 Found 635 hits with Last Name = 'human' and Initial = 'jb'
Found 635 hits with Last Name = 'human' and Initial = 'jb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032720

(CHEMBL3354696)Show SMILES NC1=N[C@]2(CO1)c1cc(OCC3CCCCC3)ccc1Oc1ccc(cc21)-c1cncnc1 |r,t:1| Show InChI InChI=1S/C26H26N4O3/c27-25-30-26(15-32-25)21-10-18(19-12-28-16-29-13-19)6-8-23(21)33-24-9-7-20(11-22(24)26)31-14-17-4-2-1-3-5-17/h6-13,16-17H,1-5,14-15H2,(H2,27,30)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032730
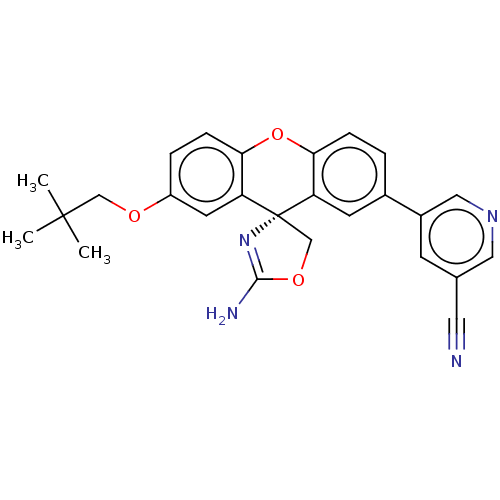
(CHEMBL3354706)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncc(c1)C#N |r,c:22| Show InChI InChI=1S/C26H24N4O3/c1-25(2,3)14-31-19-5-7-23-21(10-19)26(15-32-24(28)30-26)20-9-17(4-6-22(20)33-23)18-8-16(11-27)12-29-13-18/h4-10,12-13H,14-15H2,1-3H3,(H2,28,30)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032713
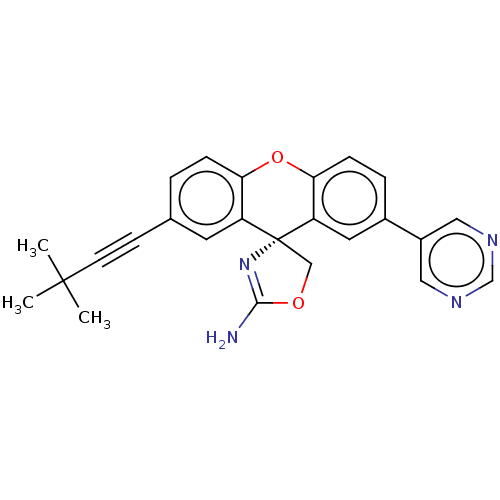
(CHEMBL3354689)Show SMILES CC(C)(C)C#Cc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C25H22N4O2/c1-24(2,3)9-8-16-4-6-21-19(10-16)25(14-30-23(26)29-25)20-11-17(5-7-22(20)31-21)18-12-27-15-28-13-18/h4-7,10-13,15H,14H2,1-3H3,(H2,26,29)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032729
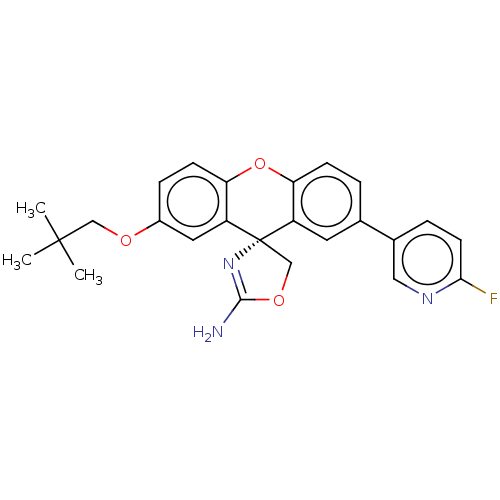
(CHEMBL3354705)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1ccc(F)nc1 |r,c:22| Show InChI InChI=1S/C25H24FN3O3/c1-24(2,3)13-30-17-6-8-21-19(11-17)25(14-31-23(27)29-25)18-10-15(4-7-20(18)32-21)16-5-9-22(26)28-12-16/h4-12H,13-14H2,1-3H3,(H2,27,29)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032731
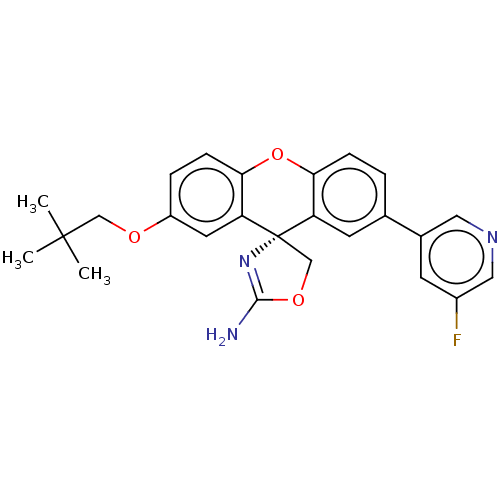
(CHEMBL3354707)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncc(F)c1 |r,c:22| Show InChI InChI=1S/C25H24FN3O3/c1-24(2,3)13-30-18-5-7-22-20(10-18)25(14-31-23(27)29-25)19-9-15(4-6-21(19)32-22)16-8-17(26)12-28-11-16/h4-12H,13-14H2,1-3H3,(H2,27,29)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032714
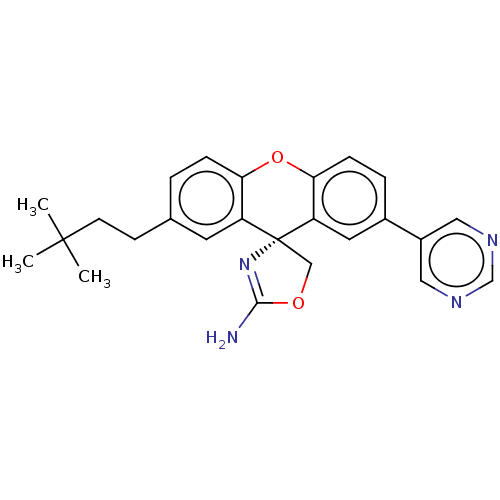
(CHEMBL3354690)Show SMILES CC(C)(C)CCc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C25H26N4O2/c1-24(2,3)9-8-16-4-6-21-19(10-16)25(14-30-23(26)29-25)20-11-17(5-7-22(20)31-21)18-12-27-15-28-13-18/h4-7,10-13,15H,8-9,14H2,1-3H3,(H2,26,29)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032726
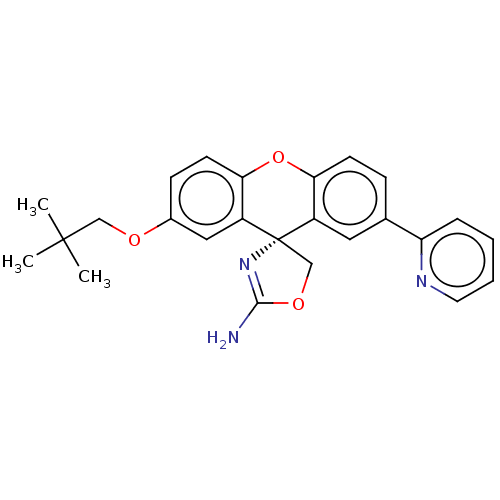
(CHEMBL3354702)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1ccccn1 |r,c:22| Show InChI InChI=1S/C25H25N3O3/c1-24(2,3)14-29-17-8-10-22-19(13-17)25(15-30-23(26)28-25)18-12-16(7-9-21(18)31-22)20-6-4-5-11-27-20/h4-13H,14-15H2,1-3H3,(H2,26,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 496 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032727

