 Found 798 hits with Last Name = 'leahy' and Initial = 'jw'
Found 798 hits with Last Name = 'leahy' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402421
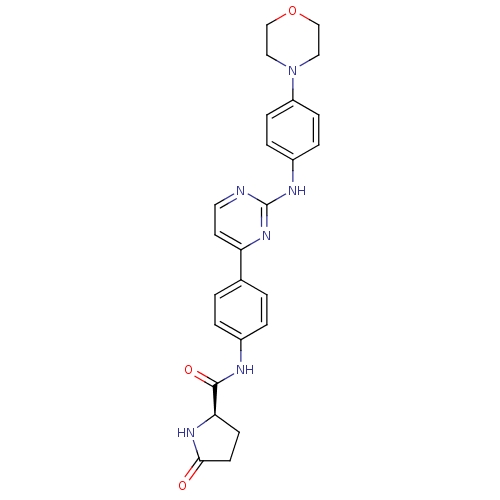
(CHEMBL2208035)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C25H26N6O3/c32-23-10-9-22(29-23)24(33)27-18-3-1-17(2-4-18)21-11-12-26-25(30-21)28-19-5-7-20(8-6-19)31-13-15-34-16-14-31/h1-8,11-12,22H,9-10,13-16H2,(H,27,33)(H,29,32)(H,26,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402413

(CHEMBL2208032)Show SMILES C[C@@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402409
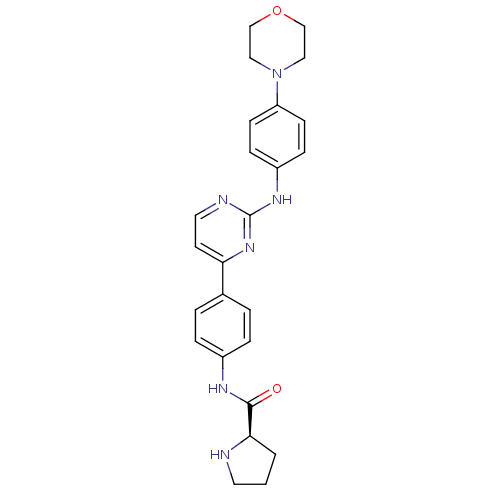
(CHEMBL2208034)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCCN1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(23-2-1-12-26-23)28-19-5-3-18(4-6-19)22-11-13-27-25(30-22)29-20-7-9-21(10-8-20)31-14-16-33-17-15-31/h3-11,13,23,26H,1-2,12,14-17H2,(H,28,32)(H,27,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402412
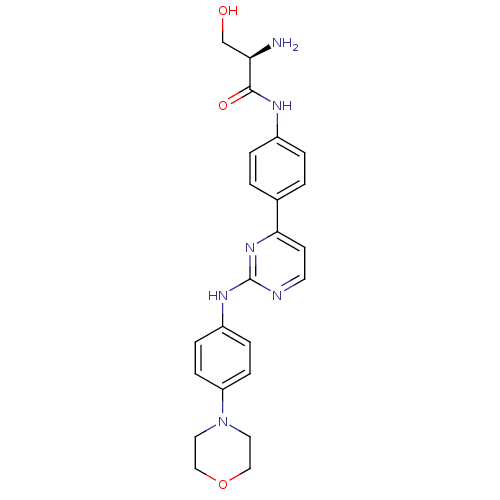
(CHEMBL2208033)Show SMILES N[C@H](CO)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O3/c24-20(15-30)22(31)26-17-3-1-16(2-4-17)21-9-10-25-23(28-21)27-18-5-7-19(8-6-18)29-11-13-32-14-12-29/h1-10,20,30H,11-15,24H2,(H,26,31)(H,25,27,28)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50310998

(CHEMBL1077458 | N-(4-(2-(4-morpholinophenylamino)p...)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-4-2-17(3-5-18)21-10-11-23-22(26-21)25-19-6-8-20(9-7-19)27-12-14-29-15-13-27/h2-11H,12-15H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402416

(CHEMBL2208025)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(c2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-7-5-17(6-8-18)21-9-10-23-22(26-21)25-19-3-2-4-20(15-19)27-11-13-29-14-12-27/h2-10,15H,11-14H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402424

