 Found 122 hits with Last Name = 'gestwicki' and Initial = 'je'
Found 122 hits with Last Name = 'gestwicki' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50613636

(CHEMBL5286207)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCCC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50613631

(CHEMBL5281156)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCCC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCCN3CCOCC3)c2)cc1OC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50613624

(CHEMBL5291300)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@H](CCc1ccccc1)C1CCCCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116636

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCCc1cccnc1 Show InChI InChI=1S/C20H28N2O3S/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50613623

(CHEMBL5280097)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCCCc1cc(OC)c(OC)c(OC)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50132556
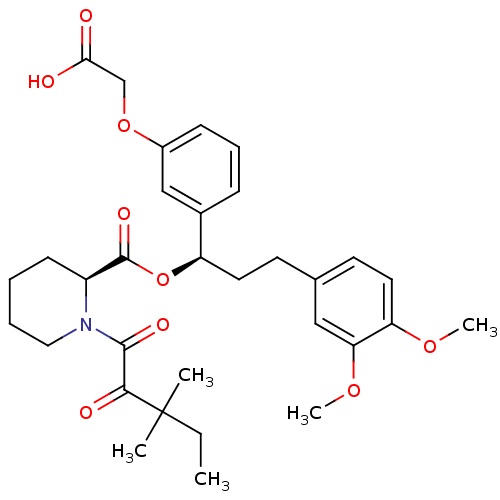
((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C32H41NO9/c1-6-32(2,3)29(36)30(37)33-17-8-7-12-24(33)31(38)42-25(22-10-9-11-23(19-22)41-20-28(34)35)15-13-21-14-16-26(39-4)27(18-21)40-5/h9-11,14,16,18-19,24-25H,6-8,12-13,15,17,20H2,1-5H3,(H,34,35)/t24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50613634
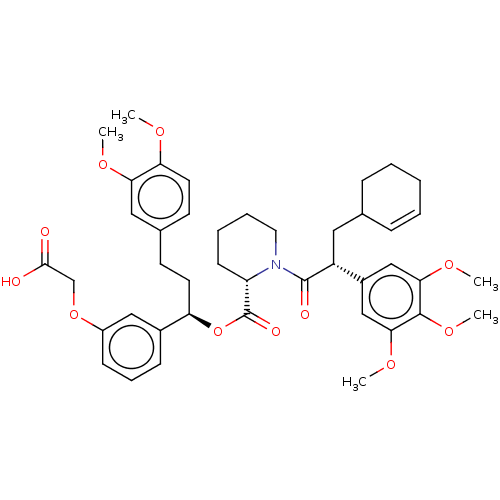
(CHEMBL5289205)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCC=C2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r,c:27| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50613635

(CHEMBL5291024)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCC=C2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCCN3CCOCC3)c2)cc1OC |r,c:27| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50030448
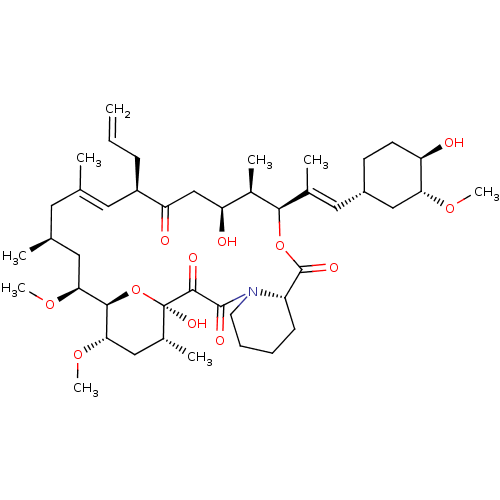
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of wild-type HIV-1 protease using fluorogenic peptide substrate incubated for 30 mins prior to substrate addition measured after 10 mins b... |
J Med Chem 57: 6468-78 (2014)
Article DOI: 10.1021/jm5008352
BindingDB Entry DOI: 10.7270/Q2V126G7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50067006
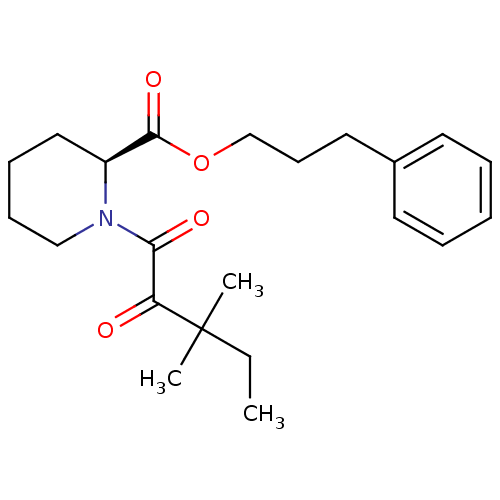
((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCCCc1ccccc1 Show InChI InChI=1S/C22H31NO4/c1-4-22(2,3)19(24)20(25)23-15-9-8-14-18(23)21(26)27-16-10-13-17-11-6-5-7-12-17/h5-7,11-12,18H,4,8-10,13-16H2,1-3H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of multidrug resistant HIV-1 protease L10I/L63P/A71V/G73S/I84V/L90M mutant using fluorogenic peptide substrate incubated for 30 mins prior... |
J Med Chem 57: 6468-78 (2014)
Article DOI: 10.1021/jm5008352
BindingDB Entry DOI: 10.7270/Q2V126G7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50613625