(CHEMBL3354703)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1ccncc1 |r,c:22| Show InChI InChI=1S/C25H25N3O3/c1-24(2,3)14-29-18-5-7-22-20(13-18)25(15-30-23(26)28-25)19-12-17(4-6-21(19)31-22)16-8-10-27-11-9-16/h4-13H,14-15H2,1-3H3,(H2,26,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 624 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031612
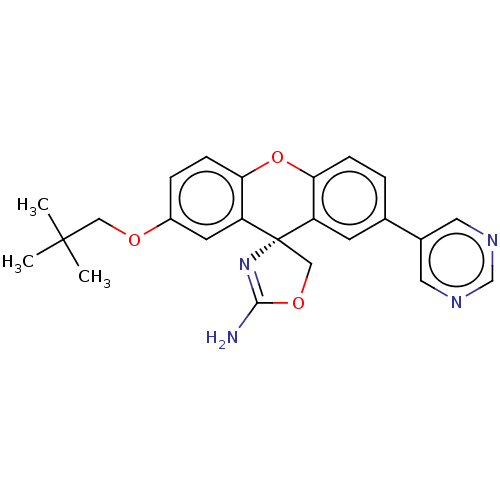
(CHEMBL3354688)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C24H24N4O3/c1-23(2,3)12-29-17-5-7-21-19(9-17)24(13-30-22(25)28-24)18-8-15(4-6-20(18)31-21)16-10-26-14-27-11-16/h4-11,14H,12-13H2,1-3H3,(H2,25,28)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032728
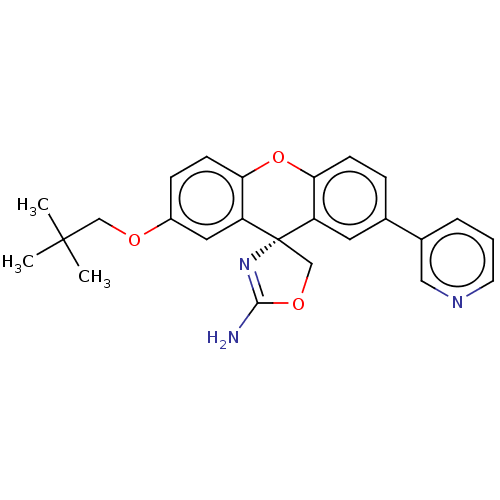
(CHEMBL3354704)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1 |r,c:22| Show InChI InChI=1S/C25H25N3O3/c1-24(2,3)14-29-18-7-9-22-20(12-18)25(15-30-23(26)28-25)19-11-16(6-8-21(19)31-22)17-5-4-10-27-13-17/h4-13H,14-15H2,1-3H3,(H2,26,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 797 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032716
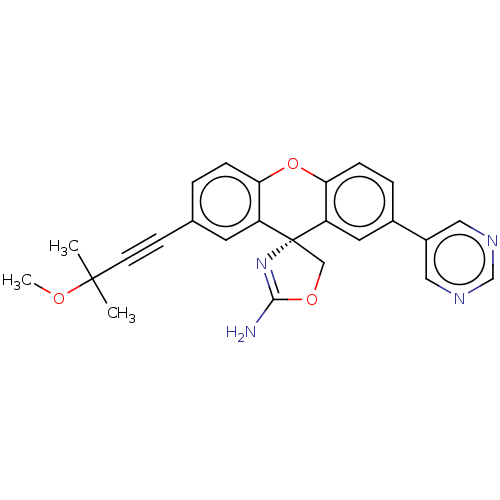
(CHEMBL3354692)Show SMILES COC(C)(C)C#Cc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:23| Show InChI InChI=1S/C25H22N4O3/c1-24(2,30-3)9-8-16-4-6-21-19(10-16)25(14-31-23(26)29-25)20-11-17(5-7-22(20)32-21)18-12-27-15-28-13-18/h4-7,10-13,15H,14H2,1-3H3,(H2,26,29)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50398311
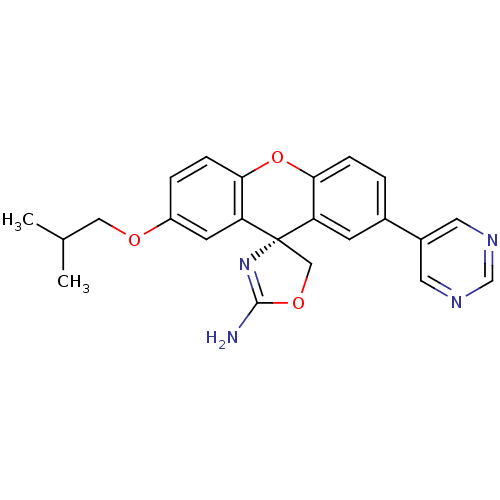
(CHEMBL2177343)Show SMILES CC(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:21| Show InChI InChI=1S/C23H22N4O3/c1-14(2)11-28-17-4-6-21-19(8-17)23(12-29-22(24)27-23)18-7-15(3-5-20(18)30-21)16-9-25-13-26-10-16/h3-10,13-14H,11-12H2,1-2H3,(H2,24,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032712
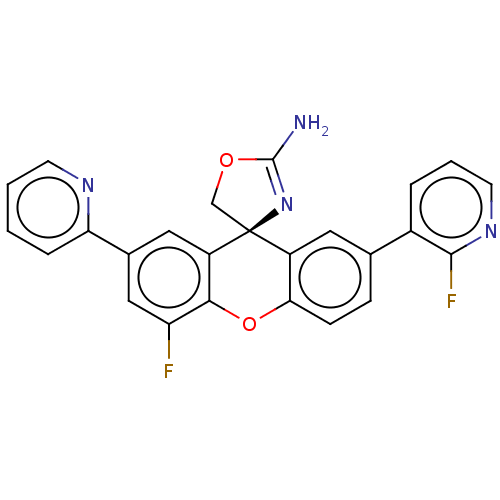
(CHEMBL3355143)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)-c1ccccn1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C25H16F2N4O2/c26-19-12-15(20-5-1-2-8-29-20)11-18-22(19)33-21-7-6-14(16-4-3-9-30-23(16)27)10-17(21)25(18)13-32-24(28)31-25/h1-12H,13H2,(H2,28,31)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032737

(CHEMBL3354718)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)C1=CCOCC1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C25H19F2N3O3/c26-20-12-16(14-5-8-31-9-6-14)11-19-22(20)33-21-4-3-15(17-2-1-7-29-23(17)27)10-18(21)25(19)13-32-24(28)30-25/h1-5,7,10-12H,6,8-9,13H2,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032723

