

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082250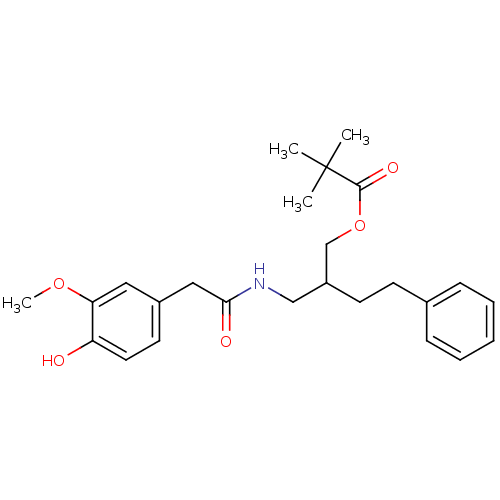 (2,2-Dimethyl-propionic acid 2-{[2-(4-hydroxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082239 (2,2-Dimethyl-propionic acid 2-benzyl-3-[2-(4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082243 ((E)-4-Benzyl-5-[2-(4-hydroxy-3-methoxy-phenyl)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082248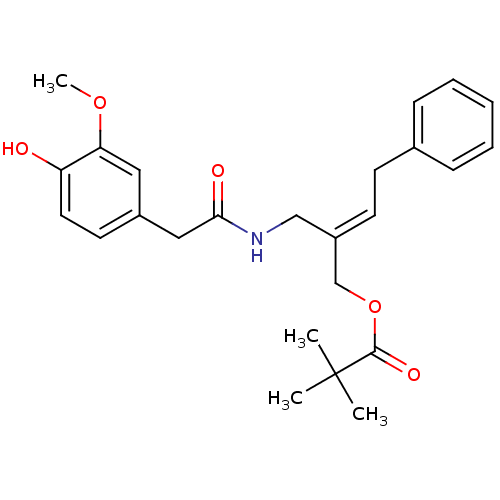 (2,2-Dimethyl-propionic acid (E)-2-{[2-(4-hydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082242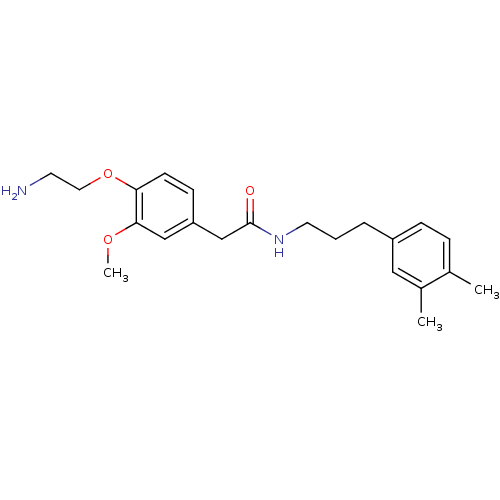 (2-[4-(2-Amino-ethoxy)-3-methoxy-phenyl]-N-[3-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082252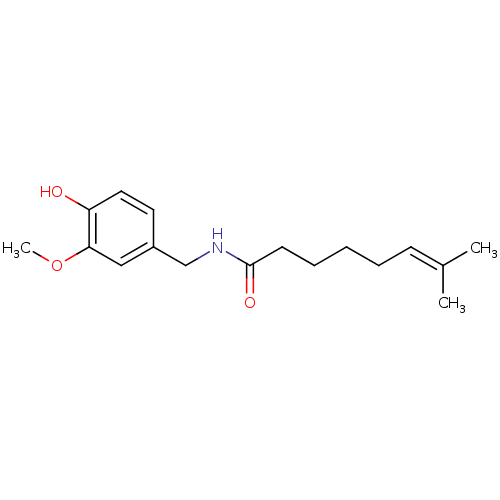 (7-Methyl-oct-6-enoic acid 4-hydroxy-3-methoxy-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082240 (Acetic acid (E)-2-{[2-(4-hydroxy-3-methoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082237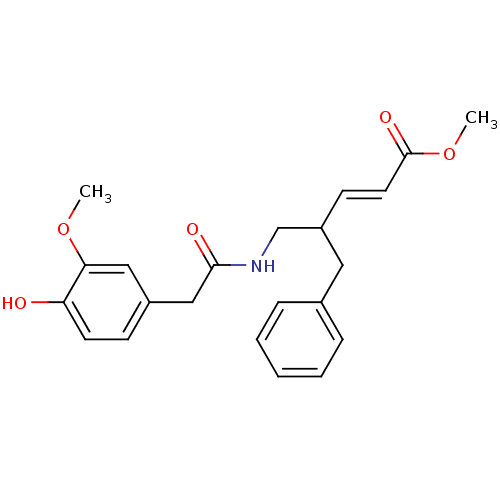 ((E)-4-Benzyl-5-[2-(4-hydroxy-3-methoxy-phenyl)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082238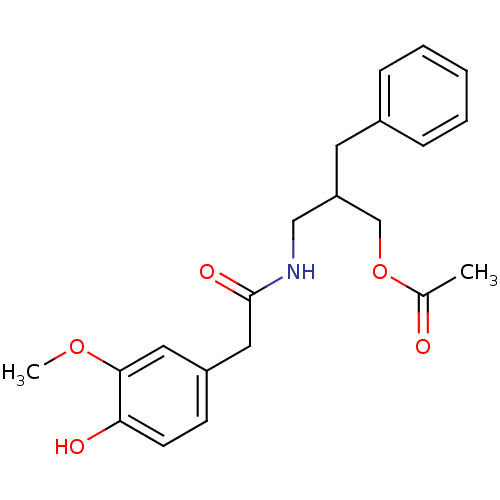 (Acetic acid 2-benzyl-3-[2-(4-hydroxy-3-methoxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082236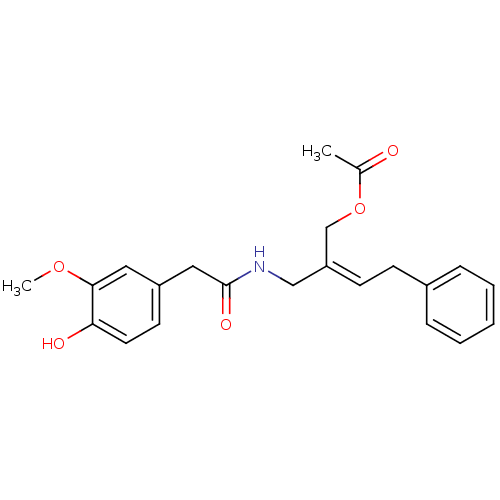 (Acetic acid (Z)-2-{[2-(4-hydroxy-3-methoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082245 (Acetic acid 2-{[2-(4-hydroxy-3-methoxy-phenyl)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082246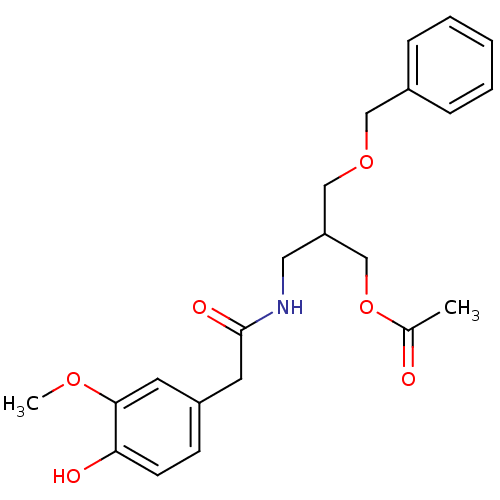 (Acetic acid 2-benzyloxymethyl-3-[2-(4-hydroxy-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082244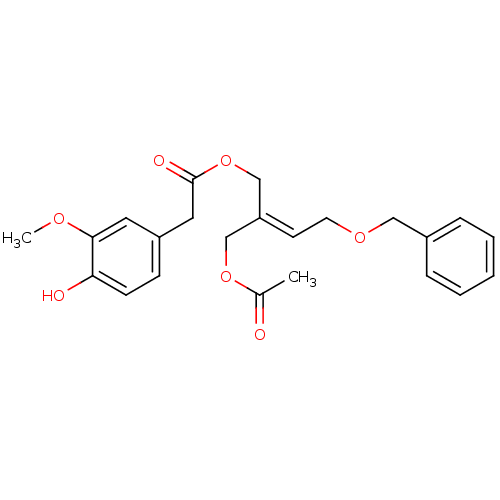 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (Z)-2-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082241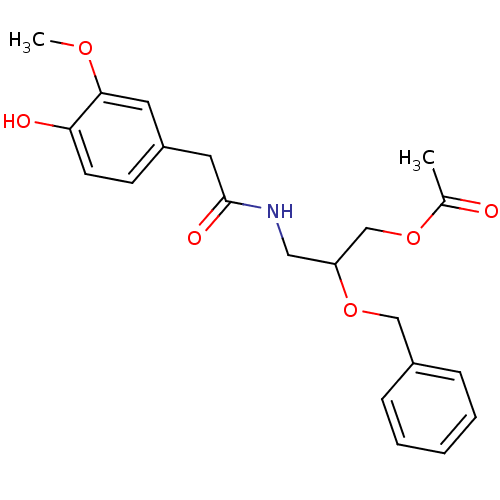 (Acetic acid 2-benzyloxy-3-[2-(4-hydroxy-3-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082249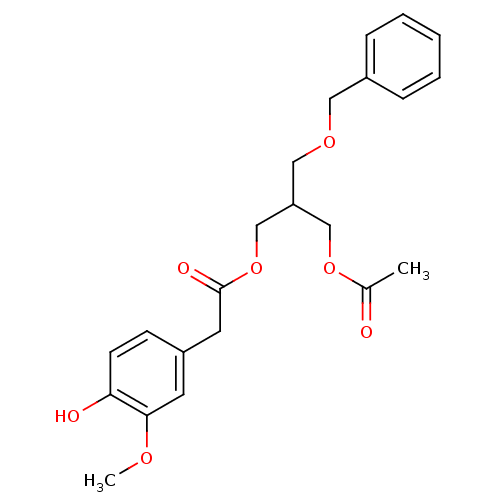 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid 3-acetoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082253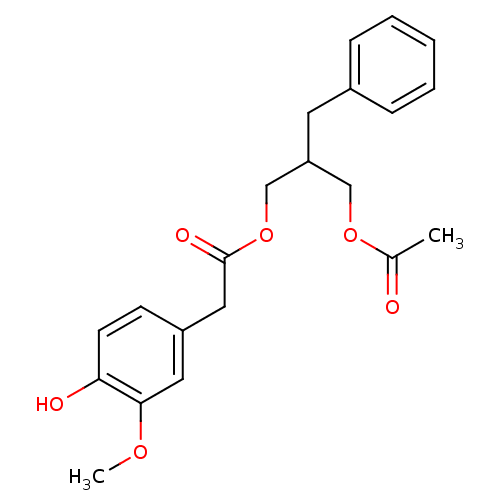 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid 2-acetoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082251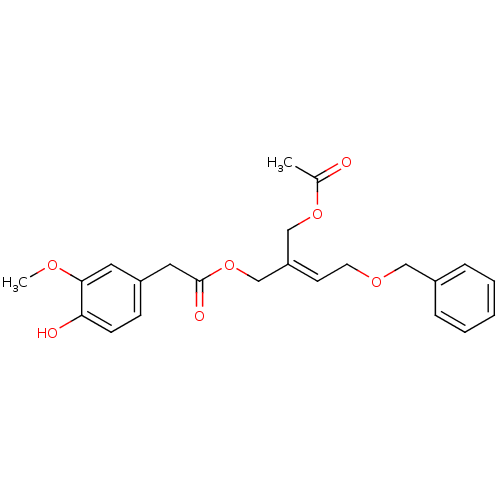 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (E)-2-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082235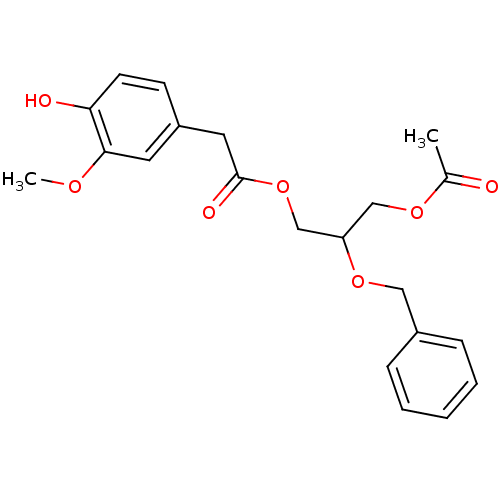 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid 3-acetoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474740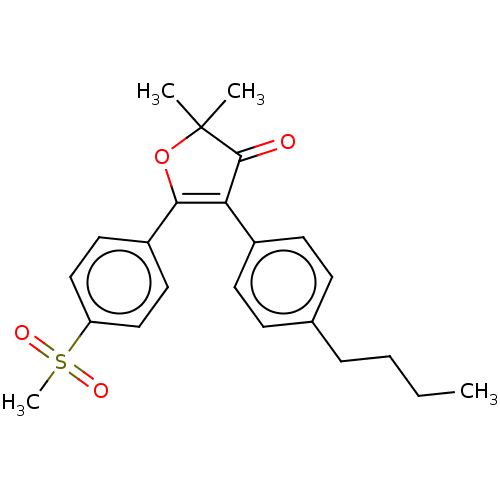 (CHEMBL355074) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.02 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474747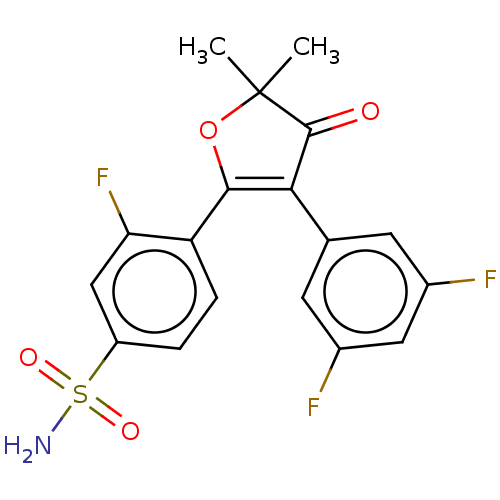 (CHEMBL167357) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.55 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474743 (CHEMBL353935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.91 