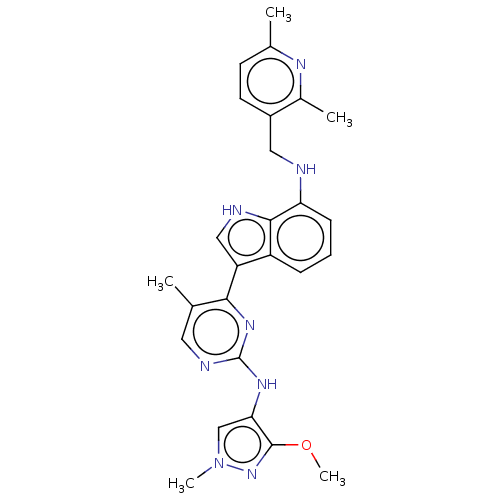 (CHEMBL4446962) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged JAK1 (866 to 1154 residues) expressed in insect cells using FITC-labeled C6-KKHTDDGYMPMSPGVA-NH... | J Med Chem 63: 4517-4527 (2020) Article DOI: 10.1021/acs.jmedchem.9b01392 BindingDB Entry DOI: 10.7270/Q21V5JDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264224 ((2R)-N-(3-{2-[(3- Methoxy-1-methyl- 1H-pyrazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10167276 (2019) BindingDB Entry DOI: 10.7270/Q20R9RG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264224 ((2R)-N-(3-{2-[(3- Methoxy-1-methyl- 1H-pyrazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264229 ((2R)-N-(3-{2-[(1,3- dimethyl-1H-pyrazol- 4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264240 ((2R)-3-Methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM313401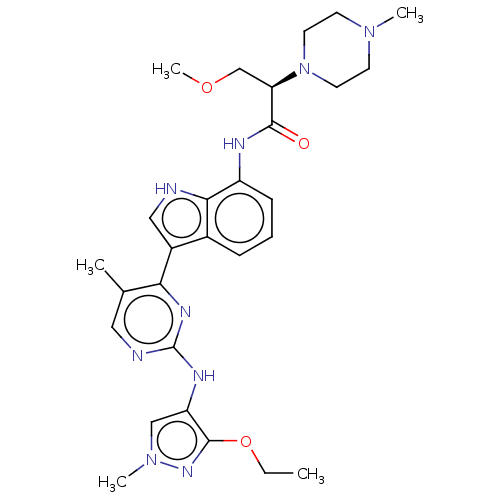 ((2R)-N-(3-{2-[(3-ethoxy-1-methyl-1H- pyrazol-4-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10167276 (2019) BindingDB Entry DOI: 10.7270/Q20R9RG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM313401 ((2R)-N-(3-{2-[(3-ethoxy-1-methyl-1H- pyrazol-4-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1816 total ) | Next | Last >> |