(CHEMBL2208027)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1F Show InChI InChI=1S/C22H22FN5O2/c1-15(29)25-17-4-2-16(3-5-17)21-20(23)14-24-22(27-21)26-18-6-8-19(9-7-18)28-10-12-30-13-11-28/h2-9,14H,10-13H2,1H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380413

(CHEMBL2018573)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2nccn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(34(17-20)27(36)18-35-31-13-14-32-35)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393357

(CHEMBL2152148)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccc(F)cc1Cl Show InChI InChI=1S/C18H14Cl2FN5O3S/c1-23-18(27)16-17(22)24-8-14(25-16)9-2-4-11(19)15(6-9)30(28,29)26-13-5-3-10(21)7-12(13)20/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402415
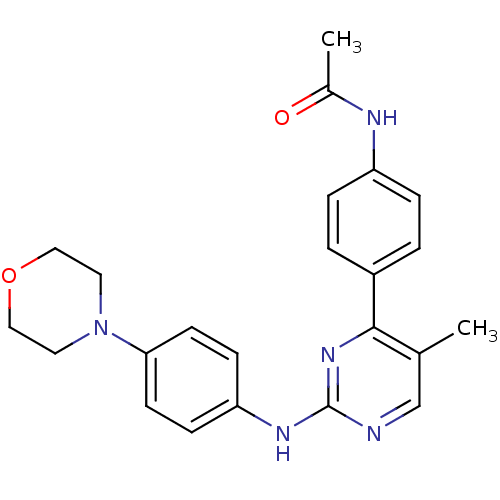
(CHEMBL2208028)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C Show InChI InChI=1S/C23H25N5O2/c1-16-15-24-23(27-22(16)18-3-5-19(6-4-18)25-17(2)29)26-20-7-9-21(10-8-20)28-11-13-30-14-12-28/h3-10,15H,11-14H2,1-2H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402411

(CHEMBL2207759)Show SMILES CN1CCC[C@@H]1C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-14-2-3-24(31)25(33)28-20-6-4-19(5-7-20)23-12-13-27-26(30-23)29-21-8-10-22(11-9-21)32-15-17-34-18-16-32/h4-13,24H,2-3,14-18H2,1H3,(H,28,33)(H,27,29,30)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402420

(CHEMBL2207758)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCNC1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(19-9-11-26-17-19)28-20-3-1-18(2-4-20)23-10-12-27-25(30-23)29-21-5-7-22(8-6-21)31-13-15-33-16-14-31/h1-8,10,12,19,26H,9,11,13-17H2,(H,28,32)(H,27,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380417
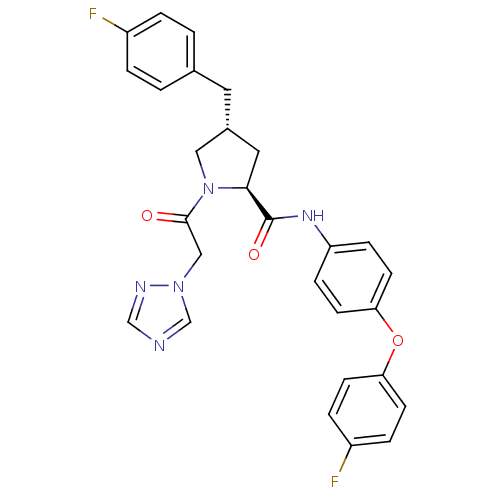
(CHEMBL2018485)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2cncn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)13-20-14-26(35(15-20)27(36)16-34-18-31-17-32-34)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-12,17-18,20,26H,13-16H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380418

