 Found 1211 hits with Last Name = 'mo' and Initial = 'jr'
Found 1211 hits with Last Name = 'mo' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283704
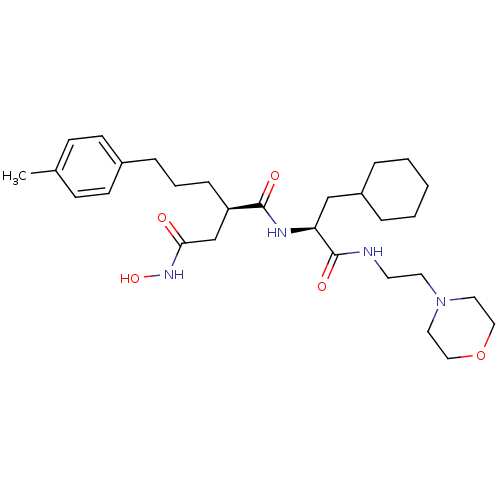
((R)-N*1*-[(S)-2-Cyclohexyl-1-(2-morpholin-4-yl-eth...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCN2CCOCC2)cc1 Show InChI InChI=1S/C29H46N4O5/c1-22-10-12-23(13-11-22)8-5-9-25(21-27(34)32-37)28(35)31-26(20-24-6-3-2-4-7-24)29(36)30-14-15-33-16-18-38-19-17-33/h10-13,24-26,37H,2-9,14-21H2,1H3,(H,30,36)(H,31,35)(H,32,34)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283703
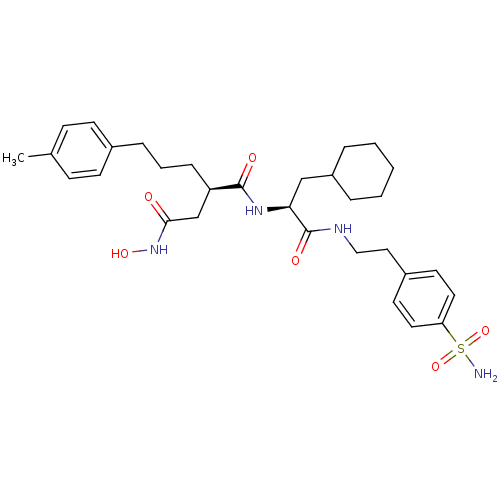
((R)-N*1*-{(S)-2-Cyclohexyl-1-[2-(4-sulfamoyl-pheny...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C31H44N4O6S/c1-22-10-12-23(13-11-22)8-5-9-26(21-29(36)35-39)30(37)34-28(20-25-6-3-2-4-7-25)31(38)33-19-18-24-14-16-27(17-15-24)42(32,40)41/h10-17,25-26,28,39H,2-9,18-21H2,1H3,(H,33,38)(H,34,37)(H,35,36)(H2,32,40,41)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283708
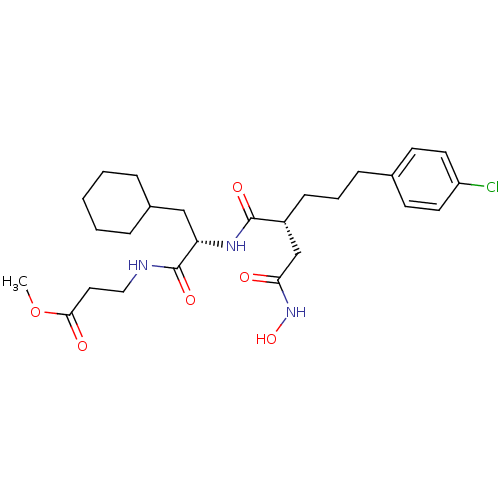
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C26H38ClN3O6/c1-36-24(32)14-15-28-26(34)22(16-19-6-3-2-4-7-19)29-25(33)20(17-23(31)30-35)9-5-8-18-10-12-21(27)13-11-18/h10-13,19-20,22,35H,2-9,14-17H2,1H3,(H,28,34)(H,29,33)(H,30,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283701
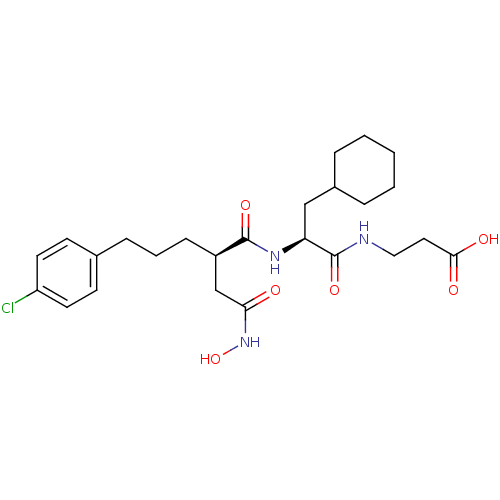
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H36ClN3O6/c26-20-11-9-17(10-12-20)7-4-8-19(16-22(30)29-35)24(33)28-21(15-18-5-2-1-3-6-18)25(34)27-14-13-23(31)32/h9-12,18-19,21,35H,1-8,13-16H2,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283705
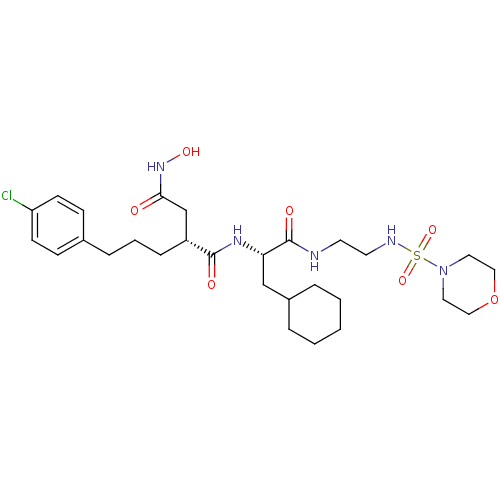
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCNS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C28H44ClN5O7S/c29-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-38)27(36)32-25(19-22-5-2-1-3-6-22)28(37)30-13-14-31-42(39,40)34-15-17-41-18-16-34/h9-12,22-23,25,31,38H,1-8,13-20H2,(H,30,37)(H,32,36)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283715
