 Found 5276 hits with Last Name = 'lyssikatos' and Initial = 'jp'
Found 5276 hits with Last Name = 'lyssikatos' and Initial = 'jp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM110961

(US8614206, 518)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@](C)(N)CC(F)(F)C1 |r| Show InChI InChI=1S/C21H23F4N7OS/c1-20(27)6-7-32(10-21(24,25)9-20)19-13(8-28-31(19)2)29-17(33)15-16(26)34-18(30-15)14-11(22)4-3-5-12(14)23/h3-5,8H,6-7,9-10,26-27H2,1-2H3,(H,29,33)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505054

(CHEMBL4455188)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC[C@@H](CC1)NCC(F)F |r| Show InChI InChI=1S/C22H25F4N7OS/c1-32-22(33-8-3-4-12(7-9-33)28-11-16(25)26)15(10-29-32)30-20(34)18-19(27)35-21(31-18)17-13(23)5-2-6-14(17)24/h2,5-6,10,12,16,28H,3-4,7-9,11,27H2,1H3,(H,30,34)/t12-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505052

(CHEMBL3623150)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@H](N)CC(F)(F)C1 |r| Show InChI InChI=1S/C20H21F4N7OS/c1-30-19(31-6-5-10(25)7-20(23,24)9-31)13(8-27-30)28-17(32)15-16(26)33-18(29-15)14-11(21)3-2-4-12(14)22/h2-4,8,10H,5-7,9,25-26H2,1H3,(H,28,32)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM227170

(US9328106, 118)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1[C@@]12CC[C@@H](O1)[C@H](N)CC2 |r| Show InChI InChI=1S/C21H22F2N6O2S/c1-29-17(21-7-5-12(24)14(31-21)6-8-21)13(9-26-29)27-19(30)16-18(25)32-20(28-16)15-10(22)3-2-4-11(15)23/h2-4,9,12,14H,5-8,24-25H2,1H3,(H,27,30)/t12-,14-,21-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131278
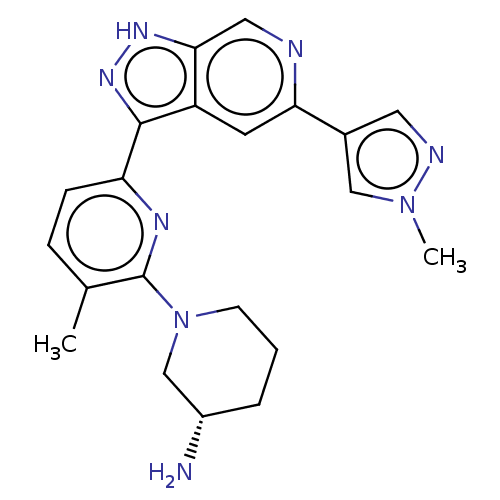
(CHEMBL3634760 | US9260425, 433)Show SMILES Cc1ccc(nc1N1CCC[C@H](N)C1)-c1n[nH]c2cnc(cc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C21H24N8/c1-13-5-6-17(25-21(13)29-7-3-4-15(22)12-29)20-16-8-18(14-9-24-28(2)11-14)23-10-19(16)26-27-20/h5-6,8-11,15H,3-4,7,12,22H2,1-2H3,(H,26,27)/t15-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM227170