(CHEMBL2018571)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2ccnn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(35(17-20)27(36)18-34-14-13-31-33-34)28(37)32-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,32,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393365
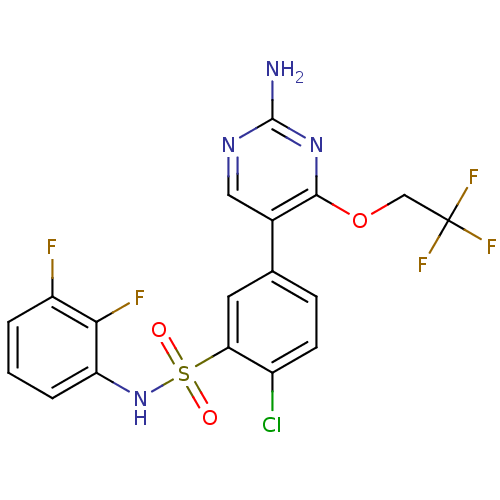
(CHEMBL2152257)Show SMILES Nc1ncc(-c2ccc(Cl)c(c2)S(=O)(=O)Nc2cccc(F)c2F)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C18H12ClF5N4O3S/c19-11-5-4-9(10-7-26-17(25)27-16(10)31-8-18(22,23)24)6-14(11)32(29,30)28-13-3-1-2-12(20)15(13)21/h1-7,28H,8H2,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402417
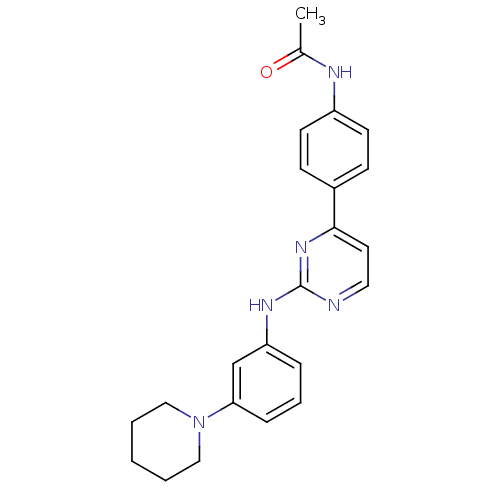
(CHEMBL2208024)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(c2)N2CCCCC2)n1 Show InChI InChI=1S/C23H25N5O/c1-17(29)25-19-10-8-18(9-11-19)22-12-13-24-23(27-22)26-20-6-5-7-21(16-20)28-14-3-2-4-15-28/h5-13,16H,2-4,14-15H2,1H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380410