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474760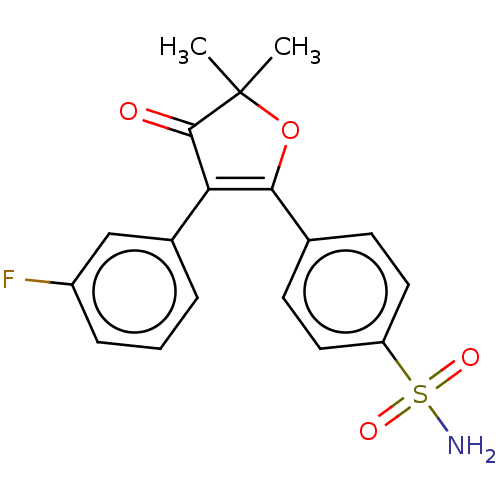 (CG-100649 | CG100649 | Polmacoxib) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50170264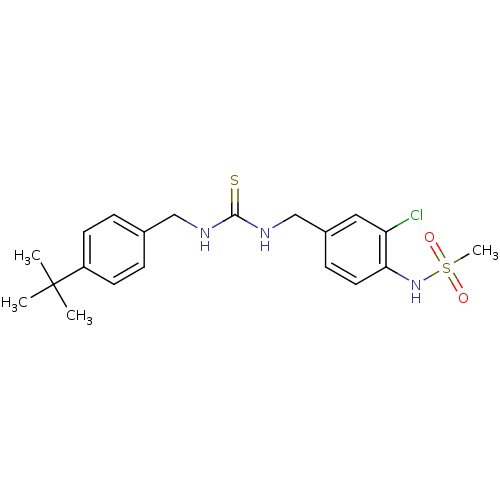 (CHEMBL361931 | N-{4-[3-(4-tert-Butyl-benzyl)-thiou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibitory effect on capsaicin (0.5 uM)-induced calcium uptake in rat DRG neurons upon incubation at RT for 10 minutes | J Med Chem 48: 5823-36 (2005) Article DOI: 10.1021/jm0502790 BindingDB Entry DOI: 10.7270/Q2057FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50170264 (CHEMBL361931 | N-{4-[3-(4-tert-Butyl-benzyl)-thiou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibitory effect on capsaicin (0.5 uM)-induced calcium uptake in rat DRG neurons upon incubation at RT for 10 minutes | J Med Chem 48: 5823-36 (2005) Article DOI: 10.1021/jm0502790 BindingDB Entry DOI: 10.7270/Q2057FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298804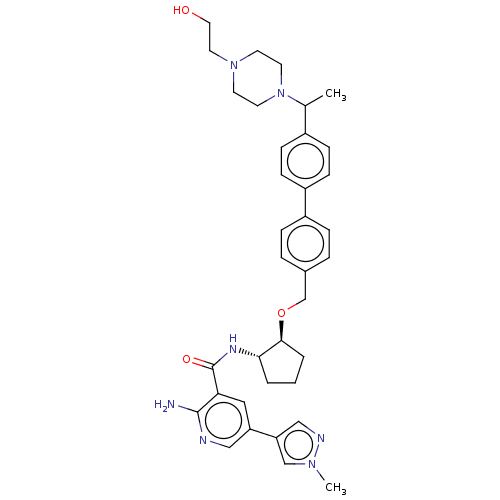 (US10125118, Example 210 | US10947215, Example 210) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298805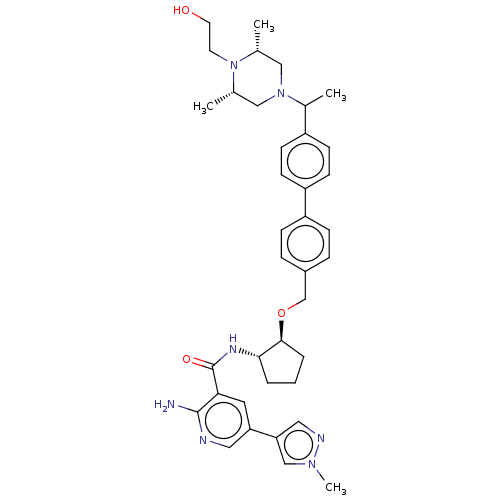 (US10125118, Example 211 | US10947215, Example 211) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298806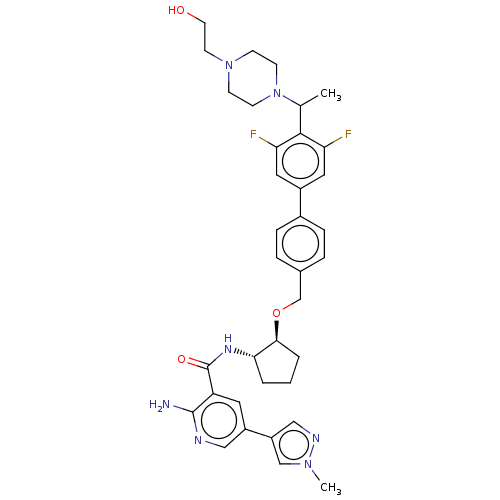 (US10125118, Example 212 | US10947215, Example 212) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298807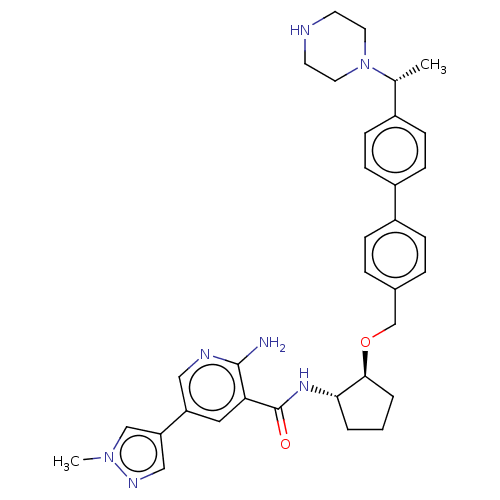 (US10125118, Example 213 | US10947215, Example 213) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298808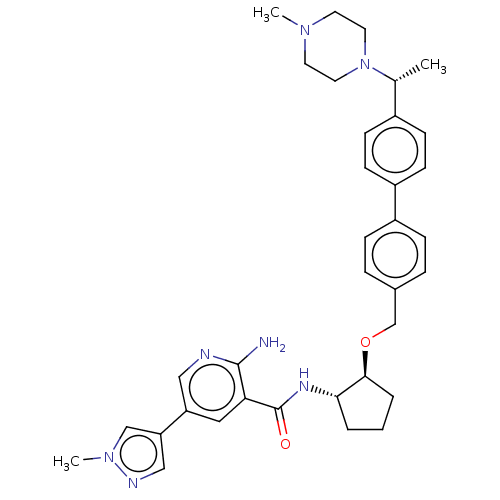 (US10125118, Example 214 | US10947215, Example 214) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298809 (US10125118, Example 215 | US10947215, Example 215) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298810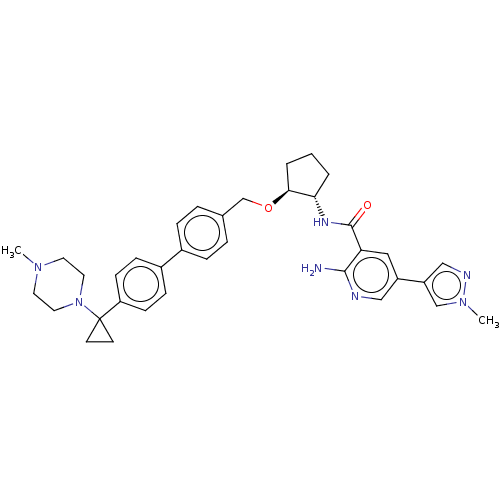 (US10125118, Example 216 | US10947215, Example 216) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298811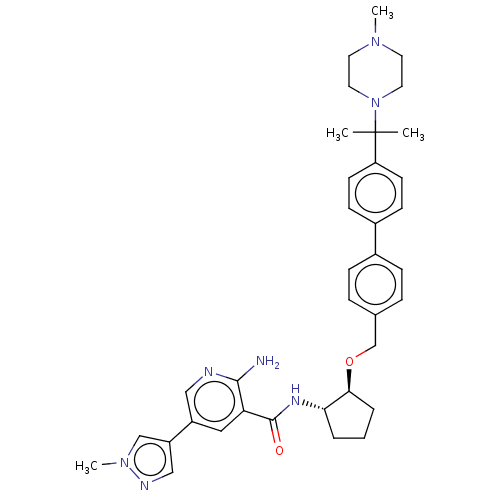 (US10125118, Example 217 | US10947215, Example 217) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298812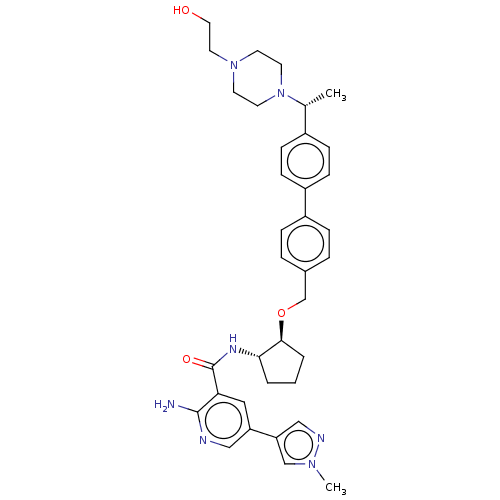 (US10125118, Example 218 | US10947215, Example 218) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298813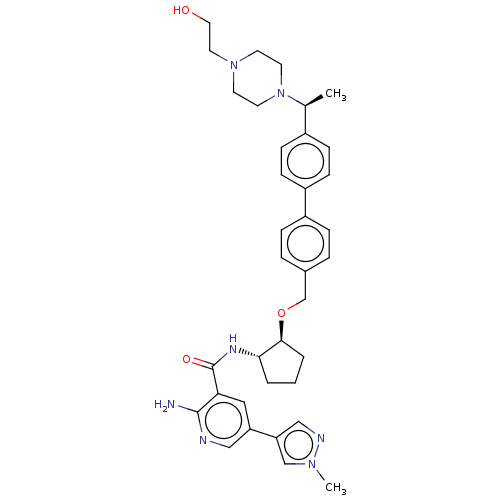 (US10125118, Example 219 | US10947215, Example 219) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298814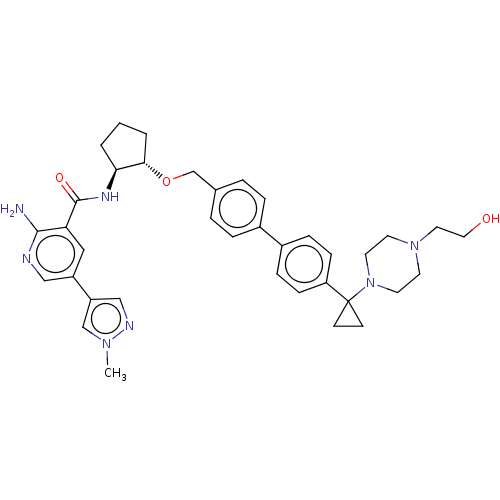 (US10125118, Example 220 | US10947215, Example 220) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298815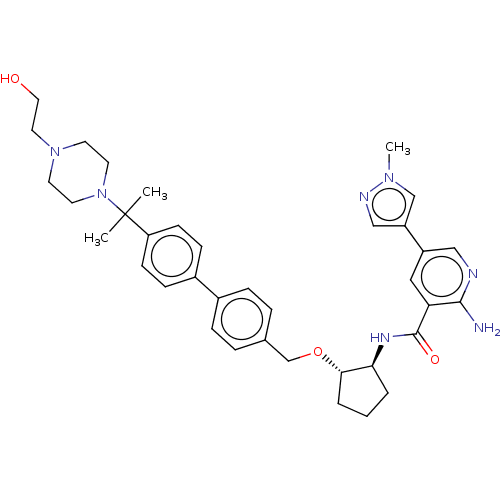 (US10125118, Example 221 | US10947215, Example 221) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298816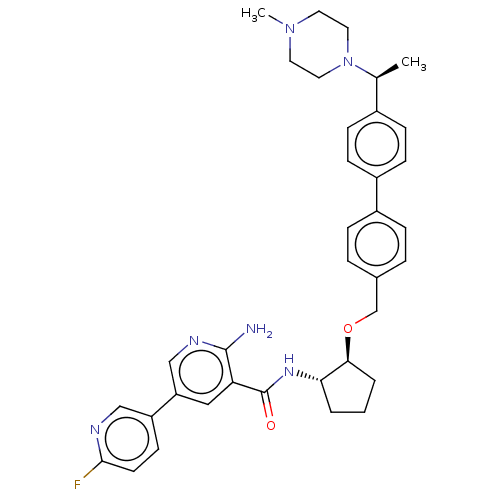 (US10125118, Example 222 | US10125118, Example 223 ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298816 (US10125118, Example 222 | US10125118, Example 223 ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298818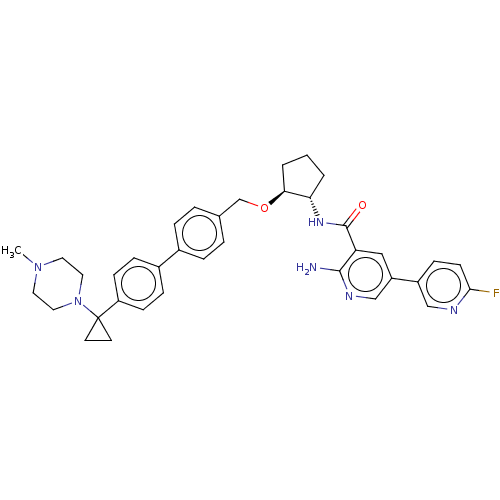 (US10125118, Example 224 | US10947215, Example 224) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298819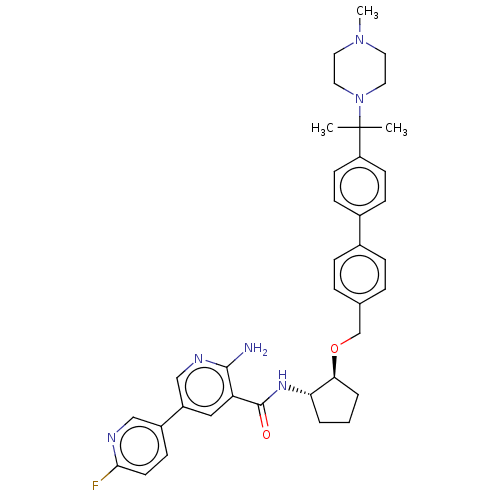 (US10125118, Example 225 | US10947215, Example 225) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298820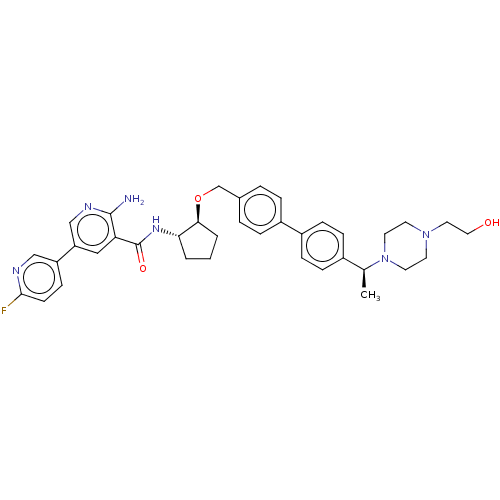 (US10125118, Example 226 | US10947215, Example 226) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298821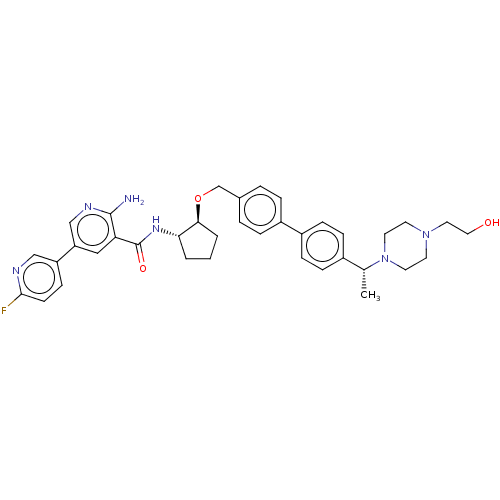 (US10125118, Example 227 | US10947215, Example 227) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298822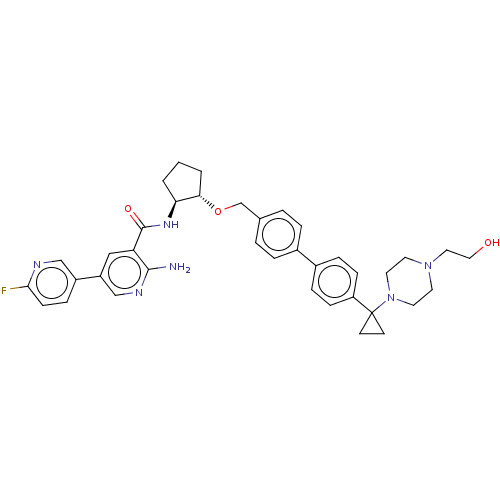 (US10125118, Example 228 | US10947215, Example 228) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298823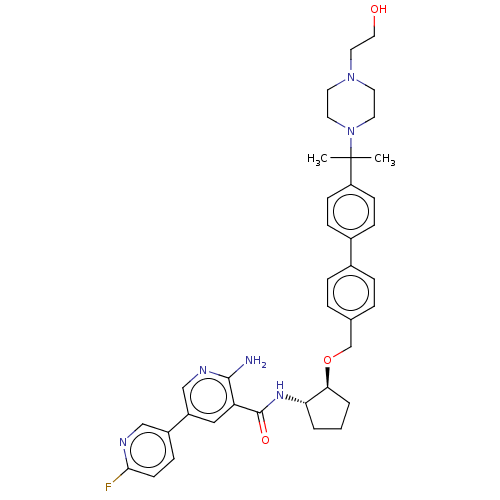 (US10125118, Example 229 | US10947215, Example 229) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298824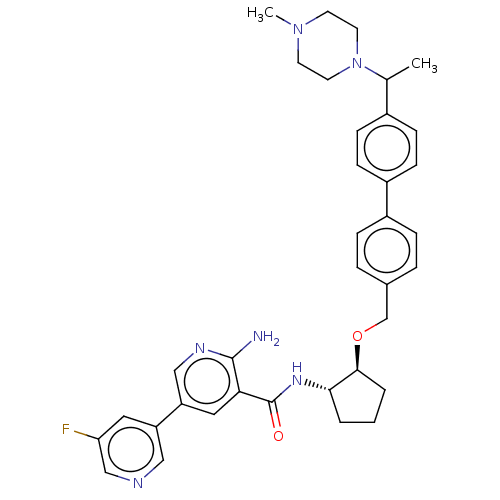 (US10125118, Example 230 | US10947215, Example 230) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298825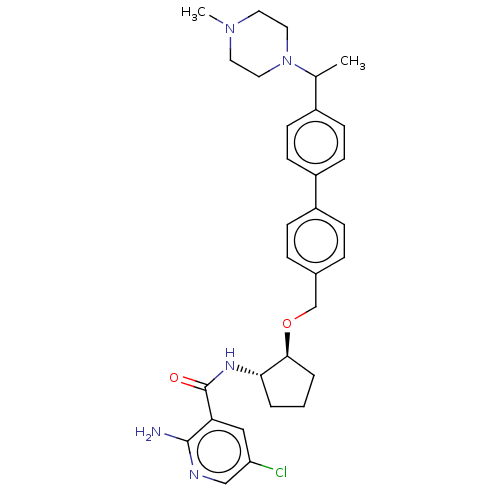 (US10125118, Example 231 | US10947215, Example 231) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298826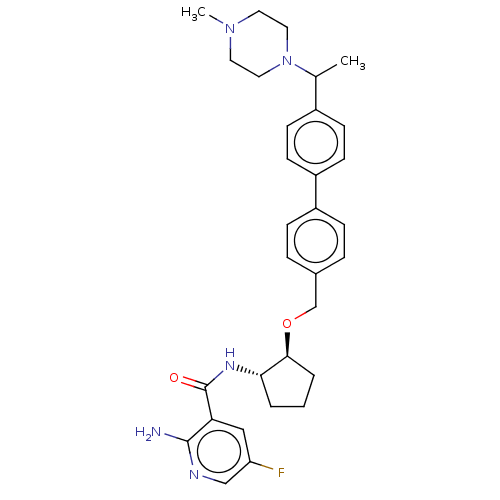 (US10125118, Example 232 | US10947215, Example 232) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM486602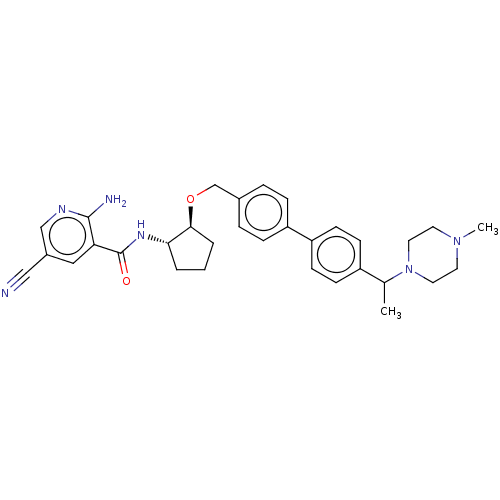 (US10947215, Example 233) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer/receptor UFO (Homo sapiens (Human)) | BDBM298829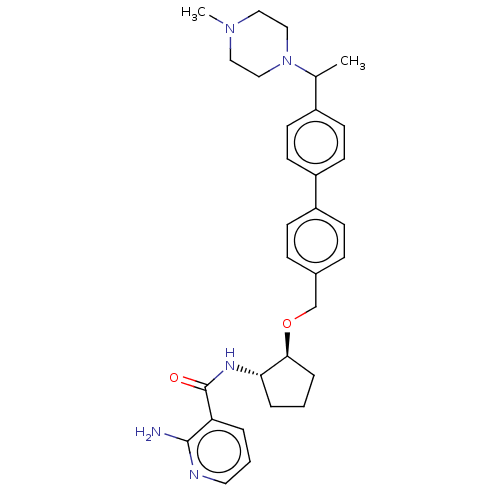 (US10125118, Example 235 | US10947215, Example 235) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Socio Holdings Co., Ltd. US Patent | Assay Description For the SAR (structure-activity relationship) and compound screening, LanthaScreen™ TR-FRET (Time-Resolved fluorescence energy transfer) assay was em... | US Patent US10947215 (2021) BindingDB Entry DOI: 10.7270/Q2862KKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1374 total ) | Next | Last >> |