(CHEMBL1235853)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)C1CCCCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 306 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 936 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50080534
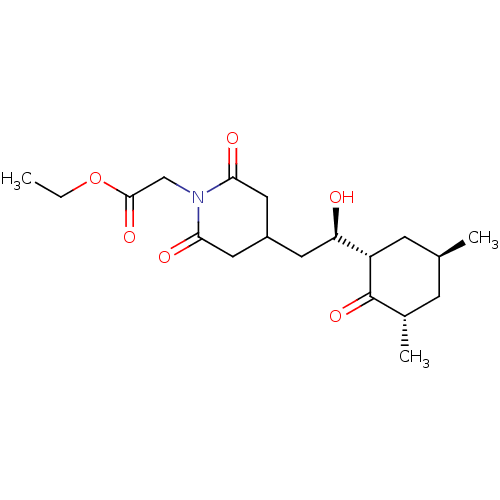
(CHEMBL333448 | ethyl (4-{(2R)-2-[(1S,3S,5S)-3,5-di...)Show SMILES CCOC(=O)CN1C(=O)CC(C[C@@H](O)[C@@H]2C[C@@H](C)C[C@H](C)C2=O)CC1=O Show InChI InChI=1S/C19H29NO6/c1-4-26-18(24)10-20-16(22)8-13(9-17(20)23)7-15(21)14-6-11(2)5-12(3)19(14)25/h11-15,21H,4-10H2,1-3H3/t11-,12-,14-,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| | 958 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50080534

(CHEMBL333448 | ethyl (4-{(2R)-2-[(1S,3S,5S)-3,5-di...)Show SMILES CCOC(=O)CN1C(=O)CC(C[C@@H](O)[C@@H]2C[C@@H](C)C[C@H](C)C2=O)CC1=O Show InChI InChI=1S/C19H29NO6/c1-4-26-18(24)10-20-16(22)8-13(9-17(20)23)7-15(21)14-6-11(2)5-12(3)19(14)25/h11-15,21H,4-10H2,1-3H3/t11-,12-,14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92915

(Aryl 1-indanylketone, 4)Show SMILES COc1ccc(cc1C(=O)C1CCc2ccccc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H19F3O3/c1-29-22-13-9-17(15-6-10-18(11-7-15)30-24(25,26)27)14-21(22)23(28)20-12-8-16-4-2-3-5-19(16)20/h2-7,9-11,13-14,20H,8,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50080534