(US9328106, 118)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1[C@@]12CC[C@@H](O1)[C@H](N)CC2 |r| Show InChI InChI=1S/C21H22F2N6O2S/c1-29-17(21-7-5-12(24)14(31-21)6-8-21)13(9-26-29)27-19(30)16-18(25)32-20(28-16)15-10(22)3-2-4-11(15)23/h2-4,9,12,14H,5-8,24-25H2,1H3,(H,27,30)/t12-,14-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505051
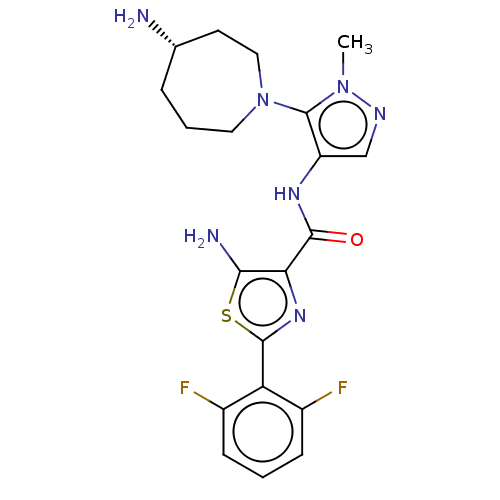
(CHEMBL4437940)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC[C@H](N)CC1 |r| Show InChI InChI=1S/C20H23F2N7OS/c1-28-20(29-8-3-4-11(23)7-9-29)14(10-25-28)26-18(30)16-17(24)31-19(27-16)15-12(21)5-2-6-13(15)22/h2,5-6,10-11H,3-4,7-9,23-24H2,1H3,(H,26,30)/t11-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505059

(CHEMBL4459538)Show SMILES CO[C@H]1C[C@H](N)CCN(C1)c1c(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)cnn1C |r| Show InChI InChI=1S/C21H25F2N7O2S/c1-29-21(30-7-6-11(24)8-12(10-30)32-2)15(9-26-29)27-19(31)17-18(25)33-20(28-17)16-13(22)4-3-5-14(16)23/h3-5,9,11-12H,6-8,10,24-25H2,1-2H3,(H,27,31)/t11-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM110700

(US8614206, 120 | US8614206, 125 | US8614206, 400)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCCC(N)C(F)C1 Show InChI InChI=1S/C20H22F3N7OS/c1-29-20(30-7-3-6-13(24)12(23)9-30)14(8-26-29)27-18(31)16-17(25)32-19(28-16)15-10(21)4-2-5-11(15)22/h2,4-5,8,12-13H,3,6-7,9,24-25H2,1H3,(H,27,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505052

(CHEMBL3623150)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@H](N)CC(F)(F)C1 |r| Show InChI InChI=1S/C20H21F4N7OS/c1-30-19(31-6-5-10(25)7-20(23,24)9-31)13(8-27-30)28-17(32)15-16(26)33-18(29-15)14-11(21)3-2-4-12(14)22/h2-4,8,10H,5-7,9,25-26H2,1H3,(H,28,32)/t10-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131224
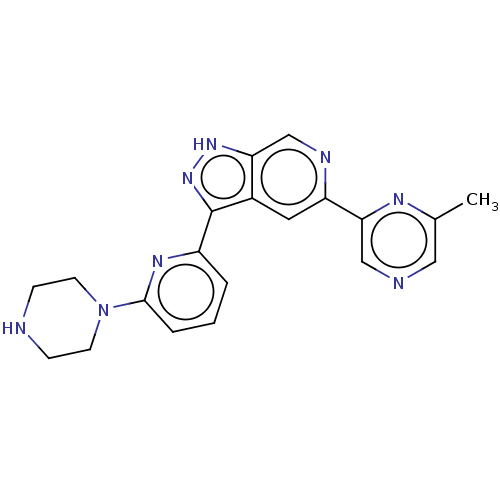
(CHEMBL3634783 | US9260425, 505)Show SMILES Cc1cncc(n1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C20H20N8/c1-13-10-22-11-18(24-13)16-9-14-17(12-23-16)26-27-20(14)15-3-2-4-19(25-15)28-7-5-21-6-8-28/h2-4,9-12,21H,5-8H2,1H3,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505053

(CHEMBL4469964)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1C1CCC(N)C(F)CC1 Show InChI InChI=1S/C21H23F3N6OS/c1-30-18(10-5-7-11(22)14(25)8-6-10)15(9-27-30)28-20(31)17-19(26)32-21(29-17)16-12(23)3-2-4-13(16)24/h2-4,9-11,14H,5-8,25-26H2,1H3,(H,28,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131377

(CHEMBL3634781 | US9260425, 162)Show SMILES C1CN(CCN1)c1cccc(n1)-c1n[nH]c2cnc(cc12)-c1cccnc1 Show InChI InChI=1S/C20H19N7/c1-4-16(24-19(5-1)27-9-7-21-8-10-27)20-15-11-17(14-3-2-6-22-12-14)23-13-18(15)25-26-20/h1-6,11-13,21H,7-10H2,(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131224

