

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114327 BindingDB Entry DOI: 10.7270/Q2NP28GR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100535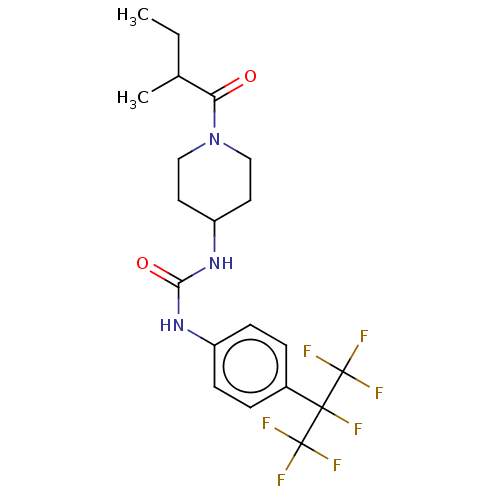 (CHEMBL3327073) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100528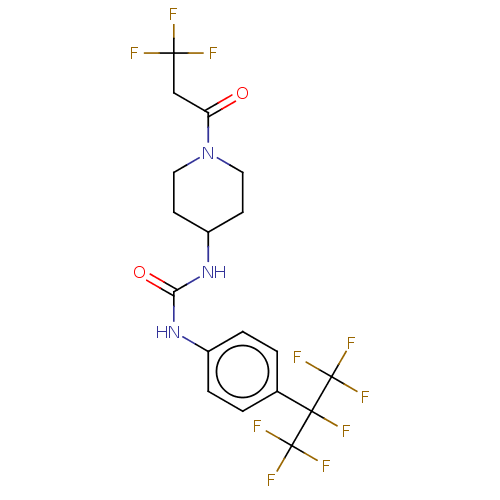 (CHEMBL3327081) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409020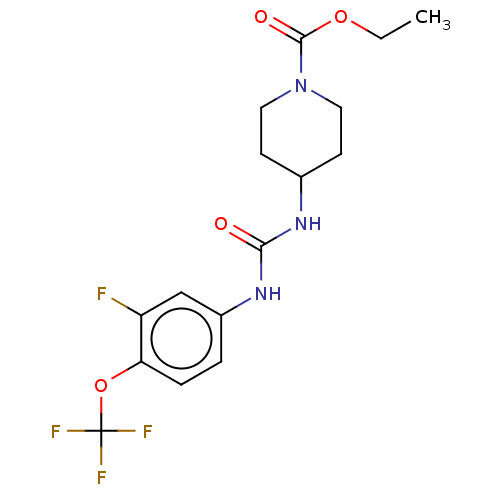 (US10377744, Compound No. 40 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409009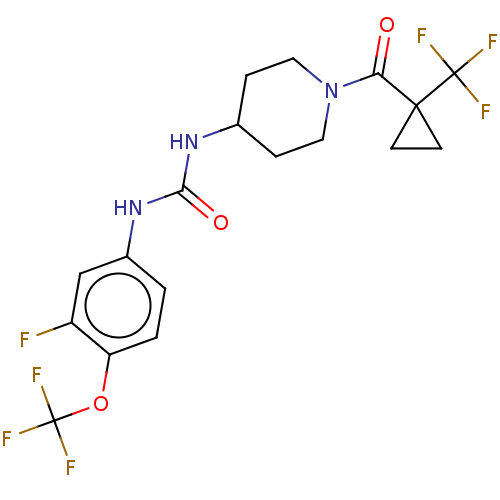 (US10377744, Compound No. 30 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409005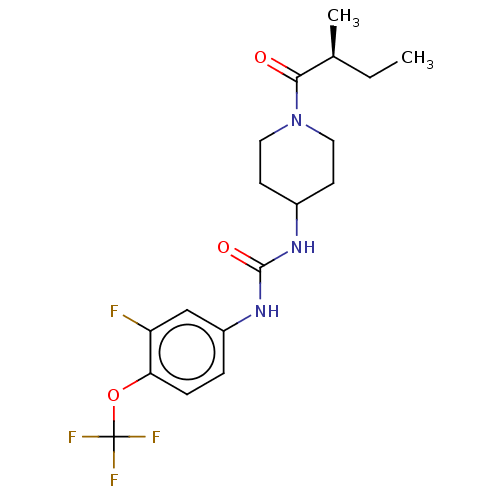 (US10377744, Compound No. 26 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409020 (US10377744, Compound No. 40 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409005 (US10377744, Compound No. 26 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409005 (US10377744, Compound No. 26 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM517699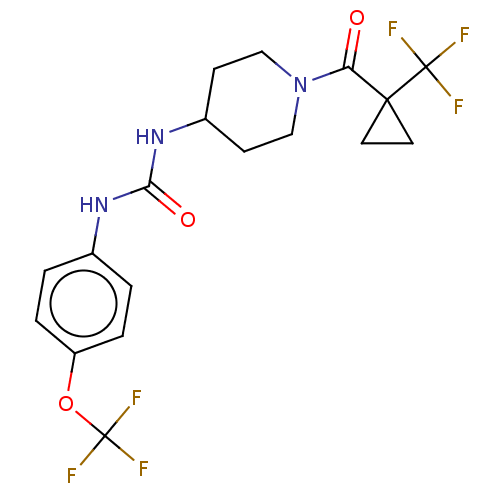 (US11123311, Compound 28) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409009 (US10377744, Compound No. 30 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409030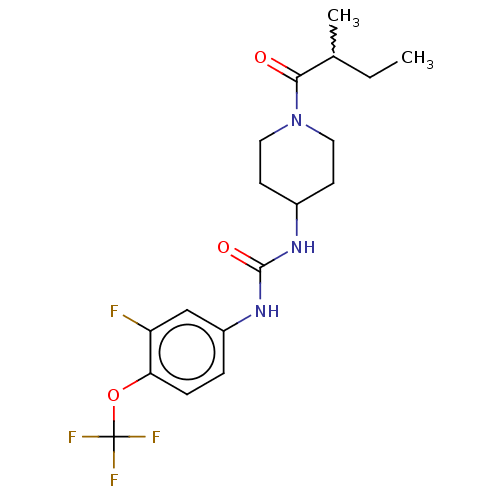 (US10377744, Compound No. 51 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409016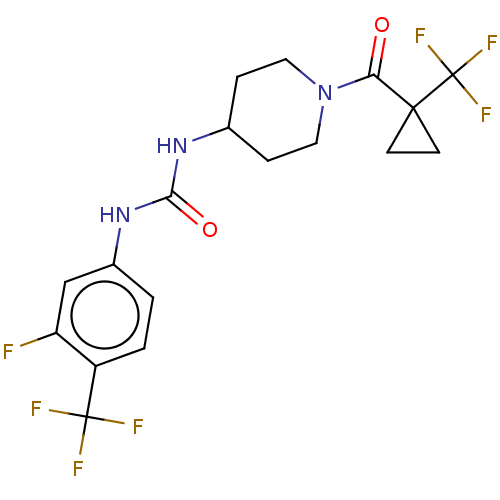 (US10377744, Compound No. 36 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19023 (1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409016 (US10377744, Compound No. 36 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50434787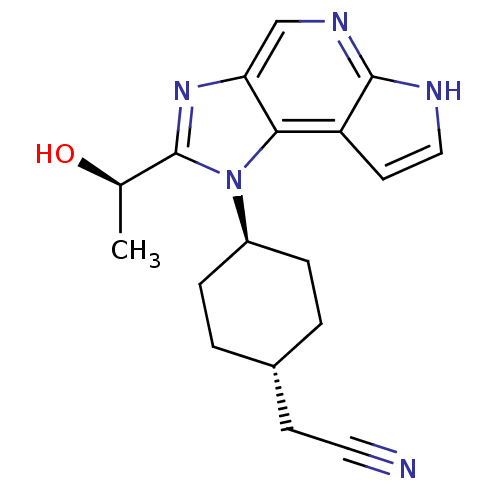 (CHEMBL2386635 | US10487083, Example C | US10703751...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant JAK1 (unknown origin) using Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 mins in presence of ATP | J Med Chem 56: 4764-85 (2013) Article DOI: 10.1021/jm4004895 BindingDB Entry DOI: 10.7270/Q2CV4K40 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409003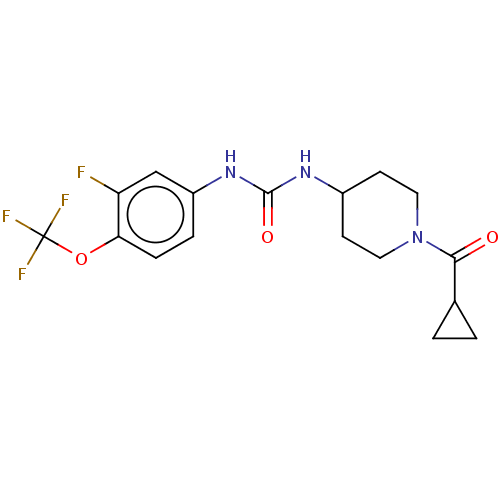 (US10377744, Compound No. 24 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409003 (US10377744, Compound No. 24 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409003 (US10377744, Compound No. 24 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100521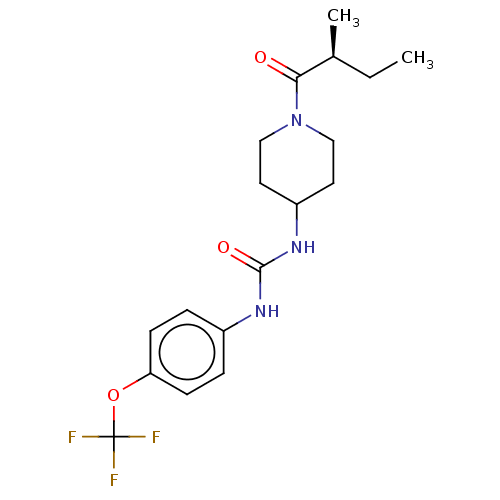 (CHEMBL3327078 | US10377744, Compound No. 