(CHEMBL3354699)Show SMILES CC(C)(F)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C23H21FN4O3/c1-22(2,24)11-29-16-4-6-20-18(8-16)23(12-30-21(25)28-23)17-7-14(3-5-19(17)31-20)15-9-26-13-27-10-15/h3-10,13H,11-12H2,1-2H3,(H2,25,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032717
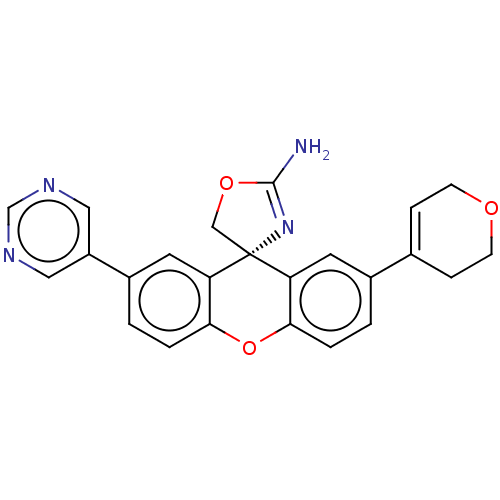
(CHEMBL3354693)Show SMILES NC1=N[C@]2(CO1)c1cc(ccc1Oc1ccc(cc21)-c1cncnc1)C1=CCOCC1 |r,t:1,30| Show InChI InChI=1S/C24H20N4O3/c25-23-28-24(13-30-23)19-9-16(15-5-7-29-8-6-15)1-3-21(19)31-22-4-2-17(10-20(22)24)18-11-26-14-27-12-18/h1-5,9-12,14H,6-8,13H2,(H2,25,28)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032724

(CHEMBL3354700)Show SMILES CC(C)(COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1)C#N |r,c:21| Show InChI InChI=1S/C24H21N5O3/c1-23(2,11-25)12-30-17-4-6-21-19(8-17)24(13-31-22(26)29-24)18-7-15(3-5-20(18)32-21)16-9-27-14-28-10-16/h3-10,14H,12-13H2,1-2H3,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032715
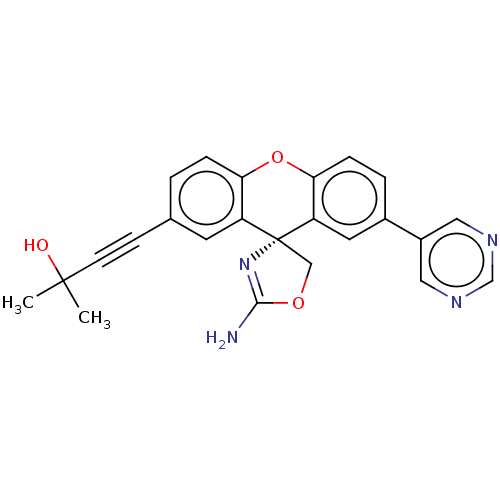
(CHEMBL3354691)Show SMILES CC(C)(O)C#Cc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C24H20N4O3/c1-23(2,29)8-7-15-3-5-20-18(9-15)24(13-30-22(25)28-24)19-10-16(4-6-21(19)31-20)17-11-26-14-27-12-17/h3-6,9-12,14,29H,13H2,1-2H3,(H2,25,28)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032738

(CHEMBL3354719)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)N1CCOCC1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H20F2N4O3/c25-19-12-15(30-6-8-31-9-7-30)11-18-21(19)33-20-4-3-14(16-2-1-5-28-22(16)26)10-17(20)24(18)13-32-23(27)29-24/h1-5,10-12H,6-9,13H2,(H2,27,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032739

(CHEMBL3354720)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)N1CC[C@H](F)C1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H19F3N4O2/c25-14-5-7-31(11-14)15-9-18-21(19(26)10-15)33-20-4-3-13(16-2-1-6-29-22(16)27)8-17(20)24(18)12-32-23(28)30-24/h1-4,6,8-10,14H,5,7,11-12H2,(H2,28,30)/t14-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032711

(CHEMBL3354721)Show SMILES Cn1cc(cn1)-c1cc(F)c2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1F |r,c:24| Show InChI InChI=1S/C24H17F2N5O2/c1-31-11-15(10-29-31)14-8-18-21(19(25)9-14)33-20-5-4-13(16-3-2-6-28-22(16)26)7-17(20)24(18)12-32-23(27)30-24/h2-11H,12H2,1H3,(H2,27,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032736

(CHEMBL3354717)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)C1=CCCOC1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C25H19F2N3O3/c26-20-11-16(15-3-2-8-31-12-15)10-19-22(20)33-21-6-5-14(17-4-1-7-29-23(17)27)9-18(21)25(19)13-32-24(28)30-25/h1,3-7,9-11H,2,8,12-13H2,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032735