(CHEMBL2018484)Show SMILES Fc1ccc(Oc2ccc(NC(=O)[C@@H]3C[C@@H](Cc4ccccc4)CN3C(=O)Cn3cncn3)cc2)cc1 |r| Show InChI InChI=1S/C28H26FN5O3/c29-22-6-10-24(11-7-22)37-25-12-8-23(9-13-25)32-28(36)26-15-21(14-20-4-2-1-3-5-20)16-34(26)27(35)17-33-19-30-18-31-33/h1-13,18-19,21,26H,14-17H2,(H,32,36)/t21-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402427
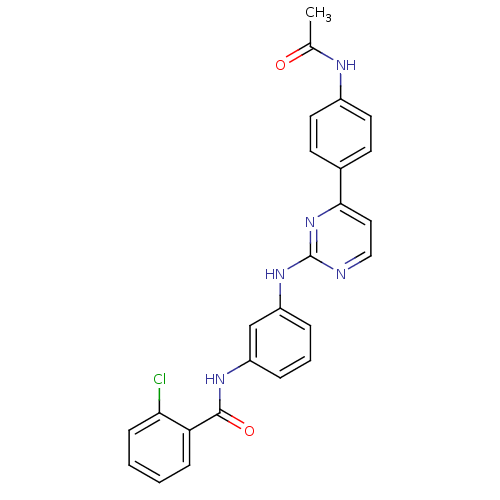
(CHEMBL2207766)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(NC(=O)c3ccccc3Cl)c2)n1 Show InChI InChI=1S/C25H20ClN5O2/c1-16(32)28-18-11-9-17(10-12-18)23-13-14-27-25(31-23)30-20-6-4-5-19(15-20)29-24(33)21-7-2-3-8-22(21)26/h2-15H,1H3,(H,28,32)(H,29,33)(H,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393359
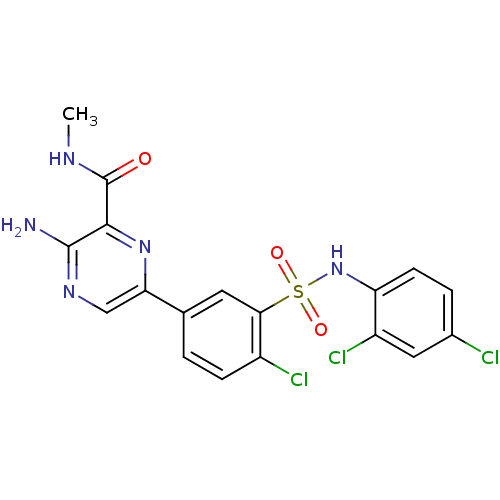
(CHEMBL2152150)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccc(Cl)cc1Cl Show InChI InChI=1S/C18H14Cl3N5O3S/c1-23-18(27)16-17(22)24-8-14(25-16)9-2-4-11(20)15(6-9)30(28,29)26-13-5-3-10(19)7-12(13)21/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402414
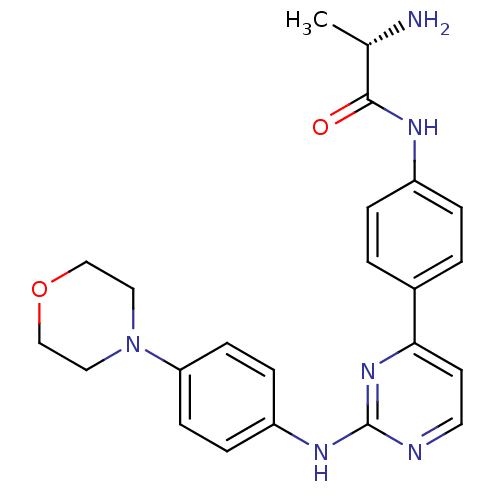
(CHEMBL2208031)Show SMILES C[C@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402410
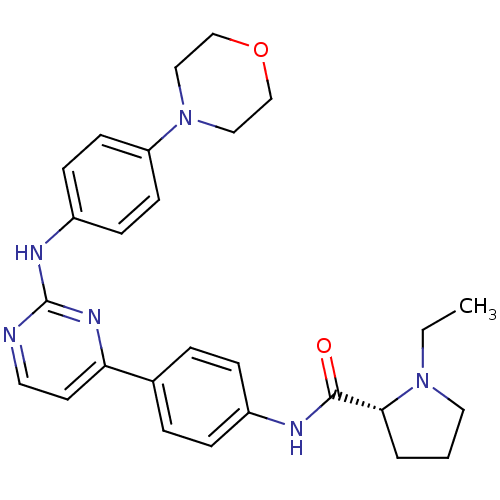
(CHEMBL2207760)Show SMILES CCN1CCC[C@@H]1C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C27H32N6O2/c1-2-32-15-3-4-25(32)26(34)29-21-7-5-20(6-8-21)24-13-14-28-27(31-24)30-22-9-11-23(12-10-22)33-16-18-35-19-17-33/h5-14,25H,2-4,15-19H2,1H3,(H,29,34)(H,28,30,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393333
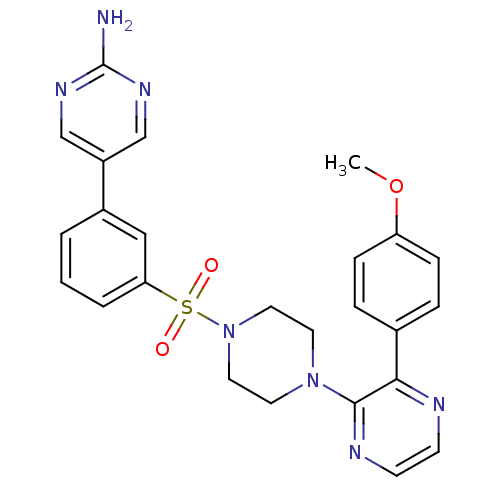
(CHEMBL2151050)Show SMILES COc1ccc(cc1)-c1nccnc1N1CCN(CC1)S(=O)(=O)c1cccc(c1)-c1cnc(N)nc1 Show InChI InChI=1S/C25H25N7O3S/c1-35-21-7-5-18(6-8-21)23-24(28-10-9-27-23)31-11-13-32(14-12-31)36(33,34)22-4-2-3-19(15-22)20-16-29-25(26)30-17-20/h2-10,15-17H,11-14H2,1H3,(H2,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393348
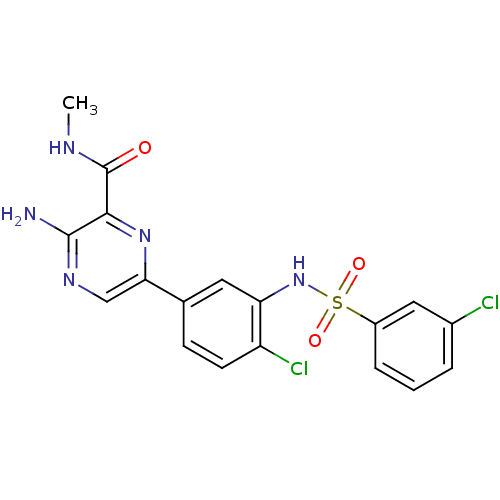
(CHEMBL2152139)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(NS(=O)(=O)c2cccc(Cl)c2)c1 Show InChI InChI=1S/C18H15Cl2N5O3S/c1-22-18(26)16-17(21)23-9-15(24-16)10-5-6-13(20)14(7-10)25-29(27,28)12-4-2-3-11(19)8-12/h2-9,25H,1H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402426