(CHEMBL3634783 | US9260425, 505)Show SMILES Cc1cncc(n1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C20H20N8/c1-13-10-22-11-18(24-13)16-9-14-17(12-23-16)26-27-20(14)15-3-2-4-19(25-15)28-7-5-21-6-8-28/h2-4,9-12,21H,5-8H2,1H3,(H,26,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505061
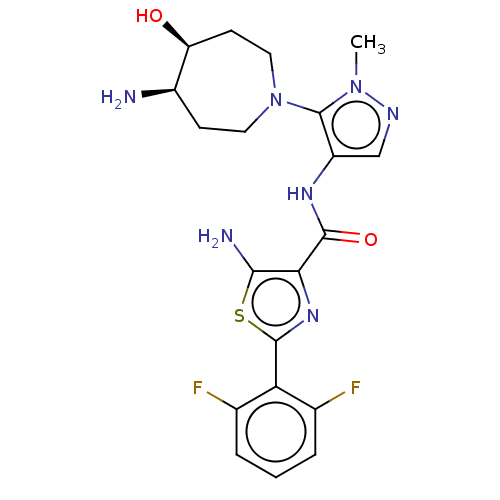
(CHEMBL4453890)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@H](N)[C@@H](O)CC1 |r| Show InChI InChI=1S/C20H23F2N7O2S/c1-28-20(29-7-5-12(23)14(30)6-8-29)13(9-25-28)26-18(31)16-17(24)32-19(27-16)15-10(21)3-2-4-11(15)22/h2-4,9,12,14,30H,5-8,23-24H2,1H3,(H,26,31)/t12-,14+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM110961

(US8614206, 518)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@](C)(N)CC(F)(F)C1 |r| Show InChI InChI=1S/C21H23F4N7OS/c1-20(27)6-7-32(10-21(24,25)9-20)19-13(8-28-31(19)2)29-17(33)15-16(26)34-18(30-15)14-11(22)4-3-5-12(14)23/h3-5,8H,6-7,9-10,26-27H2,1-2H3,(H,29,33)/t20-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505050

(CHEMBL4439756)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC(N)CC(F)C1 Show InChI InChI=1S/C20H22F3N7OS/c1-29-20(30-6-5-11(24)7-10(21)9-30)14(8-26-29)27-18(31)16-17(25)32-19(28-16)15-12(22)3-2-4-13(15)23/h2-4,8,10-11H,5-7,9,24-25H2,1H3,(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505050

(CHEMBL4439756)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC(N)CC(F)C1 Show InChI InChI=1S/C20H22F3N7OS/c1-29-20(30-6-5-11(24)7-10(21)9-30)14(8-26-29)27-18(31)16-17(25)32-19(28-16)15-12(22)3-2-4-13(15)23/h2-4,8,10-11H,5-7,9,24-25H2,1H3,(H,27,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505057

(CHEMBL3676285)Show SMILES N[C@H]1CCCN(C1)c1ccncc1NC(=O)c1nc(sc1N)-c1c(F)cccc1F Show InChI InChI=1S/C20H20F2N6OS/c21-12-4-1-5-13(22)16(12)20-27-17(18(24)30-20)19(29)26-14-9-25-7-6-15(14)28-8-2-3-11(23)10-28/h1,4-7,9,11H,2-3,8,10,23-24H2,(H,26,29)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131227