(CHEMBL3354716)Show SMILES CC(C)(F)COc1cc(F)c2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1F |r,c:23| Show InChI InChI=1S/C24H20F3N3O3/c1-23(2,27)11-31-14-9-17-20(18(25)10-14)33-19-6-5-13(15-4-3-7-29-21(15)26)8-16(19)24(17)12-32-22(28)30-24/h3-10H,11-12H2,1-2H3,(H2,28,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032734
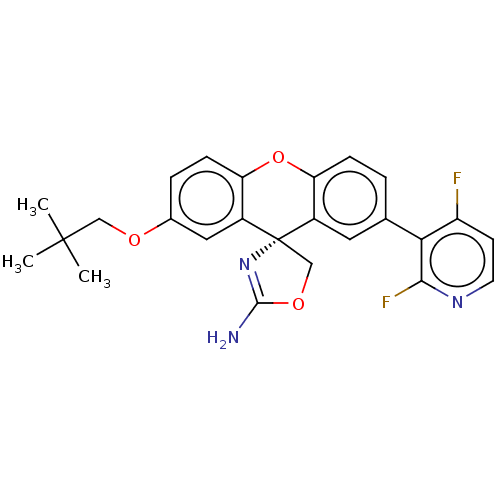
(CHEMBL3354710)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1c(F)ccnc1F |r,c:22| Show InChI InChI=1S/C25H23F2N3O3/c1-24(2,3)12-31-15-5-7-20-17(11-15)25(13-32-23(28)30-25)16-10-14(4-6-19(16)33-20)21-18(26)8-9-29-22(21)27/h4-11H,12-13H2,1-3H3,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032733
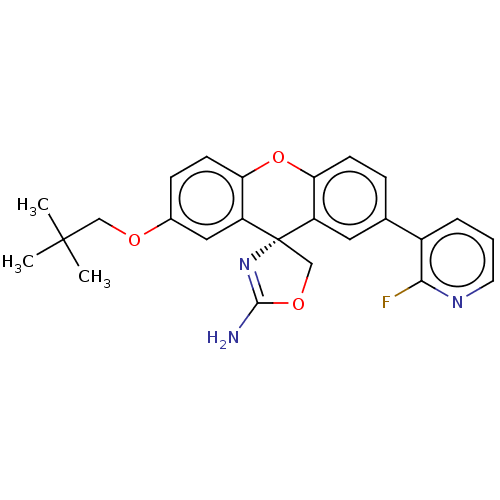
(CHEMBL3354709)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1F |r,c:22| Show InChI InChI=1S/C25H24FN3O3/c1-24(2,3)13-30-16-7-9-21-19(12-16)25(14-31-23(27)29-25)18-11-15(6-8-20(18)32-21)17-5-4-10-28-22(17)26/h4-12H,13-14H2,1-3H3,(H2,27,29)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032732
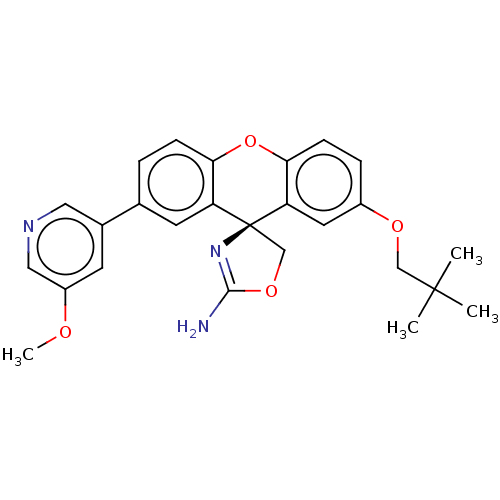
(CHEMBL3354708)Show SMILES COc1cncc(c1)-c1ccc2Oc3ccc(OCC(C)(C)C)cc3[C@]3(COC(N)=N3)c2c1 |r,c:31| Show InChI InChI=1S/C26H27N3O4/c1-25(2,3)14-31-18-6-8-23-21(11-18)26(15-32-24(27)29-26)20-10-16(5-7-22(20)33-23)17-9-19(30-4)13-28-12-17/h5-13H,14-15H2,1-4H3,(H2,27,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032725
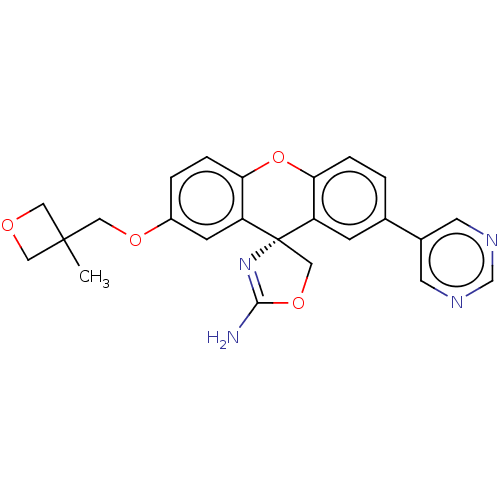
(CHEMBL3354701)Show SMILES CC1(COc2ccc3Oc4ccc(cc4[C@@]4(COC(N)=N4)c3c2)-c2cncnc2)COC1 |r,c:20| Show InChI InChI=1S/C24H22N4O4/c1-23(10-29-11-23)12-30-17-3-5-21-19(7-17)24(13-31-22(25)28-24)18-6-15(2-4-20(18)32-21)16-8-26-14-27-9-16/h2-9,14H,10-13H2,1H3,(H2,25,28)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032722
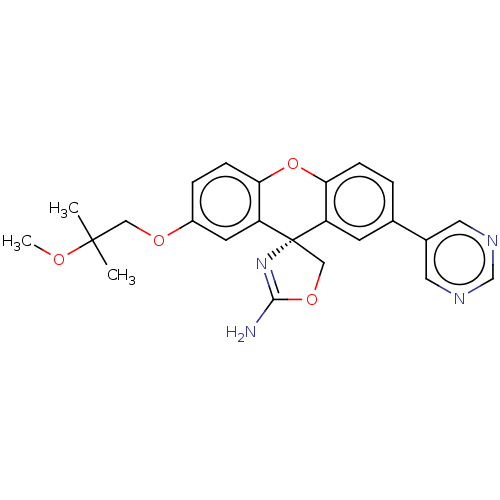
(CHEMBL3354698)Show SMILES COC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:23| Show InChI InChI=1S/C24H24N4O4/c1-23(2,29-3)12-30-17-5-7-21-19(9-17)24(13-31-22(25)28-24)18-8-15(4-6-20(18)32-21)16-10-26-14-27-11-16/h4-11,14H,12-13H2,1-3H3,(H2,25,28)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032719
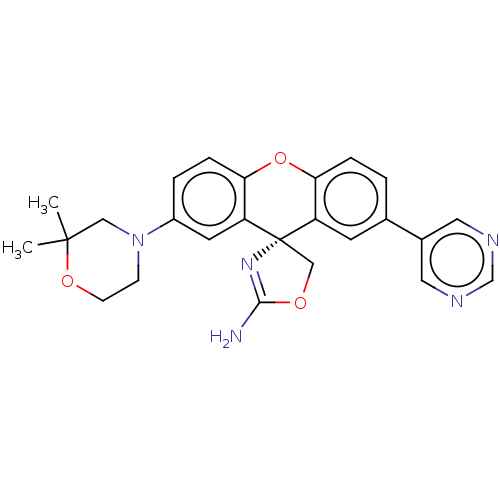
(CHEMBL3354695)Show SMILES CC1(C)CN(CCO1)c1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:25| Show InChI InChI=1S/C25H25N5O3/c1-24(2)13-30(7-8-32-24)18-4-6-22-20(10-18)25(14-31-23(26)29-25)19-9-16(3-5-21(19)33-22)17-11-27-15-28-12-17/h3-6,9-12,15H,7-8,13-14H2,1-2H3,(H2,26,29)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032718
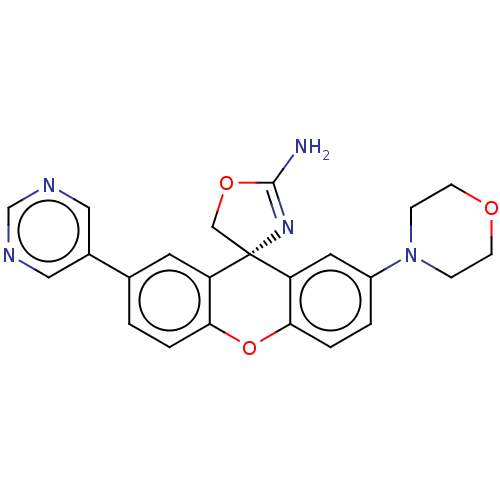