(CHEMBL333448 | ethyl (4-{(2R)-2-[(1S,3S,5S)-3,5-di...)Show SMILES CCOC(=O)CN1C(=O)CC(C[C@@H](O)[C@@H]2C[C@@H](C)C[C@H](C)C2=O)CC1=O Show InChI InChI=1S/C19H29NO6/c1-4-26-18(24)10-20-16(22)8-13(9-17(20)23)7-15(21)14-6-11(2)5-12(3)19(14)25/h11-15,21H,4-10H2,1-3H3/t11-,12-,14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase D
(Homo sapiens (Human)) | BDBM50495808
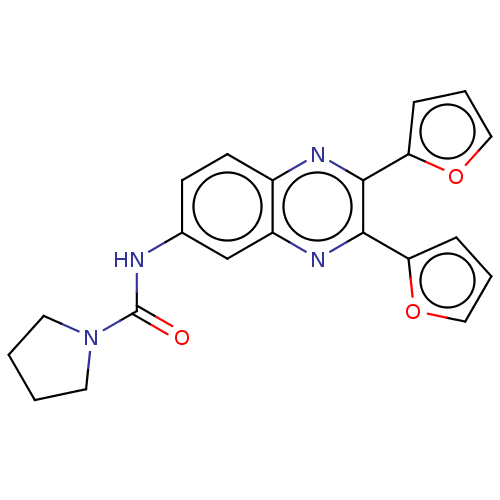
(CHEMBL1557710)Show SMILES O=C(Nc1ccc2nc(-c3ccco3)c(nc2c1)-c1ccco1)N1CCCC1 Show InChI InChI=1S/C21H18N4O3/c26-21(25-9-1-2-10-25)22-14-7-8-15-16(13-14)24-20(18-6-4-12-28-18)19(23-15)17-5-3-11-27-17/h3-8,11-13H,1-2,9-10H2,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92915

(Aryl 1-indanylketone, 4)Show SMILES COc1ccc(cc1C(=O)C1CCc2ccccc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H19F3O3/c1-29-22-13-9-17(15-6-10-18(11-7-15)30-24(25,26)27)14-21(22)23(28)20-12-8-16-4-2-3-5-19(16)20/h2-7,9-11,13-14,20H,8,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase D
(Homo sapiens (Human)) | BDBM50613627

(CHEMBL2006156)Show SMILES CC(C)N(C(C)C)C(=O)Nc1ccc2nc(-c3ccco3)c(nc2c1)-c1ccco1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase D
(Homo sapiens (Human)) | BDBM50613626
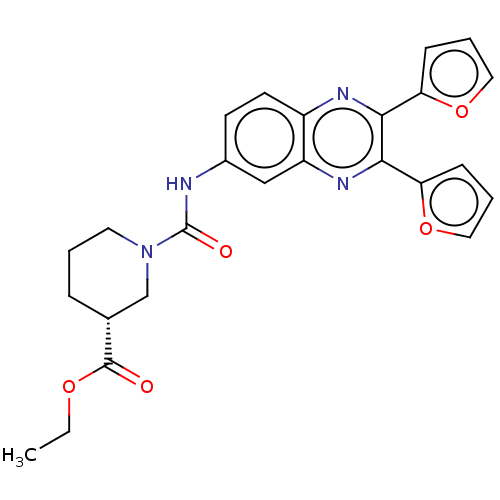
(CHEMBL5275318)Show SMILES CCOC(=O)[C@@H]1CCCN(C1)C(=O)Nc1ccc2nc(-c3ccco3)c(nc2c1)-c1ccco1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50613626

(CHEMBL5275318)Show SMILES CCOC(=O)[C@@H]1CCCN(C1)C(=O)Nc1ccc2nc(-c3ccco3)c(nc2c1)-c1ccco1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50613627

(CHEMBL2006156)Show SMILES CC(C)N(C(C)C)C(=O)Nc1ccc2nc(-c3ccco3)c(nc2c1)-c1ccco1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50080528

(3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...)Show SMILES C[C@H]1C[C@H](C)C(=O)[C@@H](C1)[C@H](O)CC1CC(=O)NC(=O)C1 |r| Show InChI InChI=1S/C15H23NO4/c1-8-3-9(2)15(20)11(4-8)12(17)5-10-6-13(18)16-14(19)7-10/h8-12,17H,3-7H2,1-2H3,(H,16,18,19)/t8-,9-,11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50613621

(CHEMBL5268083)Show SMILES [H][C@]1(C[C@@H](C)C[C@H](C)C1=O)[C@H](O)CC1CC(=O)N(CC(=O)N(C)C)C(=O)C1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50613621

(CHEMBL5268083)Show SMILES [H][C@]1(C[C@@H](C)C[C@H](C)C1=O)[C@H](O)CC1CC(=O)N(CC(=O)N(C)C)C(=O)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50613621

(CHEMBL5268083)Show SMILES [H][C@]1(C[C@@H](C)C[C@H](C)C1=O)[C@H](O)CC1CC(=O)N(CC(=O)N(C)C)C(=O)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50495808