(CHEMBL3634771 | US9260425, 473)Show SMILES N#CCn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C20H19N9/c21-4-7-29-13-14(11-24-29)17-10-15-18(12-23-17)26-27-20(15)16-2-1-3-19(25-16)28-8-5-22-6-9-28/h1-3,10-13,22H,5-9H2,(H,26,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131261
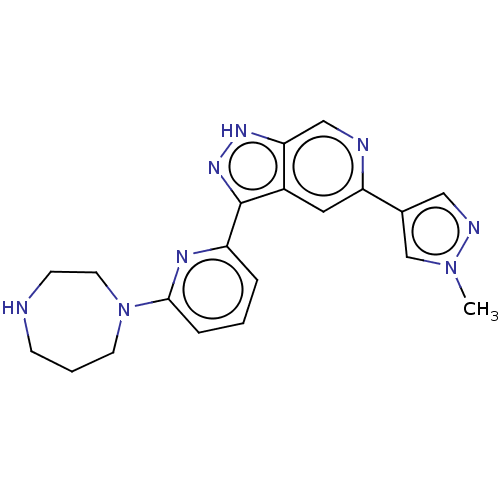
(CHEMBL3634767)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCCNCC1 Show InChI InChI=1S/C20H22N8/c1-27-13-14(11-23-27)17-10-15-18(12-22-17)25-26-20(15)16-4-2-5-19(24-16)28-8-3-6-21-7-9-28/h2,4-5,10-13,21H,3,6-9H2,1H3,(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131278

(CHEMBL3634760 | US9260425, 433)Show SMILES Cc1ccc(nc1N1CCC[C@H](N)C1)-c1n[nH]c2cnc(cc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C21H24N8/c1-13-5-6-17(25-21(13)29-7-3-4-15(22)12-29)20-16-8-18(14-9-24-28(2)11-14)23-10-19(16)26-27-20/h5-6,8-11,15H,3-4,7,12,22H2,1-2H3,(H,26,27)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131266
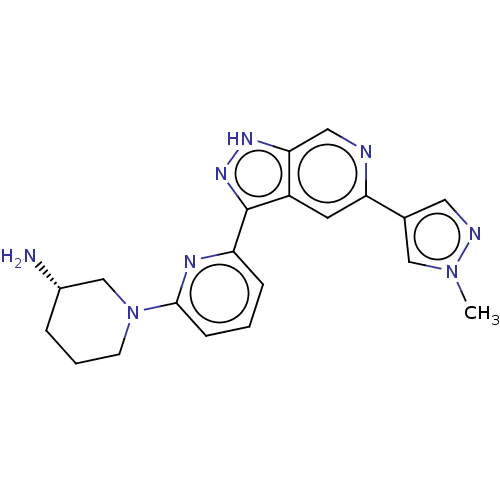
(CHEMBL3634758 | US9260425, 173)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCC[C@H](N)C1 |r| Show InChI InChI=1S/C20H22N8/c1-27-11-13(9-23-27)17-8-15-18(10-22-17)25-26-20(15)16-5-2-6-19(24-16)28-7-3-4-14(21)12-28/h2,5-6,8-11,14H,3-4,7,12,21H2,1H3,(H,25,26)/t14-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131265

(CHEMBL3634757 | US9260425, 189)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C19H20N8/c1-26-10-12(8-22-26)16-7-14-17(9-21-16)24-25-19(14)15-3-2-4-18(23-15)27-6-5-13(20)11-27/h2-4,7-10,13H,5-6,11,20H2,1H3,(H,24,25)/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131225