2696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50554114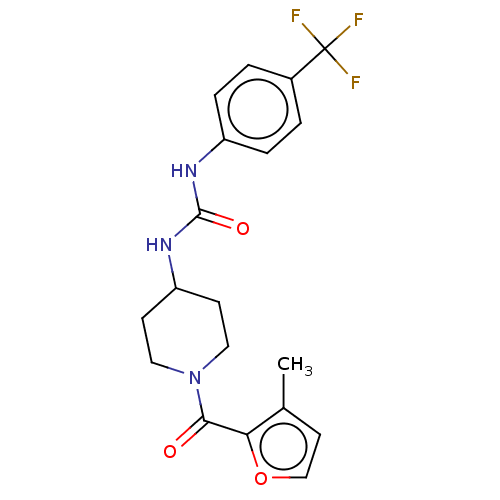 (CHEMBL4781745) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100519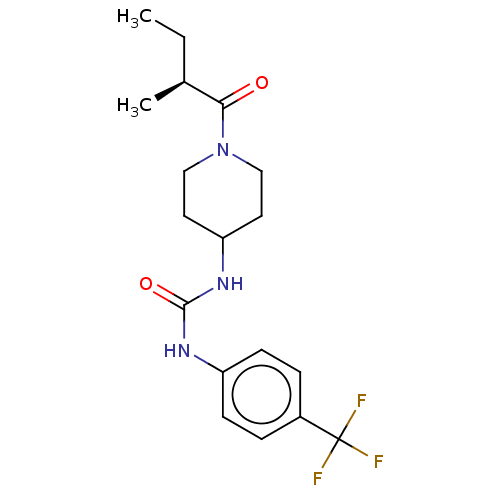 (CHEMBL3327067 | US10377744, Compound No. 2391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100519 (CHEMBL3327067 | US10377744, Compound No. 2391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408992 (US10377744, Compound No. 13 | US10377744, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100531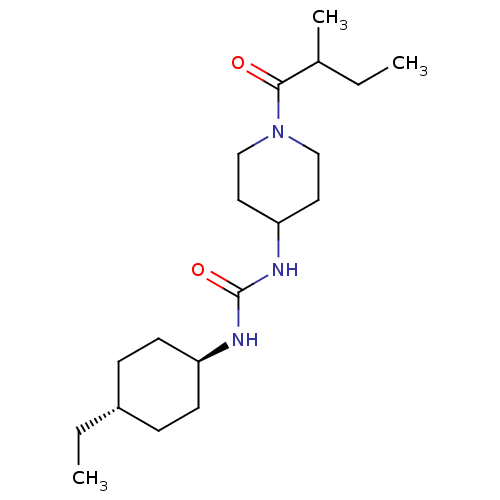 (CHEMBL3325465) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAS guanyl-releasing protein 1 (Homo sapiens (Human)) | BDBM50244449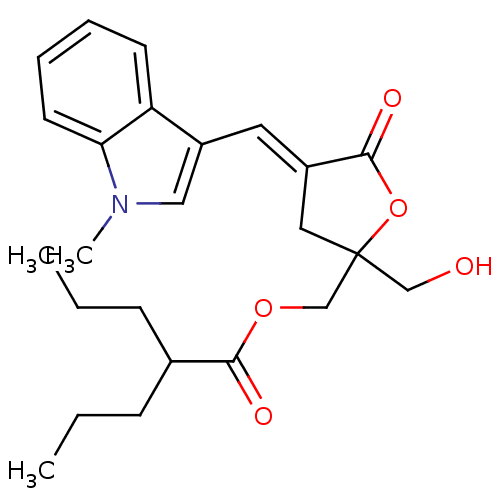 ((E)-(2-(hydroxymethyl)-4-((1-methyl-1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Industrial Technology Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from recombinant RasGRP1 C1 domain (unknown origin) after 10 mins by scintillation counting analysis in presence of phosphat... | Bioorg Med Chem 22: 3123-40 (2014) Article DOI: 10.1016/j.bmc.2014.04.024 BindingDB Entry DOI: 10.7270/Q2P270QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408986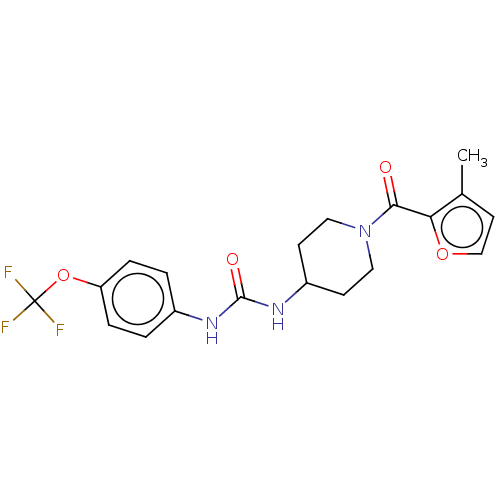 (US10377744, Compound No. 7 