(CHEMBL1557710)Show SMILES O=C(Nc1ccc2nc(-c3ccco3)c(nc2c1)-c1ccco1)N1CCCC1 Show InChI InChI=1S/C21H18N4O3/c26-21(25-9-1-2-10-25)22-14-7-8-15-16(13-14)24-20(18-6-4-12-28-18)19(23-15)17-5-3-11-27-17/h3-8,11-13H,1-2,9-10H2,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50613633
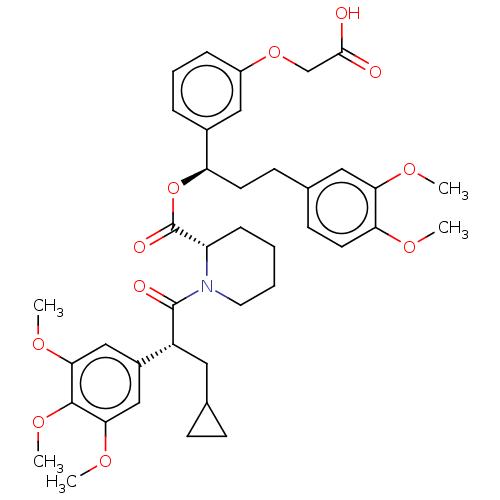
(CHEMBL5271713)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50613635

(CHEMBL5291024)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCC=C2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCCN3CCOCC3)c2)cc1OC |r,c:27| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50613636

(CHEMBL5286207)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCCC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50613634

(CHEMBL5289205)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCC=C2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r,c:27| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50613631

(CHEMBL5281156)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CCCCC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCCN3CCOCC3)c2)cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP5
(Homo sapiens (Human)) | BDBM50613632
(CHEMBL5270994)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC=C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92920

(Benzofuranone, 9)Show InChI InChI=1S/C17H14O3/c1-19-13-6-7-15-14(8-13)16(18)17(20-15)9-11-4-2-3-5-12(11)10-17/h2-8H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50058936

(CHEMBL3393116)Show SMILES [O-][N+](=O)c1cccc2C(=O)N(C(=O)c12)c1ccc2[nH]c(=S)sc2c1 Show InChI InChI=1S/C15H7N3O4S2/c19-13-8-2-1-3-10(18(21)22)12(8)14(20)17(13)7-4-5-9-11(6-7)24-15(23)16-9/h1-6H,(H,16,23) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of multidrug resistant HIV-1 protease L10I/L63P/A71V/G73S/I84V/L90M mutant using fluorogenic peptide substrate incubated for 30 mins prior... |
J Med Chem 57: 6468-78 (2014)
Article DOI: 10.1021/jm5008352
BindingDB Entry DOI: 10.7270/Q2V126G7 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50058936

(CHEMBL3393116)Show SMILES [O-][N+](=O)c1cccc2C(=O)N(C(=O)c12)c1ccc2[nH]c(=S)sc2c1 Show InChI InChI=1S/C15H7N3O4S2/c19-13-8-2-1-3-10(18(21)22)12(8)14(20)17(13)7-4-5-9-11(6-7)24-15(23)16-9/h1-6H,(H,16,23) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of wild-type HIV-1 protease using fluorogenic peptide substrate incubated for 30 mins prior to substrate addition measured after 10 mins b... |
J Med Chem 57: 6468-78 (2014)
Article DOI: 10.1021/jm5008352
BindingDB Entry DOI: 10.7270/Q2V126G7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50613632

(CHEMBL5270994)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC=C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP4
(Homo sapiens (Human)) | BDBM50613633

(CHEMBL5271713)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50613629
(CHEMBL5268140)Show SMILES [O-][N+](=O)c1ccc(NC(=O)Nc2ccccc2OCc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50381013

(CHEMBL2017129)Show SMILES Oc1c(cc(cc1[N+]([O-])=O)-c1cc(F)cc(c1O)[N+]([O-])=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50613628

(CHEMBL5279925) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34010

(benzothiophene carboxamide, 18b)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H18NO5PS/c20-18(17-11-14-8-4-5-9-16(14)26-17)19-15(12-24-25(21,22)23)10-13-6-2-1-3-7-13/h1-9,11,15H,10,12H2,(H,19,20)(H2,21,22,23)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of FITC-WFYpSPFLE from human Pin1 (45-163) catalytic domain by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00170
BindingDB Entry DOI: 10.7270/Q2FN19WZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50381013

(CHEMBL2017129)Show SMILES Oc1c(cc(cc1[N+]([O-])=O)-c1cc(F)cc(c1O)[N+]([O-])=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data