(CHEMBL3634769 | US9260425, 515)Show SMILES CCn1cncc(-c2cc3c(n[nH]c3cn2)-c2cccc(n2)N2CCNCC2)c1=O Show InChI InChI=1S/C21H22N8O/c1-2-28-13-23-11-15(21(28)30)17-10-14-18(12-24-17)26-27-20(14)16-4-3-5-19(25-16)29-8-6-22-7-9-29/h3-5,10-13,22H,2,6-9H2,1H3,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131228
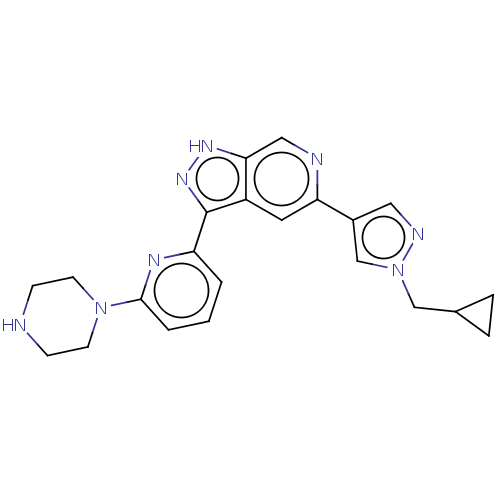
(CHEMBL3634772 | US9260425, 497)Show SMILES C(C1CC1)n1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C22H24N8/c1-2-18(26-21(3-1)29-8-6-23-7-9-29)22-17-10-19(24-12-20(17)27-28-22)16-11-25-30(14-16)13-15-4-5-15/h1-3,10-12,14-15,23H,4-9,13H2,(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131226
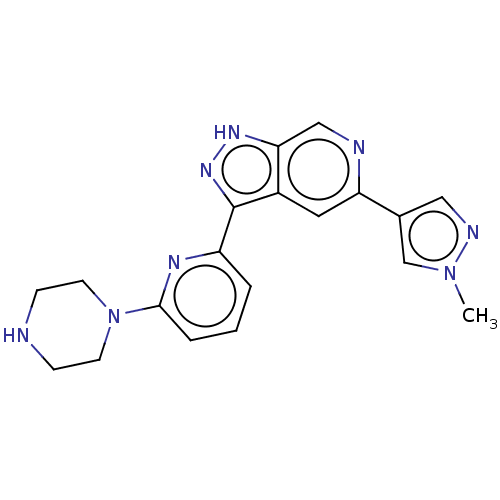
(CHEMBL3634770 | US9260425, 234)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C19H20N8/c1-26-12-13(10-22-26)16-9-14-17(11-21-16)24-25-19(14)15-3-2-4-18(23-15)27-7-5-20-6-8-27/h2-4,9-12,20H,5-8H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112421

(US8623889, 420)Show SMILES Cc1c(F)cncc1-c1ccc2cc(NC(=O)[C@@H]3C[C@@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H15F2N3O/c1-10-15(8-22-9-17(10)21)12-3-2-11-5-18(23-7-13(11)4-12)24-19(25)14-6-16(14)20/h2-5,7-9,14,16H,6H2,1H3,(H,23,24,25)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112359

(US8623889, 358)Show SMILES Cc1ccc2[nH]ncc2c1-c1cc2cnc(NC(=O)[C@@H]3C[C@@H]3F)cc2cn1 |r| Show InChI InChI=1S/C20H16FN5O/c1-10-2-3-16-14(9-24-26-16)19(10)17-4-11-8-23-18(5-12(11)7-22-17)25-20(27)13-6-15(13)21/h2-5,7-9,13,15H,6H2,1H3,(H,24,26)(H,23,25,27)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505059

(CHEMBL4459538)Show SMILES CO[C@H]1C[C@H](N)CCN(C1)c1c(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)cnn1C |r| Show InChI InChI=1S/C21H25F2N7O2S/c1-29-21(30-7-6-11(24)8-12(10-30)32-2)15(9-26-29)27-19(31)17-18(25)33-20(28-17)16-13(22)4-3-5-14(16)23/h3-5,9,11-12H,6-8,10,24-25H2,1-2H3,(H,27,31)/t11-,12+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112111
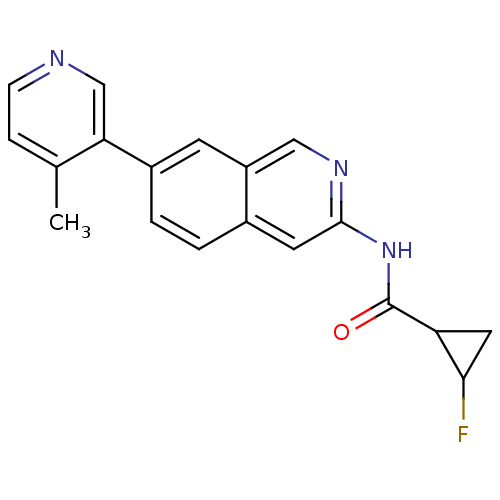
(US8623889, 109)Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112161