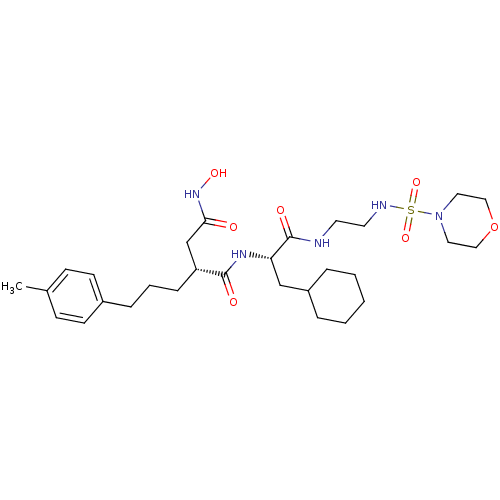
((R)-N*1*-{(S)-2-Cyclohexyl-1-[2-(morpholine-4-sulf...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCNS(=O)(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C29H47N5O7S/c1-22-10-12-23(13-11-22)8-5-9-25(21-27(35)33-38)28(36)32-26(20-24-6-3-2-4-7-24)29(37)30-14-15-31-42(39,40)34-16-18-41-19-17-34/h10-13,24-26,31,38H,2-9,14-21H2,1H3,(H,30,37)(H,32,36)(H,33,35)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283710
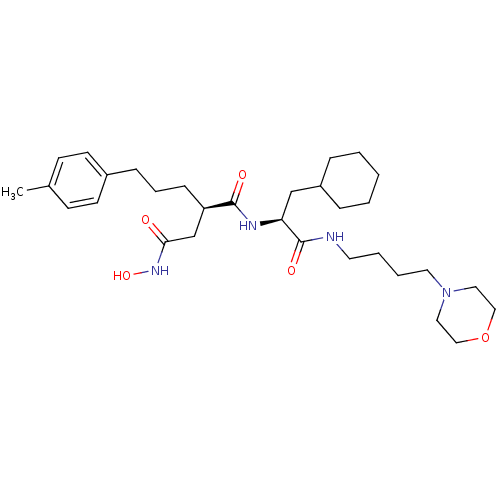
((R)-N*1*-[(S)-2-Cyclohexyl-1-(4-morpholin-4-yl-but...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCCCN2CCOCC2)cc1 Show InChI InChI=1S/C31H50N4O5/c1-24-12-14-25(15-13-24)10-7-11-27(23-29(36)34-39)30(37)33-28(22-26-8-3-2-4-9-26)31(38)32-16-5-6-17-35-18-20-40-21-19-35/h12-15,26-28,39H,2-11,16-23H2,1H3,(H,32,38)(H,33,37)(H,34,36)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283713
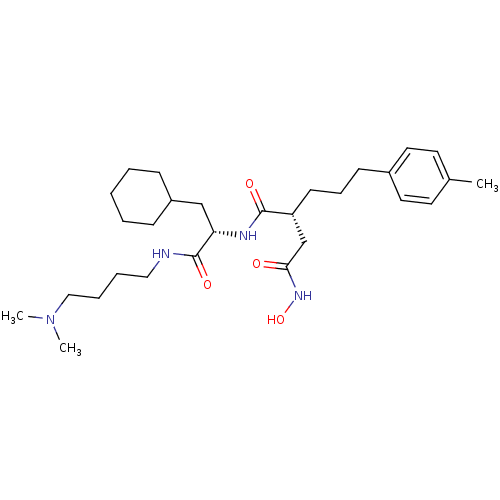
((R)-N*1*-[(S)-2-Cyclohexyl-1-(4-dimethylamino-buty...)Show SMILES CN(C)CCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(C)cc1)CC(=O)NO Show InChI InChI=1S/C29H48N4O4/c1-22-14-16-23(17-15-22)12-9-13-25(21-27(34)32-37)28(35)31-26(20-24-10-5-4-6-11-24)29(36)30-18-7-8-19-33(2)3/h14-17,24-26,37H,4-13,18-21H2,1-3H3,(H,30,36)(H,31,35)(H,32,34)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283711
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C29H43ClN4O6/c30-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-39)28(37)32-25(19-22-5-2-1-3-6-22)29(38)31-14-13-27(36)34-15-17-40-18-16-34/h9-12,22-23,25,39H,1-8,13-20H2,(H,31,38)(H,32,37)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101495
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-((S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40ClN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283707