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408986 (US10377744, Compound No. 7 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50434786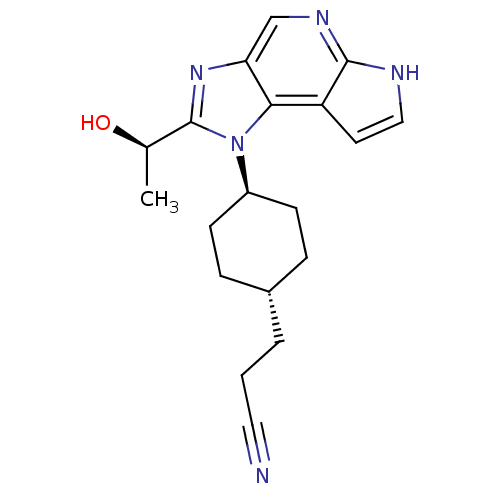 (CHEMBL2386636) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant JAK1 (unknown origin) using Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 mins in presence of ATP | J Med Chem 56: 4764-85 (2013) Article DOI: 10.1021/jm4004895 BindingDB Entry DOI: 10.7270/Q2CV4K40 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pro-neuropeptide Y (RAT) | BDBM82286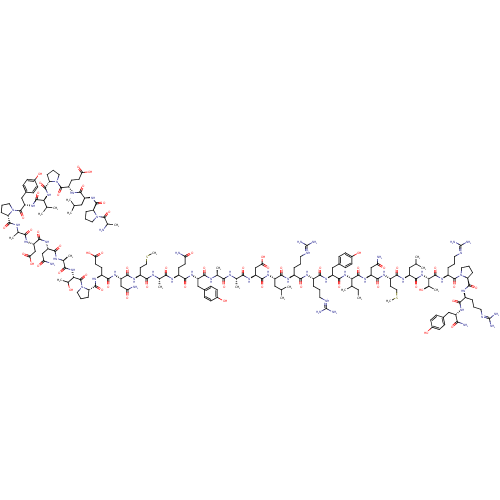 (CAS_59763-91-6 | PP, human | PP,SALMON) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 93: 4661-5 (1996) Article DOI: 10.1073/pnas.93.10.4661 BindingDB Entry DOI: 10.7270/Q22B8WJN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408998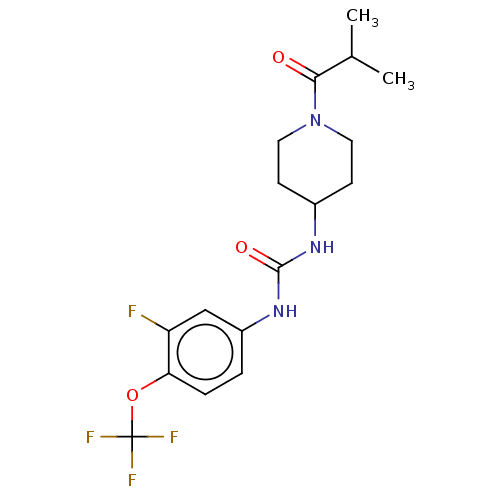 (US10377744, Compound No. 19 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100520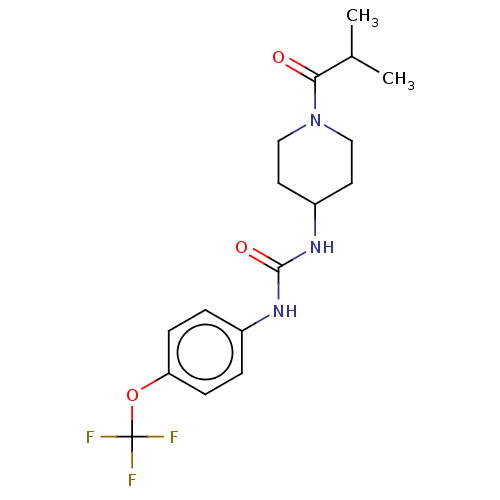 (CHEMBL3327077 | US10377744, Compound No. 2422 | US...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408998 (US10377744, Compound No. 19 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100520 (CHEMBL3327077 | US10377744, Compound No. 2422 | US...