(US8623889, 159)Show InChI InChI=1S/C19H16FN3O/c1-11-16(9-21-10-17(11)20)14-5-4-13-7-18(22-8-15(13)6-14)23-19(24)12-2-3-12/h4-10,12H,2-3H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112066

(US8623889, 64)Show InChI InChI=1S/C20H18N2O2/c1-12-2-7-17(23)10-18(12)15-6-5-14-9-19(21-11-16(14)8-15)22-20(24)13-3-4-13/h2,5-11,13,23H,3-4H2,1H3,(H,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112230
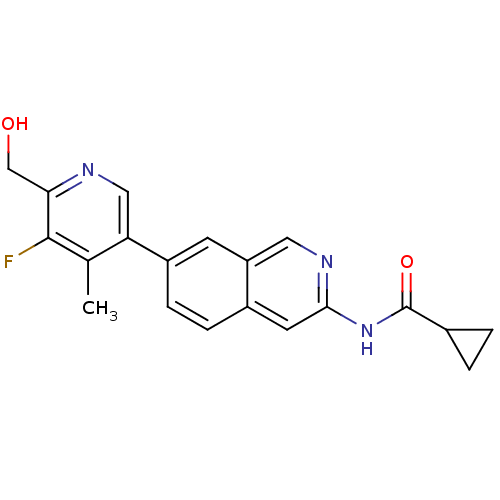
(US8623889, 228)Show SMILES Cc1c(F)c(CO)ncc1-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C20H18FN3O2/c1-11-16(9-22-17(10-25)19(11)21)14-5-4-13-7-18(23-8-15(13)6-14)24-20(26)12-2-3-12/h4-9,12,25H,2-3,10H2,1H3,(H,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112193
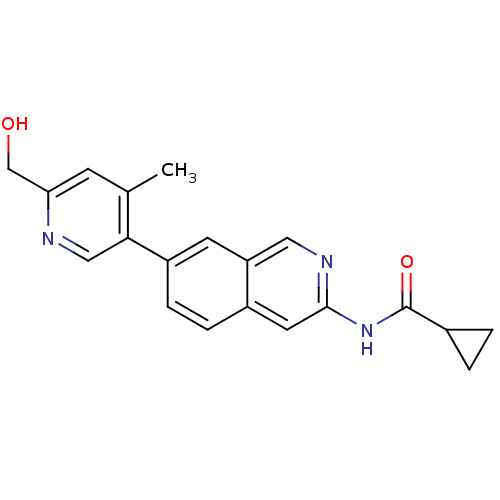
(US8623889, 191)Show InChI InChI=1S/C20H19N3O2/c1-12-6-17(11-24)21-10-18(12)15-5-4-14-8-19(22-9-16(14)7-15)23-20(25)13-2-3-13/h4-10,13,24H,2-3,11H2,1H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112312

(US8623889, 311)Show SMILES Cc1cc(ncc1-c1ccc2cc(NC(=O)C3C[C@@H]3F)ncc2c1)[C@H](O)C(F)(F)F |r| Show InChI InChI=1S/C21H17F4N3O2/c1-10-4-17(19(29)21(23,24)25)26-9-15(10)12-3-2-11-6-18(27-8-13(11)5-12)28-20(30)14-7-16(14)22/h2-6,8-9,14,16,19,29H,7H2,1H3,(H,27,28,30)/t14?,16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505055
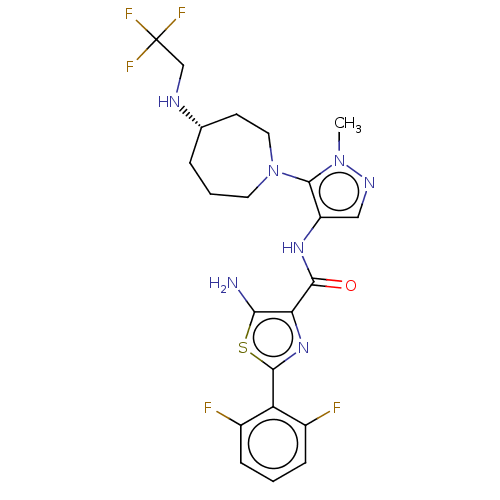
(CHEMBL4449930)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC[C@@H](CC1)NCC(F)(F)F |r| Show InChI InChI=1S/C22H24F5N7OS/c1-33-21(34-8-3-4-12(7-9-34)29-11-22(25,26)27)15(10-30-33)31-19(35)17-18(28)36-20(32-17)16-13(23)5-2-6-14(16)24/h2,5-6,10,12,29H,3-4,7-9,11,28H2,1H3,(H,31,35)/t12-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505057