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCCCN1CCOCC1 Show InChI InChI=1S/C30H47ClN4O5/c31-26-13-11-23(12-14-26)9-6-10-25(22-28(36)34-39)29(37)33-27(21-24-7-2-1-3-8-24)30(38)32-15-4-5-16-35-17-19-40-20-18-35/h11-14,24-25,27,39H,1-10,15-22H2,(H,32,38)(H,33,37)(H,34,36)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283702
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{(S)-2-cyc...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)[C@H](CC2CCCCC2)NC(=O)[C@H](CCCc2ccc(Cl)cc2)CC(=O)NO)cc1 Show InChI InChI=1S/C30H41ClN4O6S/c31-25-13-9-21(10-14-25)7-4-8-24(20-28(36)35-39)29(37)34-27(19-23-5-2-1-3-6-23)30(38)33-18-17-22-11-15-26(16-12-22)42(32,40)41/h9-16,23-24,27,39H,1-8,17-20H2,(H,33,38)(H,34,37)(H,35,36)(H2,32,40,41)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283714
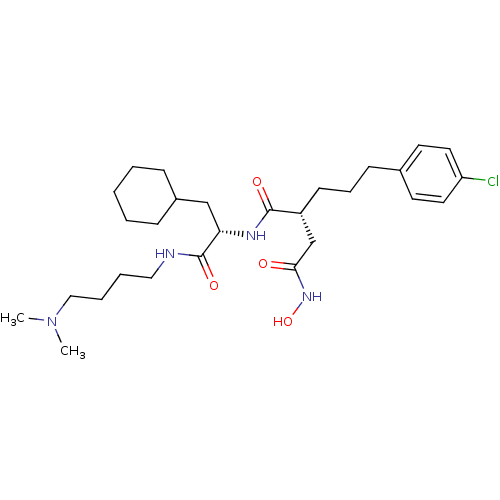
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES CN(C)CCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C28H45ClN4O4/c1-33(2)18-7-6-17-30-28(36)25(19-22-9-4-3-5-10-22)31-27(35)23(20-26(34)32-37)12-8-11-21-13-15-24(29)16-14-21/h13-16,22-23,25,37H,3-12,17-20H2,1-2H3,(H,30,36)(H,31,35)(H,32,34)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101530
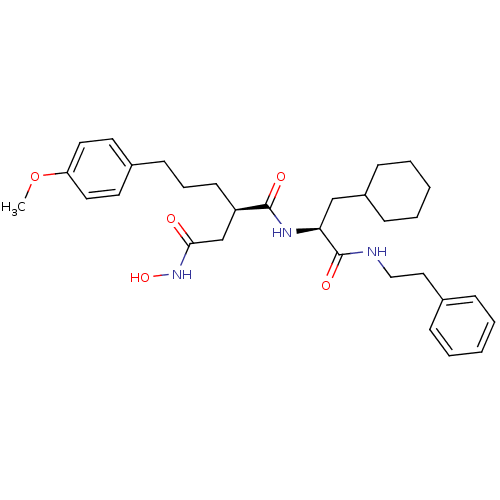
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES COc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O5/c1-39-27-17-15-24(16-18-27)13-8-14-26(22-29(35)34-38)30(36)33-28(21-25-11-6-3-7-12-25)31(37)32-20-19-23-9-4-2-5-10-23/h2,4-5,9-10,15-18,25-26,28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101526
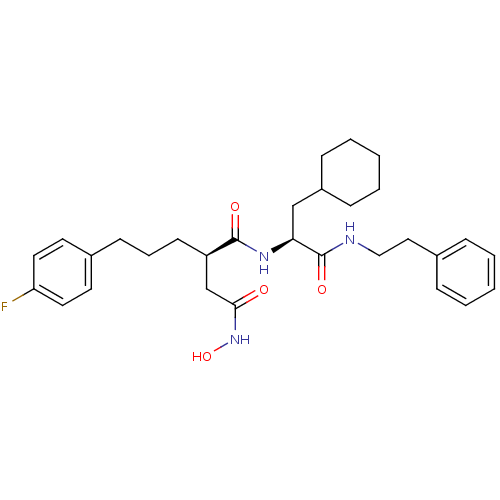
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(F)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40FN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101492
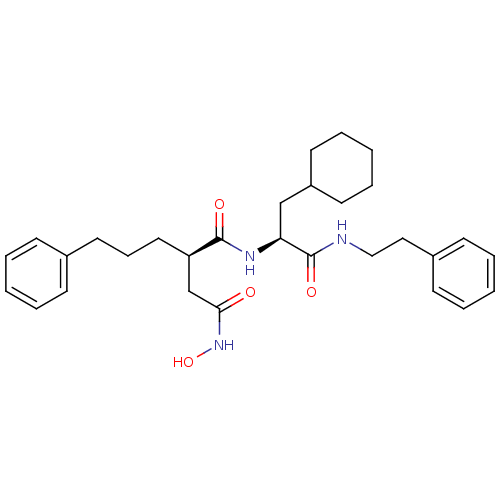
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c34-28(33-37)22-26(18-10-17-23-11-4-1-5-12-23)29(35)32-27(21-25-15-8-3-9-16-25)30(36)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-27,37H,3,8-10,15-22H2,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101492