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408998 (US10377744, Compound No. 19 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50244449 ((E)-(2-(hydroxymethyl)-4-((1-methyl-1H-indol-3-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Industrial Technology Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from recombinant human GFP-tagged RasGRP3 expressed in Escherichia coli BL21 after 5 mins by scintillation counting analysis... | Bioorg Med Chem 22: 3123-40 (2014) Article DOI: 10.1016/j.bmc.2014.04.024 BindingDB Entry DOI: 10.7270/Q2P270QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408982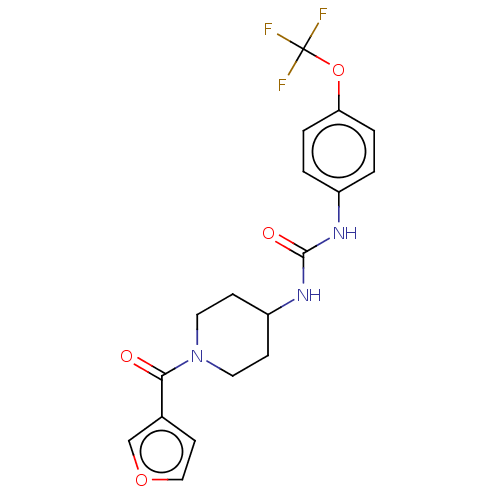 (US10377744, Compound No. 3 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408982 (US10377744, Compound No. 3 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50017890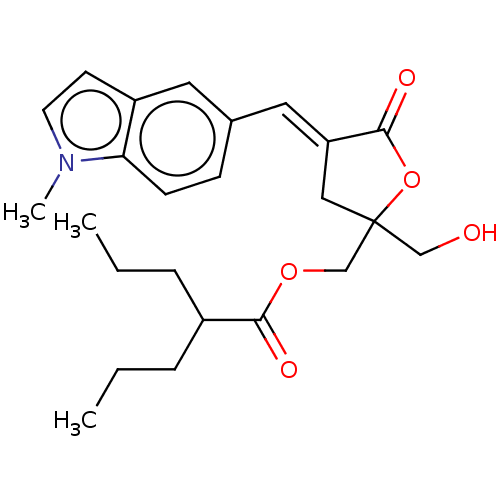 (CHEMBL3289012) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Industrial Technology Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from recombinant human GFP-tagged RasGRP3 expressed in Escherichia coli BL21 after 5 mins by scintillation counting analysis... | Bioorg Med Chem 22: 3123-40 (2014) Article DOI: 10.1016/j.bmc.2014.04.024 BindingDB Entry DOI: 