(CHEMBL3354694)Show SMILES NC1=N[C@]2(CO1)c1cc(ccc1Oc1ccc(cc21)-c1cncnc1)N1CCOCC1 |r,t:1| Show InChI InChI=1S/C23H21N5O3/c24-22-27-23(13-30-22)18-9-15(16-11-25-14-26-12-16)1-3-20(18)31-21-4-2-17(10-19(21)23)28-5-7-29-8-6-28/h1-4,9-12,14H,5-8,13H2,(H2,24,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032721
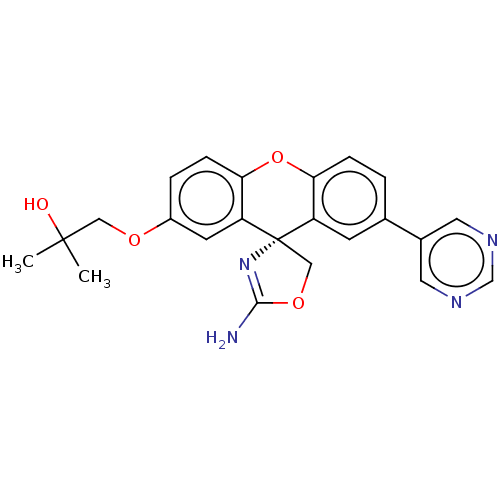
(CHEMBL3354697)Show SMILES CC(C)(O)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C23H22N4O4/c1-22(2,28)11-29-16-4-6-20-18(8-16)23(12-30-21(24)27-23)17-7-14(3-5-19(17)31-20)15-9-25-13-26-10-15/h3-10,13,28H,11-12H2,1-2H3,(H2,24,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM228440
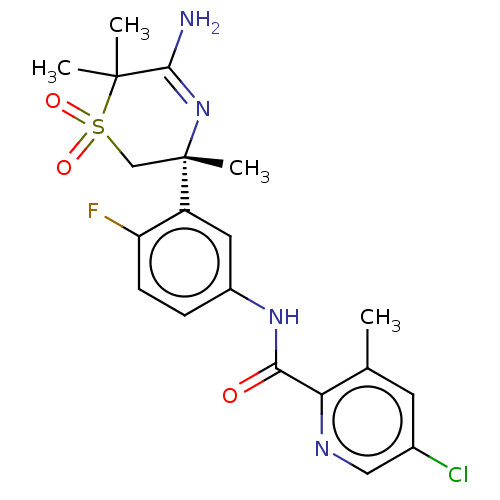
(US9556135, 25)Show SMILES Cc1cc(Cl)cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CS(=O)(=O)C(C)(C)C(N)=N1 |r,c:30| Show InChI InChI=1S/C20H22ClFN4O3S/c1-11-7-12(21)9-24-16(11)17(27)25-13-5-6-15(22)14(8-13)20(4)10-30(28,29)19(2,3)18(23)26-20/h5-9H,10H2,1-4H3,(H2,23,26)(H,25,27)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM149348
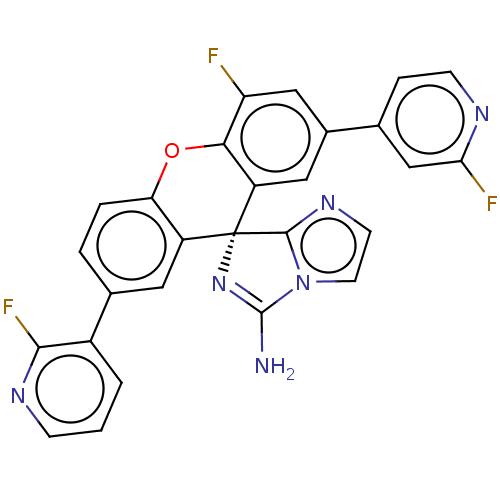
(US8962859, 8B)Show SMILES NC1=N[C@]2(c3nccn13)c1cc(ccc1Oc1c(F)cc(cc21)-c1ccnc(F)c1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C27H15F3N6O/c28-20-12-16(14-5-7-32-22(29)13-14)11-19-23(20)37-21-4-3-15(17-2-1-6-33-24(17)30)10-18(21)27(19)25-34-8-9-36(25)26(31)35-27/h1-13H,(H2,31,35)/t27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 4.2 | n/a |
Amgen Inc.
US Patent
| Assay Description
The assay buffer used in this screen is 0.05 M acetate, pH 4.2, 10% DMSO final, 100 uM genapol (which is a nonionic detergent, below its Critical Mic... |
US Patent US8962859 (2015)
BindingDB Entry DOI: 10.7270/Q2G15ZJZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM229582
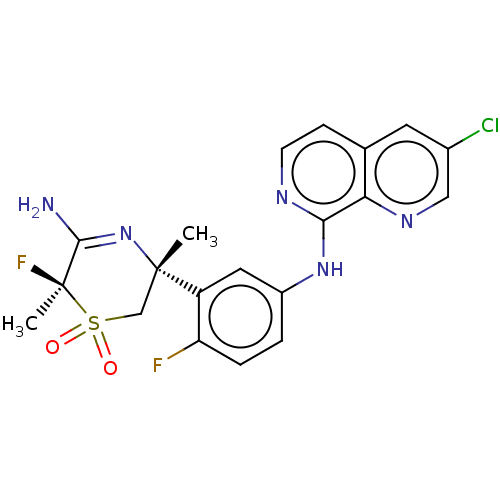
(US9556135, 174)Show SMILES C[C@]1(CS(=O)(=O)[C@@](C)(F)C(N)=N1)c1cc(Nc2nccc3cc(Cl)cnc23)ccc1F |r,c:10| Show InChI InChI=1S/C20H18ClF2N5O2S/c1-19(10-31(29,30)20(2,23)18(24)28-19)14-8-13(3-4-15(14)22)27-17-16-11(5-6-25-17)7-12(21)9-26-16/h3-9H,10H2,1-2H3,(H2,24,28)(H,25,27)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM230011
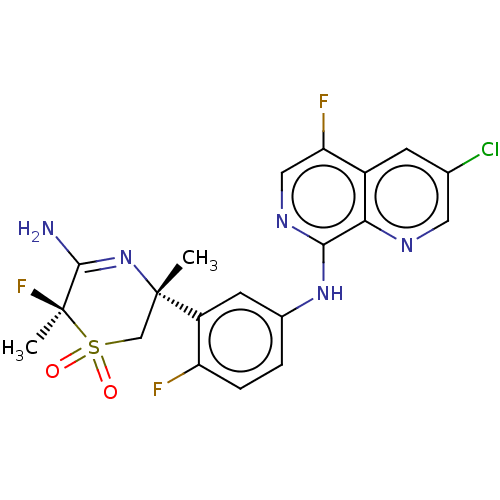
(US9556135, 178)Show SMILES C[C@]1(CS(=O)(=O)[C@@](C)(F)C(N)=N1)c1cc(Nc2ncc(F)c3cc(Cl)cnc23)ccc1F |r,c:10| Show InChI InChI=1S/C20H17ClF3N5O2S/c1-19(9-32(30,31)20(2,24)18(25)29-19)13-6-11(3-4-14(13)22)28-17-16-12(15(23)8-27-17)5-10(21)7-26-16/h3-8H,9H2,1-2H3,(H2,25,29)(H,27,28)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM228441
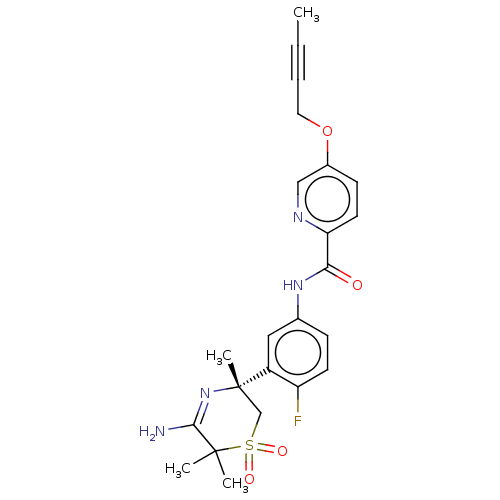