(CHEMBL3676285)Show SMILES N[C@H]1CCCN(C1)c1ccncc1NC(=O)c1nc(sc1N)-c1c(F)cccc1F Show InChI InChI=1S/C20H20F2N6OS/c21-12-4-1-5-13(22)16(12)20-27-17(18(24)30-20)19(29)26-14-9-25-7-6-15(14)28-8-2-3-11(23)10-28/h1,4-7,9,11H,2-3,8,10,23-24H2,(H,26,29)/t11-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131261

(CHEMBL3634767)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCCNCC1 Show InChI InChI=1S/C20H22N8/c1-27-13-14(11-23-27)17-10-15-18(12-22-17)25-26-20(15)16-4-2-5-19(24-16)28-8-3-6-21-7-9-28/h2,4-5,10-13,21H,3,6-9H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM110706

(US8614206, 139)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@H](N)[C@H](F)CC1 |r| Show InChI InChI=1S/C20H22F3N7OS/c1-29-20(30-7-5-10(21)13(24)6-8-30)14(9-26-29)27-18(31)16-17(25)32-19(28-16)15-11(22)3-2-4-12(15)23/h2-4,9-10,13H,5-8,24-25H2,1H3,(H,27,31)/t10-,13-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505051

(CHEMBL4437940)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC[C@H](N)CC1 |r| Show InChI InChI=1S/C20H23F2N7OS/c1-28-20(29-8-3-4-11(23)7-9-29)14(10-25-28)26-18(30)16-17(24)31-19(27-16)15-12(21)5-2-6-13(15)22/h2,5-6,10-11H,3-4,7-9,23-24H2,1H3,(H,26,30)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131228

(CHEMBL3634772 | US9260425, 497)Show SMILES C(C1CC1)n1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C22H24N8/c1-2-18(26-21(3-1)29-8-6-23-7-9-29)22-17-10-19(24-12-20(17)27-28-22)16-11-25-30(14-16)13-15-4-5-15/h1-3,10-12,14-15,23H,4-9,13H2,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131264
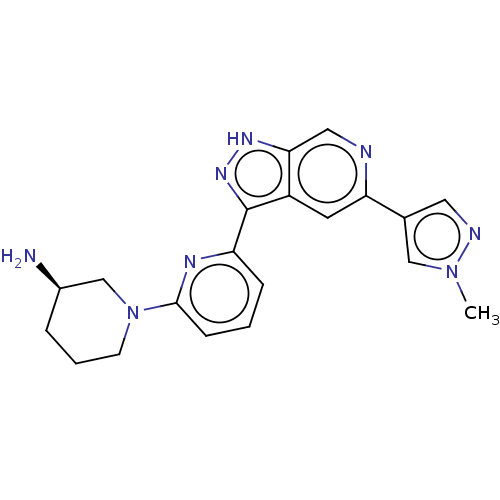
(CHEMBL3634756 | US9260425, 177)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C20H22N8/c1-27-11-13(9-23-27)17-8-15-18(10-22-17)25-26-20(15)16-5-2-6-19(24-16)28-7-3-4-14(21)12-28/h2,5-6,8-11,14H,3-4,7,12,21H2,1H3,(H,25,26)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131227

(CHEMBL3634771 | US9260425, 473)Show SMILES N#CCn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C20H19N9/c21-4-7-29-13-14(11-24-29)17-10-15-18(12-23-17)26-27-20(15)16-2-1-3-19(25-16)28-8-5-22-6-9-28/h1-3,10-13,22H,5-9H2,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112274