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c34-28(33-37)22-26(18-10-17-23-11-4-1-5-12-23)29(35)32-27(21-25-15-8-3-9-16-25)30(36)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-27,37H,3,8-10,15-22H2,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101513

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O4/c1-23-15-17-25(18-16-23)13-8-14-27(22-29(35)34-38)30(36)33-28(21-26-11-6-3-7-12-26)31(37)32-20-19-24-9-4-2-5-10-24/h2,4-5,9-10,15-18,26-28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101494
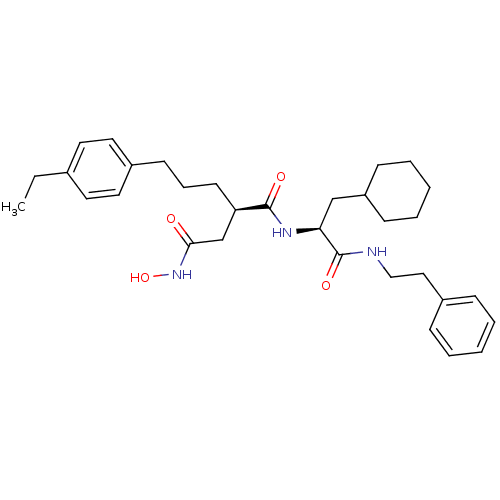
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES CCc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C32H45N3O4/c1-2-24-16-18-26(19-17-24)14-9-15-28(23-30(36)35-39)31(37)34-29(22-27-12-7-4-8-13-27)32(38)33-21-20-25-10-5-3-6-11-25/h3,5-6,10-11,16-19,27-29,39H,2,4,7-9,12-15,20-23H2,1H3,(H,33,38)(H,34,37)(H,35,36)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854
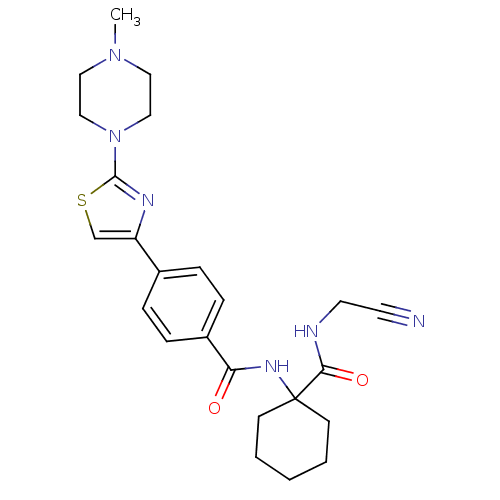
(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
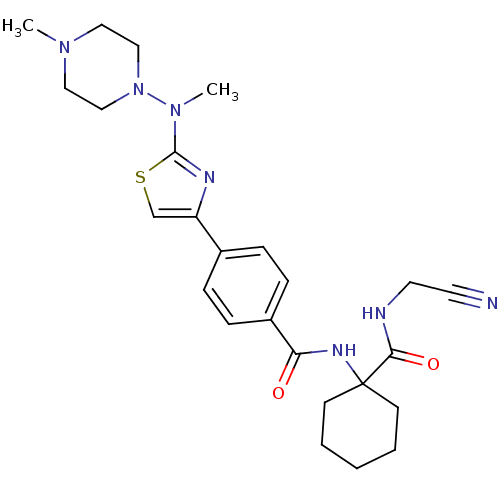
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101528
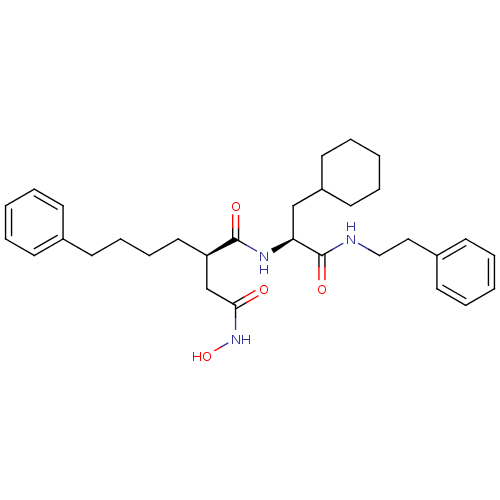
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H43N3O4/c35-29(34-38)23-27(19-11-10-16-24-12-4-1-5-13-24)30(36)33-28(22-26-17-8-3-9-18-26)31(37)32-21-20-25-14-6-2-7-15-25/h1-2,4-7,12-15,26-28,38H,3,8-11,16-23H2,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
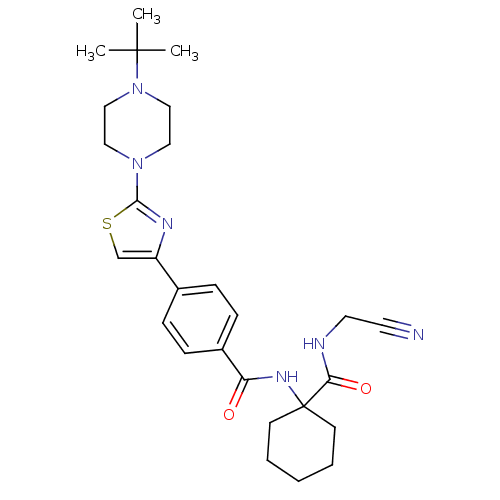
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101510

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(cc1)C(F)(F)F)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H40F3N3O4/c32-31(33,34)26-16-14-23(15-17-26)12-7-13-25(21-28(38)37-41)29(39)36-27(20-24-10-5-2-6-11-24)30(40)35-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,41H,2,5-7,10-13,18-21H2,(H,35,40)(H,36,39)(H,37,38)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101529