(US9556135, 26)Show SMILES CC#CCOc1ccc(nc1)C(=O)Nc1ccc(F)c(c1)[C@]1(C)CS(=O)(=O)C(C)(C)C(N)=N1 |r,c:33| Show InChI InChI=1S/C23H25FN4O4S/c1-5-6-11-32-16-8-10-19(26-13-16)20(29)27-15-7-9-18(24)17(12-15)23(4)14-33(30,31)22(2,3)21(25)28-23/h7-10,12-13H,11,14H2,1-4H3,(H2,25,28)(H,27,29)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM149352
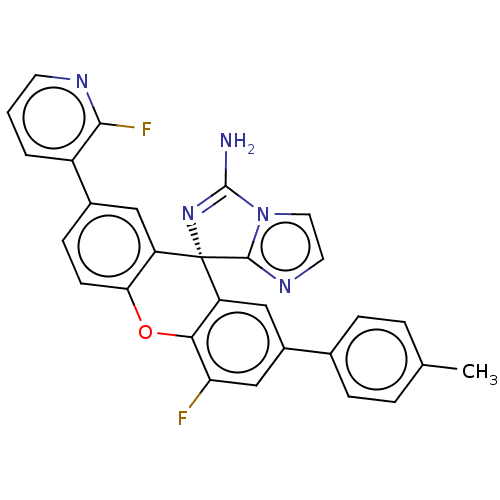
(US8962859, 15)Show SMILES Cc1ccc(cc1)-c1cc(F)c2Oc3ccc(cc3[C@]3(N=C(N)n4ccnc34)c2c1)-c1cccnc1F |r,t:22| Show InChI InChI=1S/C29H19F2N5O/c1-16-4-6-17(7-5-16)19-14-22-25(23(30)15-19)37-24-9-8-18(20-3-2-10-33-26(20)31)13-21(24)29(22)27-34-11-12-36(27)28(32)35-29/h2-15H,1H3,(H2,32,35)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 4.2 | n/a |
Amgen Inc.
US Patent
| Assay Description
The assay buffer used in this screen is 0.05 M acetate, pH 4.2, 10% DMSO final, 100 uM genapol (which is a nonionic detergent, below its Critical Mic... |
US Patent US8962859 (2015)
BindingDB Entry DOI: 10.7270/Q2G15ZJZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM149350
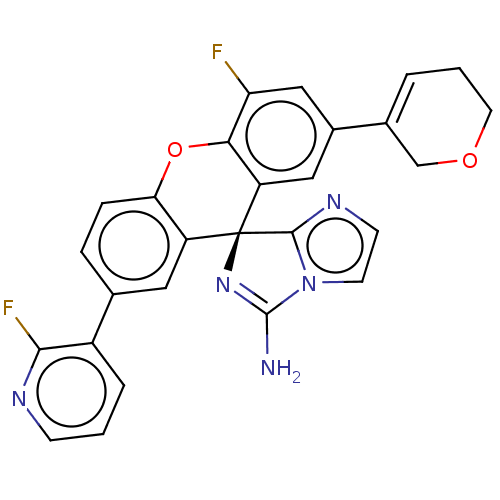
(US8962859, 13)Show SMILES NC1=N[C@@]2(c3nccn13)c1cc(ccc1Oc1c(F)cc(cc21)C1=CCCOC1)-c1cccnc1F |r,t:1,28| Show InChI InChI=1S/C27H19F2N5O2/c28-21-13-17(16-3-2-10-35-14-16)12-20-23(21)36-22-6-5-15(18-4-1-7-31-24(18)29)11-19(22)27(20)25-32-8-9-34(25)26(30)33-27/h1,3-9,11-13H,2,10,14H2,(H2,30,33)/t27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 4.2 | n/a |
Amgen Inc.
US Patent
| Assay Description
The assay buffer used in this screen is 0.05 M acetate, pH 4.2, 10% DMSO final, 100 uM genapol (which is a nonionic detergent, below its Critical Mic... |
US Patent US8962859 (2015)
BindingDB Entry DOI: 10.7270/Q2G15ZJZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50032744
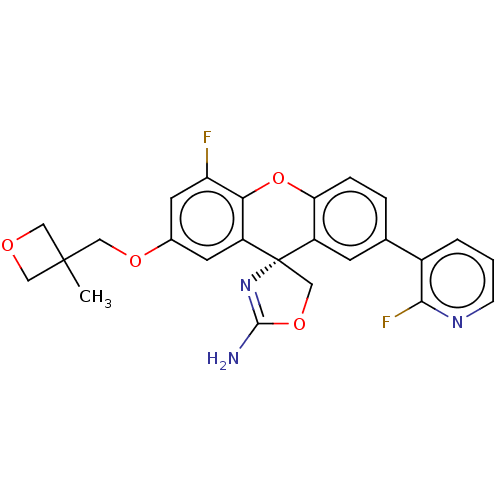
(CHEMBL3354715)Show SMILES CC1(COc2cc(F)c3Oc4ccc(cc4[C@@]4(COC(N)=N4)c3c2)-c2cccnc2F)COC1 |r,c:21| Show InChI InChI=1S/C25H21F2N3O4/c1-24(10-31-11-24)12-32-15-8-18-21(19(26)9-15)34-20-5-4-14(16-3-2-6-29-22(16)27)7-17(20)25(18)13-33-23(28)30-25/h2-9H,10-13H2,1H3,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) assessed as enhancement of fluorescence intensity |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM220775
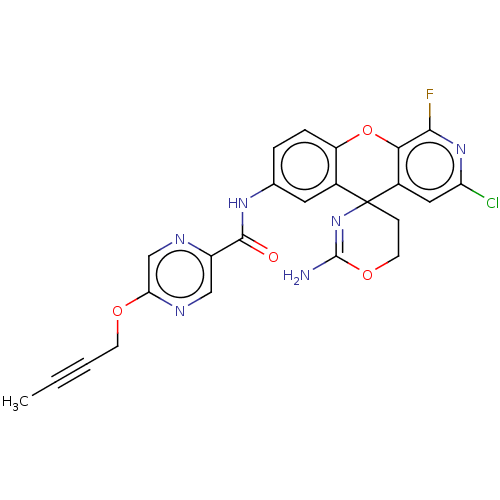
(US9296759, 48)Show SMILES CC#CCOc1cnc(cn1)C(=O)Nc1ccc2Oc3c(F)nc(Cl)cc3C3(CCOC(N)=N3)c2c1 |c:34| Show InChI InChI=1S/C24H18ClFN6O4/c1-2-3-7-34-19-12-28-16(11-29-19)22(33)30-13-4-5-17-14(9-13)24(6-8-35-23(27)32-24)15-10-18(25)31-21(26)20(15)36-17/h4-5,9-12H,6-8H2,1H3,(H2,27,32)(H,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 4.2 | n/a |
Amgen Inc.