(US8623889, 272)Show SMILES Cc1cc(ncc1-c1ccc2cc(NC(=O)C3CC3)ncc2c1)[C@H](O)C(F)(F)F |r| Show InChI InChI=1S/C21H18F3N3O2/c1-11-6-17(19(28)21(22,23)24)25-10-16(11)14-5-4-13-8-18(26-9-15(13)7-14)27-20(29)12-2-3-12/h4-10,12,19,28H,2-3H2,1H3,(H,26,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50005209
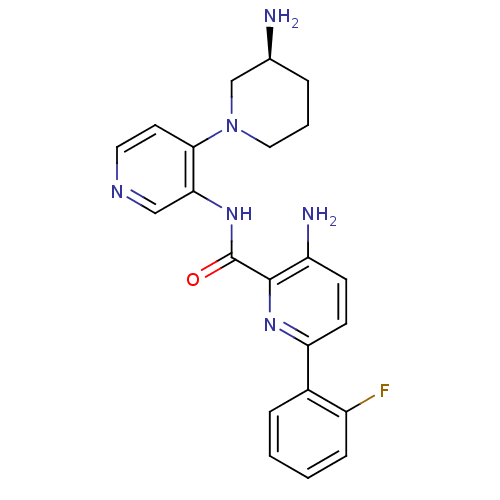
(CHEMBL3103867)Show SMILES N[C@H]1CCCN(C1)c1ccncc1NC(=O)c1nc(ccc1N)-c1ccccc1F |r| Show InChI InChI=1S/C22H23FN6O/c23-16-6-2-1-5-15(16)18-8-7-17(25)21(27-18)22(30)28-19-12-26-10-9-20(19)29-11-3-4-14(24)13-29/h1-2,5-10,12,14H,3-4,11,13,24-25H2,(H,28,30)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112288
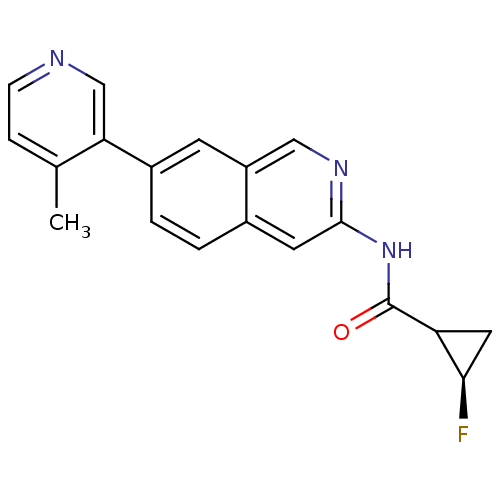
(US8623889, 286)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)C3C[C@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15?,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112289
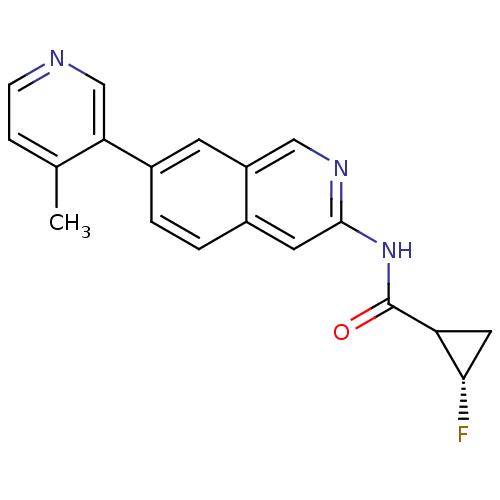
(US8623889, 287)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)C3C[C@@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112029

(US8623889, 27)Show InChI InChI=1S/C19H17N3O/c1-12-6-7-20-11-17(12)15-5-4-14-9-18(21-10-16(14)8-15)22-19(23)13-2-3-13/h4-11,13H,2-3H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112105

(US8623889, 103)Show SMILES Cc1cc(F)c(O)cc1-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C20H17FN2O2/c1-11-6-17(21)18(24)9-16(11)14-5-4-13-8-19(22-10-15(13)7-14)23-20(25)12-2-3-12/h4-10,12,24H,2-3H2,1H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data