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H39N3O4/c1-18(2)15-21(17-23(29)28-32)24(30)27-22(16-20-11-7-4-8-12-20)25(31)26-14-13-19-9-5-3-6-10-19/h3,5-6,9-10,18,20-22,32H,4,7-8,11-17H2,1-2H3,(H,26,31)(H,27,30)(H,28,29)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410609
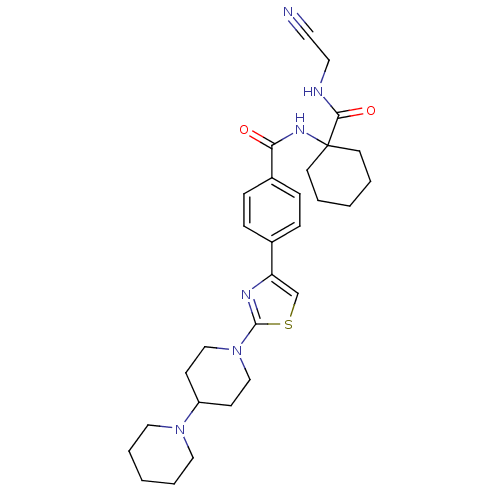
(CHEMBL198798)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C29H38N6O2S/c30-15-16-31-27(37)29(13-3-1-4-14-29)33-26(36)23-9-7-22(8-10-23)25-21-38-28(32-25)35-19-11-24(12-20-35)34-17-5-2-6-18-34/h7-10,21,24H,1-6,11-14,16-20H2,(H,31,37)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410590
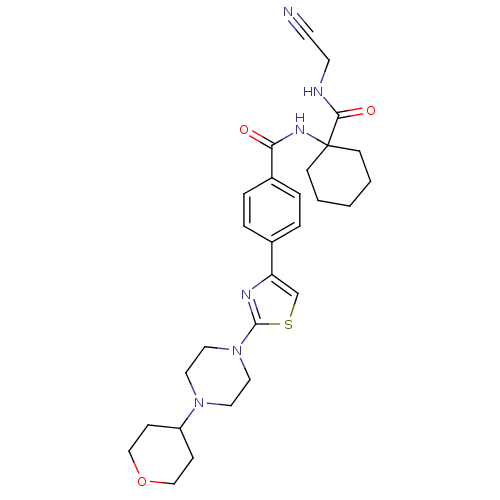
(CHEMBL200543)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCN(CC1)C1CCOCC1 Show InChI InChI=1S/C28H36N6O3S/c29-12-13-30-26(36)28(10-2-1-3-11-28)32-25(35)22-6-4-21(5-7-22)24-20-38-27(31-24)34-16-14-33(15-17-34)23-8-18-37-19-9-23/h4-7,20,23H,1-3,8-11,13-19H2,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410587
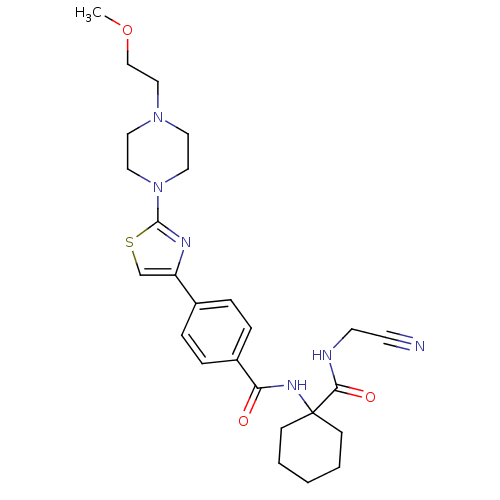
(CHEMBL200602)Show SMILES COCCN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H34N6O3S/c1-35-18-17-31-13-15-32(16-14-31)25-29-22(19-36-25)20-5-7-21(8-6-20)23(33)30-26(9-3-2-4-10-26)24(34)28-12-11-27/h5-8,19H,2-4,9-10,12-18H2,1H3,(H,28,34)(H,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410607
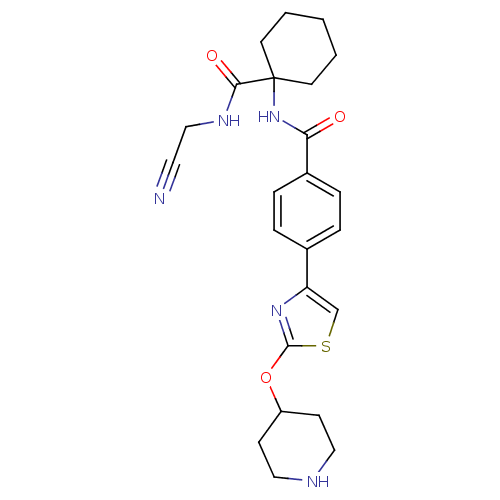
(CHEMBL200744)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(OC2CCNCC2)n1 Show InChI InChI=1S/C24H29N5O3S/c25-12-15-27-22(31)24(10-2-1-3-11-24)29-21(30)18-6-4-17(5-7-18)20-16-33-23(28-20)32-19-8-13-26-14-9-19/h4-7,16,19,26H,1-3,8-11,13-15H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410571
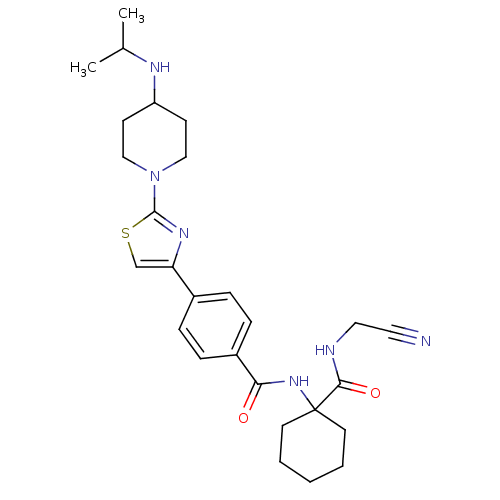
(CHEMBL200287)Show SMILES CC(C)NC1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-19(2)30-22-10-16-33(17-11-22)26-31-23(18-36-26)20-6-8-21(9-7-20)24(34)32-27(12-4-3-5-13-27)25(35)29-15-14-28/h6-9,18-19,22,30H,3-5,10-13,15-17H2,1-2H3,(H,29,35)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283712