10.7270/Q2P270QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100534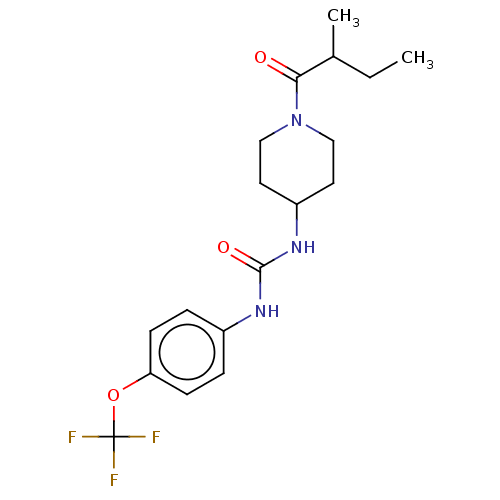 (CHEMBL3327074) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409014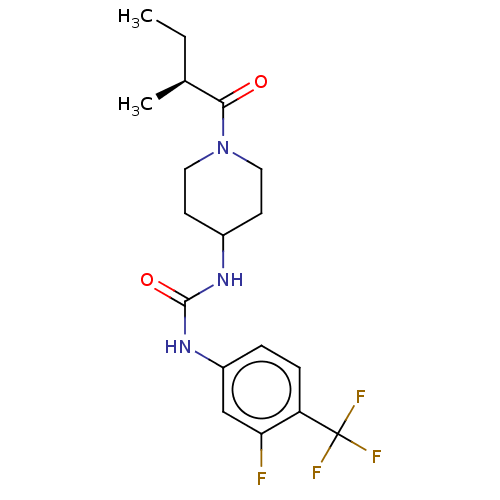 (US10377744, Compound No. 34 | US10377744, Syn34 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100541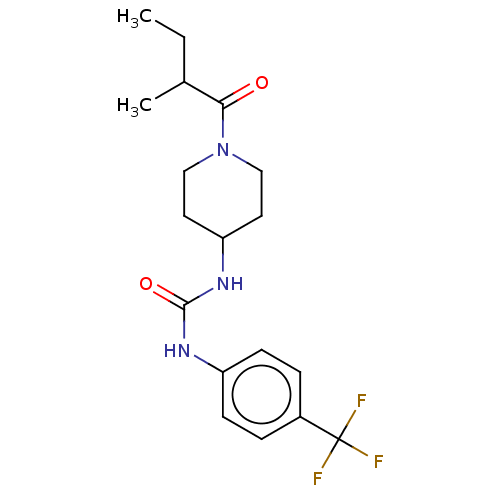 (CHEMBL3327066) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409014 (US10377744, Compound No. 34 | US10377744, Syn34 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409014 (US10377744, Compound No. 34 | US10377744, Syn34 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409021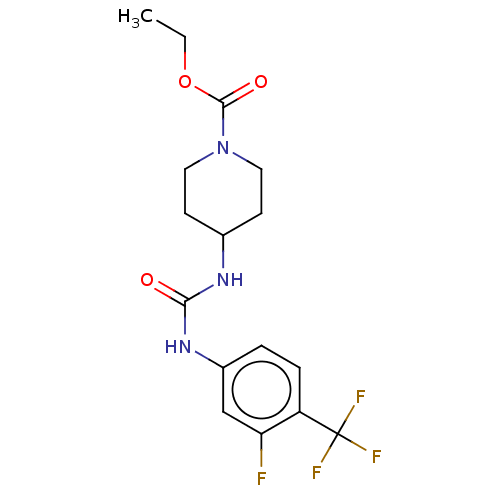 (US10377744, Compound No. 41 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409012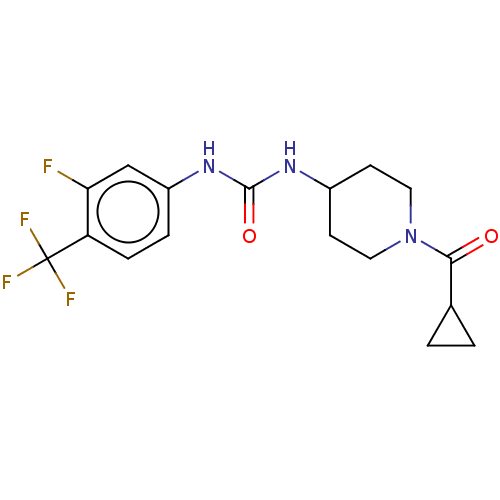 (US10377744, Compound No. 32 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q81H7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409021 (US10377744, Compound No. 41 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409012 (US10377744, Compound No. 32 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora B ATP binding site by rapid dilution method | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11517 total ) | Next | Last >> |