US Patent
| Assay Description
The assay buffer used in this screen is 0.05 M acetate, pH 4.2, 10% DMSO final, 100 uM genapol (which is a nonionic detergent, below its Critical Mic... |
US Patent US9296759 (2016)
BindingDB Entry DOI: 10.7270/Q2348J7Q |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM220852
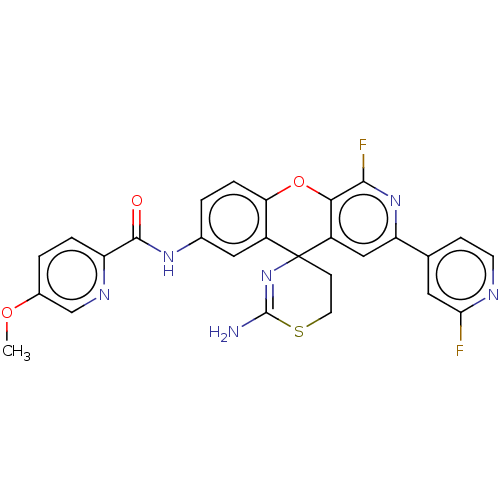
(US9296759, 115)Show SMILES COc1ccc(nc1)C(=O)Nc1ccc2Oc3c(F)nc(cc3C3(CCSC(N)=N3)c2c1)-c1ccnc(F)c1 |c:30| Show InChI InChI=1S/C27H20F2N6O3S/c1-37-16-3-4-19(32-13-16)25(36)33-15-2-5-21-17(11-15)27(7-9-39-26(30)35-27)18-12-20(34-24(29)23(18)38-21)14-6-8-31-22(28)10-14/h2-6,8,10-13H,7,9H2,1H3,(H2,30,35)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 4.2 | n/a |
Amgen Inc.
US Patent
| Assay Description
The assay buffer used in this screen is 0.05 M acetate, pH 4.2, 10% DMSO final, 100 uM genapol (which is a nonionic detergent, below its Critical Mic... |
US Patent US9296759 (2016)
BindingDB Entry DOI: 10.7270/Q2348J7Q |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM228479
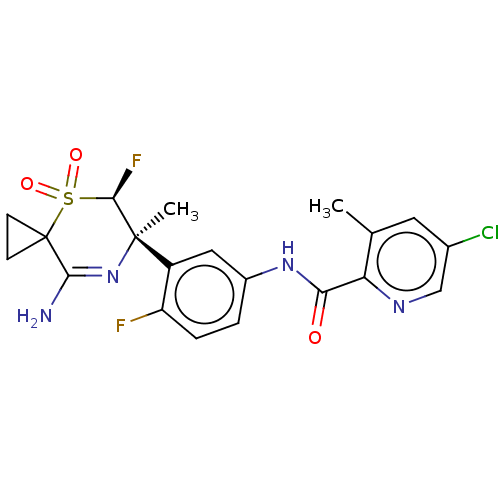
(US9556135, 205)Show SMILES Cc1cc(Cl)cnc1C(=O)Nc1ccc(F)c(c1)[C@@]1(C)N=C(N)C2(CC2)S(=O)(=O)[C@H]1F |r,t:22| Show InChI InChI=1S/C20H19ClF2N4O3S/c1-10-7-11(21)9-25-15(10)16(28)26-12-3-4-14(22)13(8-12)19(2)17(23)31(29,30)20(5-6-20)18(24)27-19/h3-4,7-9,17H,5-6H2,1-2H3,(H2,24,27)(H,26,28)/t17-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM230020
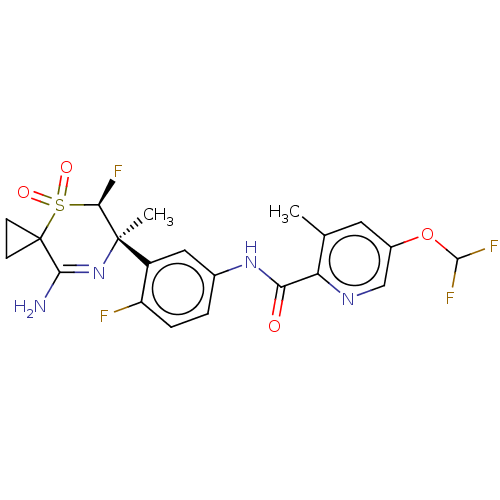
(US9556135, 203)Show SMILES Cc1cc(OC(F)F)cnc1C(=O)Nc1ccc(F)c(c1)[C@@]1(C)N=C(N)C2(CC2)S(=O)(=O)[C@H]1F |r,t:25| Show InChI InChI=1S/C21H20F4N4O4S/c1-10-7-12(33-19(24)25)9-27-15(10)16(30)28-11-3-4-14(22)13(8-11)20(2)17(23)34(31,32)21(5-6-21)18(26)29-20/h3-4,7-9,17,19H,5-6H2,1-2H3,(H2,26,29)(H,28,30)/t17-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM229989
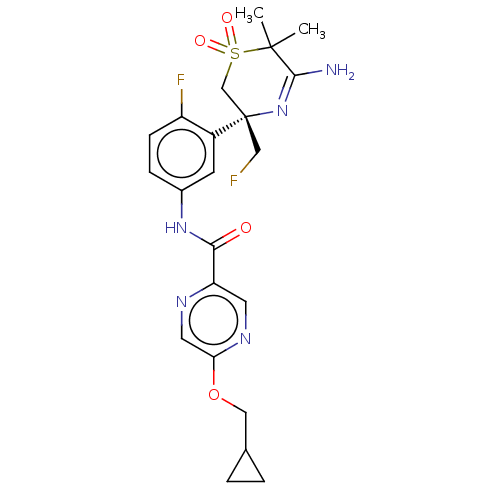
(US9556135, 156)Show SMILES CC1(C)C(N)=N[C@@](CF)(CS1(=O)=O)c1cc(NC(=O)c2cnc(OCC3CC3)cn2)ccc1F |r,c:4| Show InChI InChI=1S/C22H25F2N5O4S/c1-21(2)20(25)29-22(11-23,12-34(21,31)32)15-7-14(5-6-16(15)24)28-19(30)17-8-27-18(9-26-17)33-10-13-3-4-13/h5-9,13H,3-4,10-12H2,1-2H3,(H2,25,29)(H,28,30)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50032734