(4-{2-[(S)-3-Cyclohexyl-2-((R)-2-hydroxycarbamoylme...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccc(cc1)S(O)(=O)=O Show InChI InChI=1S/C30H41N3O7S/c34-28(33-37)21-25(13-7-12-22-8-3-1-4-9-22)29(35)32-27(20-24-10-5-2-6-11-24)30(36)31-19-18-23-14-16-26(17-15-23)41(38,39)40/h1,3-4,8-9,14-17,24-25,27,37H,2,5-7,10-13,18-21H2,(H,31,36)(H,32,35)(H,33,34)(H,38,39,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410580
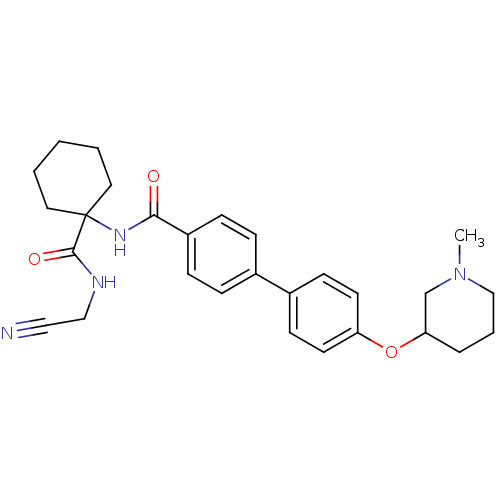
(CHEMBL435913)Show SMILES CN1CCCC(C1)Oc1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H34N4O3/c1-32-19-5-6-25(20-32)35-24-13-11-22(12-14-24)21-7-9-23(10-8-21)26(33)31-28(15-3-2-4-16-28)27(34)30-18-17-29/h7-14,25H,2-6,15-16,18-20H2,1H3,(H,30,34)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410575
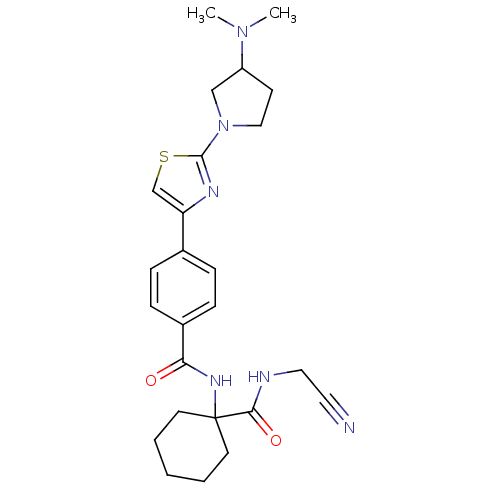
(CHEMBL199470)Show SMILES CN(C)C1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H32N6O2S/c1-30(2)20-10-15-31(16-20)24-28-21(17-34-24)18-6-8-19(9-7-18)22(32)29-25(11-4-3-5-12-25)23(33)27-14-13-26/h6-9,17,20H,3-5,10-12,14-16H2,1-2H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283701

(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H36ClN3O6/c26-20-11-9-17(10-12-20)7-4-8-19(16-22(30)29-35)24(33)28-21(15-18-5-2-1-3-6-18)25(34)27-14-13-23(31)32/h9-12,18-19,21,35H,1-8,13-16H2,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101523
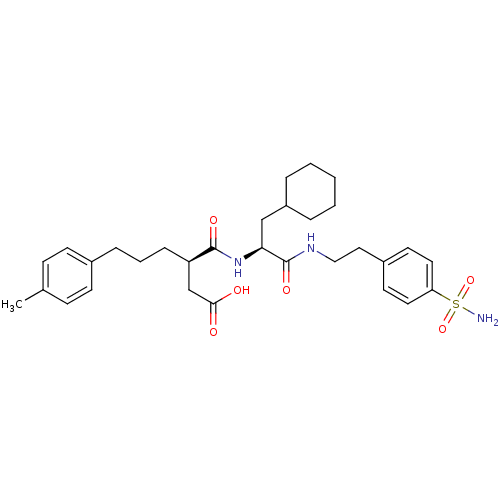
((R)-3-{(S)-2-Cyclohexyl-1-[2-(4-sulfamoyl-phenyl)-...)Show SMILES Cc1ccc(CCC[C@H](CC(O)=O)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C31H43N3O6S/c1-22-10-12-23(13-11-22)8-5-9-26(21-29(35)36)30(37)34-28(20-25-6-3-2-4-7-25)31(38)33-19-18-24-14-16-27(17-15-24)41(32,39)40/h10-17,25-26,28H,2-9,18-21H2,1H3,(H,33,38)(H,34,37)(H,35,36)(H2,32,39,40)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410591

(CHEMBL200506)Show SMILES CN1CCN(Cc2nc(cs2)-c2ccc(cc2)C(=O)NC2(CCCCC2)C(=O)NCC#N)CC1 Show InChI InChI=1S/C25H32N6O2S/c1-30-13-15-31(16-14-30)17-22-28-21(18-34-22)19-5-7-20(8-6-19)23(32)29-25(9-3-2-4-10-25)24(33)27-12-11-26/h5-8,18H,2-4,9-10,12-17H2,1H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410612
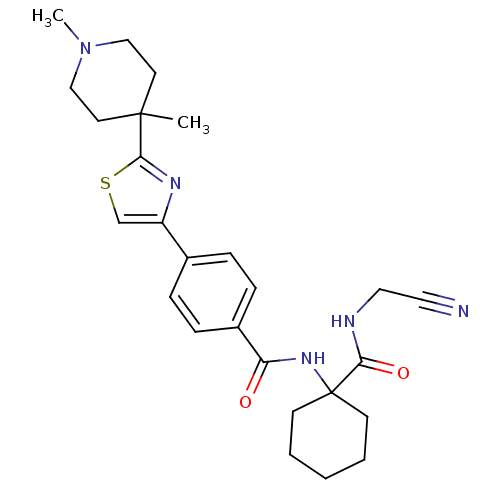
(CHEMBL200166)Show SMILES CN1CCC(C)(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H33N5O2S/c1-25(12-16-31(2)17-13-25)24-29-21(18-34-24)19-6-8-20(9-7-19)22(32)30-26(10-4-3-5-11-26)23(33)28-15-14-27/h6-9,18H,3-5,10-13,15-17H2,1-2H3,(H,28,33)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283708

(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C26H38ClN3O6/c1-36-24(32)14-15-28-26(34)22(16-19-6-3-2-4-7-19)29-25(33)20(17-23(31)30-35)9-5-8-18-10-12-21(27)13-11-18/h10-13,19-20,22,35H,2-9,14-17H2,1H3,(H,28,34)(H,29,33)(H,30,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101525

((R)-6-(4-Chloro-phenyl)-3-{(S)-2-cyclohexyl-1-[2-(...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)[C@H](CC2CCCCC2)NC(=O)[C@H](CCCc2ccc(Cl)cc2)CC(O)=O)cc1 Show InChI InChI=1S/C30H40ClN3O6S/c31-25-13-9-21(10-14-25)7-4-8-24(20-28(35)36)29(37)34-27(19-23-5-2-1-3-6-23)30(38)33-18-17-22-11-15-26(16-12-22)41(32,39)40/h9-16,23-24,27H,1-8,17-20H2,(H,33,38)(H,34,37)(H,35,36)(H2,32,39,40)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101515

((R)-3-(((S)-3-cyclohexyl-1-oxo-1-(phenethylamino)p...)Show SMILES Cc1ccc(CCC[C@H](CC(O)=O)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H42N2O4/c1-23-15-17-25(18-16-23)13-8-14-27(22-29(34)35)30(36)33-28(21-26-11-6-3-7-12-26)31(37)32-20-19-24-9-4-2-5-10-24/h2,4-5,9-10,15-18,26-28H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101496
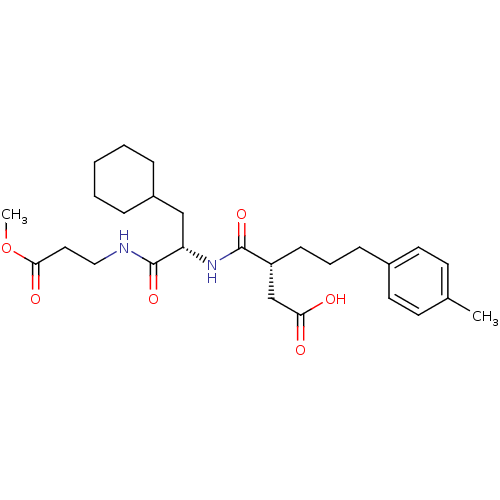