(CHEMBL3354710)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1c(F)ccnc1F |r,c:22| Show InChI InChI=1S/C25H23F2N3O3/c1-24(2,3)12-31-15-5-7-20-17(11-15)25(13-32-23(28)30-25)16-10-14(4-6-19(16)33-20)21-18(26)8-9-29-22(21)27/h4-11H,12-13H2,1-3H3,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) assessed as enhancement of fluorescence intensity |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM228404
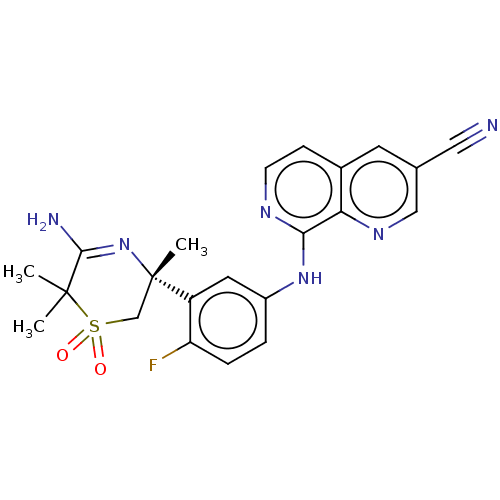
(US9556135, 48)Show SMILES CC1(C)C(N)=N[C@@](C)(CS1(=O)=O)c1cc(Nc2nccc3cc(cnc23)C#N)ccc1F |r,c:4| Show InChI InChI=1S/C22H21FN6O2S/c1-21(2)20(25)29-22(3,12-32(21,30)31)16-9-15(4-5-17(16)23)28-19-18-14(6-7-26-19)8-13(10-24)11-27-18/h4-9,11H,12H2,1-3H3,(H2,25,29)(H,26,28)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM230028
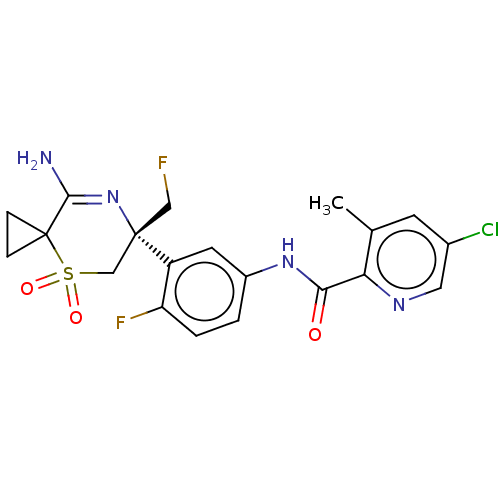
(US9556135, 216)Show SMILES Cc1cc(Cl)cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(CF)CS(=O)(=O)C2(CC2)C(N)=N1 |r,c:32| Show InChI InChI=1S/C20H19ClF2N4O3S/c1-11-6-12(21)8-25-16(11)17(28)26-13-2-3-15(23)14(7-13)19(9-22)10-31(29,30)20(4-5-20)18(24)27-19/h2-3,6-8H,4-5,9-10H2,1H3,(H2,24,27)(H,26,28)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM229992
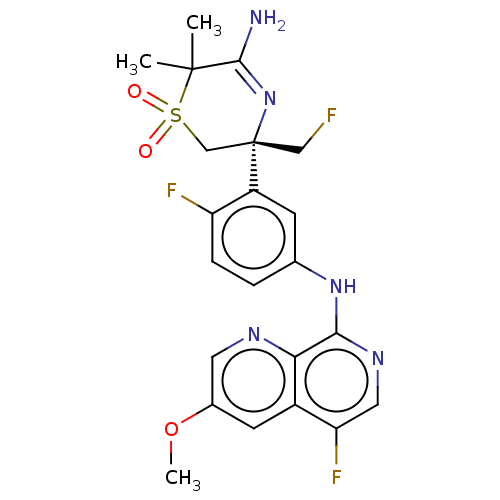
(US9556135, 159)Show SMILES COc1cnc2c(Nc3ccc(F)c(c3)[C@]3(CF)CS(=O)(=O)C(C)(C)C(N)=N3)ncc(F)c2c1 |r,c:27| Show InChI InChI=1S/C22H22F3N5O3S/c1-21(2)20(26)30-22(10-23,11-34(21,31)32)15-6-12(4-5-16(15)24)29-19-18-14(17(25)9-28-19)7-13(33-3)8-27-18/h4-9H,10-11H2,1-3H3,(H2,26,30)(H,28,29)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM228414
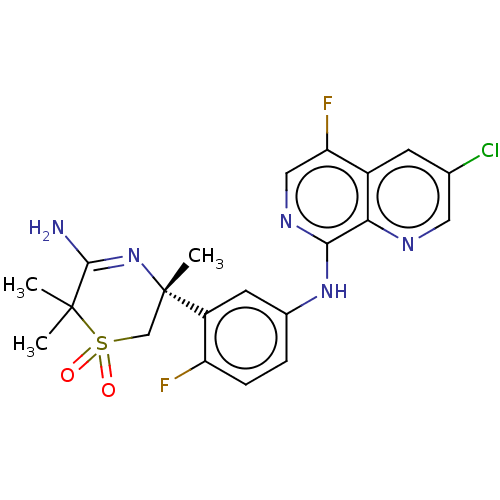
(US9556135, 49)Show SMILES CC1(C)C(N)=N[C@@](C)(CS1(=O)=O)c1cc(Nc2ncc(F)c3cc(Cl)cnc23)ccc1F |r,c:4| Show InChI InChI=1S/C21H20ClF2N5O2S/c1-20(2)19(25)29-21(3,10-32(20,30)31)14-7-12(4-5-15(14)23)28-18-17-13(16(24)9-27-18)6-11(22)8-26-17/h4-9H,10H2,1-3H3,(H2,25,29)(H,27,28)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM229990
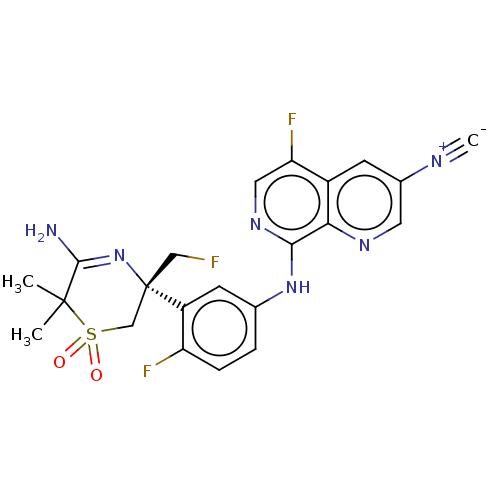
(US9556135, 157)Show SMILES CC1(C)C(N)=N[C@@](CF)(CS1(=O)=O)c1cc(Nc2ncc(F)c3cc(cnc23)[N+]#[C-])ccc1F |r,c:4| Show InChI InChI=1S/C22H19F3N6O2S/c1-21(2)20(26)31-22(10-23,11-34(21,32)33)15-7-12(4-5-16(15)24)30-19-18-14(17(25)9-29-19)6-13(27-3)8-28-18/h4-9H,10-11H2,1-2H3,(H2,26,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... |
US Patent US9556135 (2017)
BindingDB Entry DOI: 10.7270/Q2X0691F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data