((R)-3-(((S)-3-cyclohexyl-1-(3-methoxy-3-oxopropyla...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(C)cc1)CC(O)=O Show InChI InChI=1S/C27H40N2O6/c1-19-11-13-20(14-12-19)9-6-10-22(18-24(30)31)26(33)29-23(17-21-7-4-3-5-8-21)27(34)28-16-15-25(32)35-2/h11-14,21-23H,3-10,15-18H2,1-2H3,(H,28,34)(H,29,33)(H,30,31)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283711

((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C29H43ClN4O6/c30-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-39)28(37)32-25(19-22-5-2-1-3-6-22)29(38)31-14-13-27(36)34-15-17-40-18-16-34/h9-12,22-23,25,39H,1-8,13-20H2,(H,31,38)(H,32,37)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410595
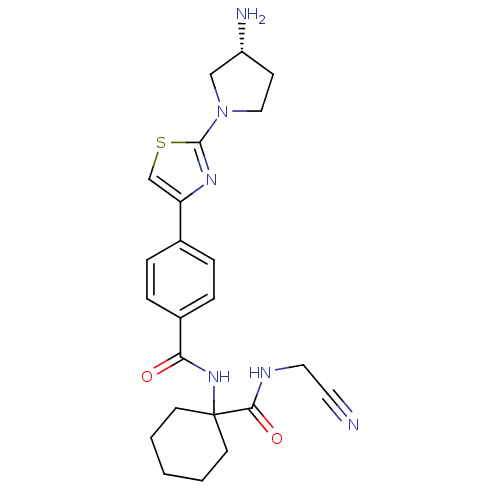
(CHEMBL200507)Show SMILES N[C@@H]1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H28N6O2S/c24-11-12-26-21(31)23(9-2-1-3-10-23)28-20(30)17-6-4-16(5-7-17)19-15-32-22(27-19)29-13-8-18(25)14-29/h4-7,15,18H,1-3,8-10,12-14,25H2,(H,26,31)(H,28,30)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19855
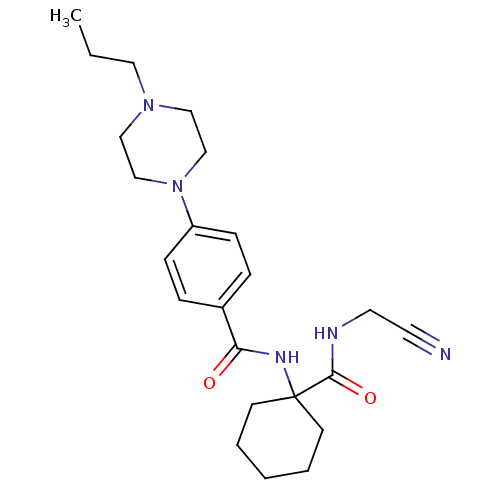
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187167
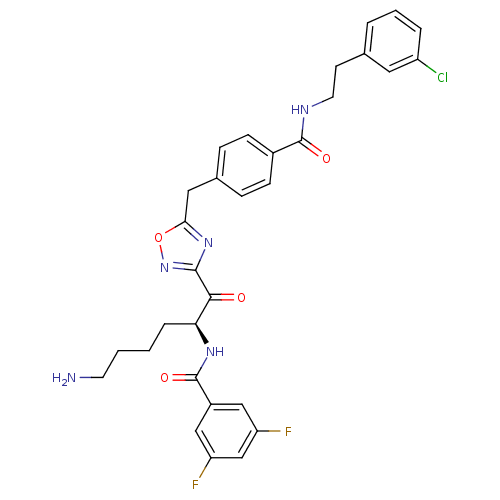
(CHEMBL211357 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1cc(F)cc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(14-23)11-13-36-30(41)21-9-7-20(8-10-21)15-27-38-29(39-43-27)28(40)26(6-1-2-12-35)37-31(42)22-16-24(33)18-25(34)17-22/h3-5,7-10,14,16-18,26H,1-2,6,11-13,15,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410572
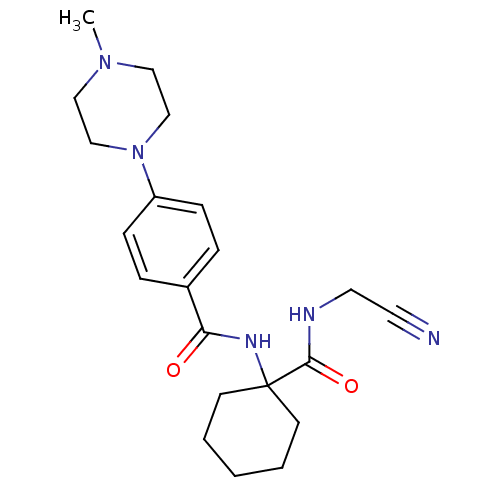
(CHEMBL440035)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C21H29N5O2/c1-25-13-15-26(16-14-25)18-7-5-17(6-8-18)19(27)24-21(9-3-2-4-10-21)20(28)23-12-11-22/h5-8H,2-4,9-10,12-16H2,1H3,(H,23,28)(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410592
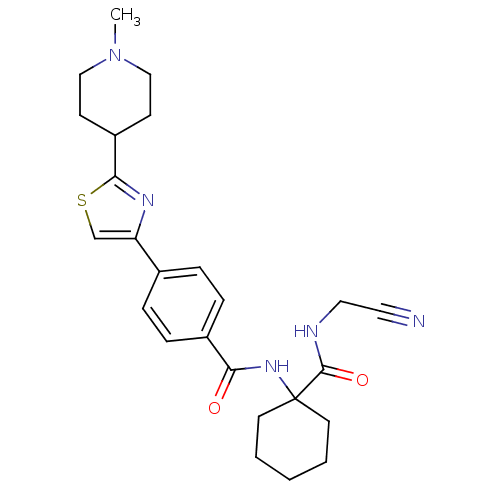
(CHEMBL200455)Show SMILES CN1CCC(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H31N5O2S/c1-30-15-9-20(10-16-30)23-28-21(17-33-23)18-5-7-19(8-6-18)22(31)29-25(11-3-2-4-12-25)24(32)27-14-13-26/h5-8,17,20H,2-4,9-12,14-16H2,1H3,(H,27,32)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data