(CHEMBL2207767)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(NC(=O)c3ccccc3)c2)n1 Show InChI InChI=1S/C25H21N5O2/c1-17(31)27-20-12-10-18(11-13-20)23-14-15-26-25(30-23)29-22-9-5-8-21(16-22)28-24(32)19-6-3-2-4-7-19/h2-16H,1H3,(H,27,31)(H,28,32)(H,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402419
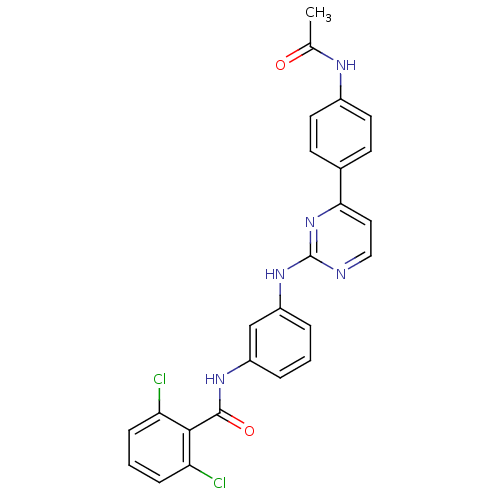
(CHEMBL2207764)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(NC(=O)c3c(Cl)cccc3Cl)c2)n1 Show InChI InChI=1S/C25H19Cl2N5O2/c1-15(33)29-17-10-8-16(9-11-17)22-12-13-28-25(32-22)31-19-5-2-4-18(14-19)30-24(34)23-20(26)6-3-7-21(23)27/h2-14H,1H3,(H,29,33)(H,30,34)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402418
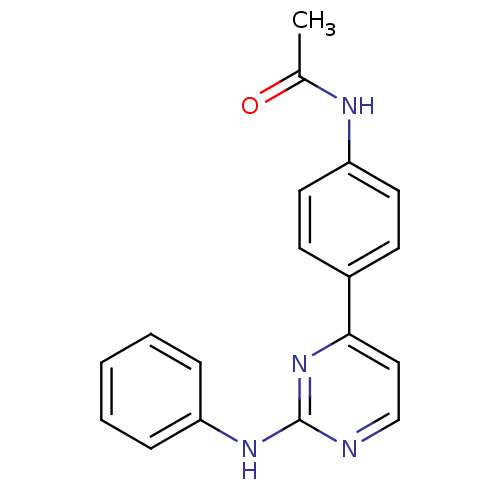
(CHEMBL2208023)Show InChI InChI=1S/C18H16N4O/c1-13(23)20-16-9-7-14(8-10-16)17-11-12-19-18(22-17)21-15-5-3-2-4-6-15/h2-12H,1H3,(H,20,23)(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380414

(CHEMBL2018467)Show SMILES Fc1ccc(Oc2ccc(NC(=O)[C@H](COCc3ccccc3)NC(=O)Cc3cnc[nH]3)cc2)cc1 |r| Show InChI InChI=1S/C27H25FN4O4/c28-20-6-10-23(11-7-20)36-24-12-8-21(9-13-24)31-27(34)25(17-35-16-19-4-2-1-3-5-19)32-26(33)14-22-15-29-18-30-22/h1-13,15,18,25H,14,16-17H2,(H,29,30)(H,31,34)(H,32,33)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380417

(CHEMBL2018485)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2cncn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)13-20-14-26(35(15-20)27(36)16-34-18-31-17-32-34)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-12,17-18,20,26H,13-16H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for... |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380437
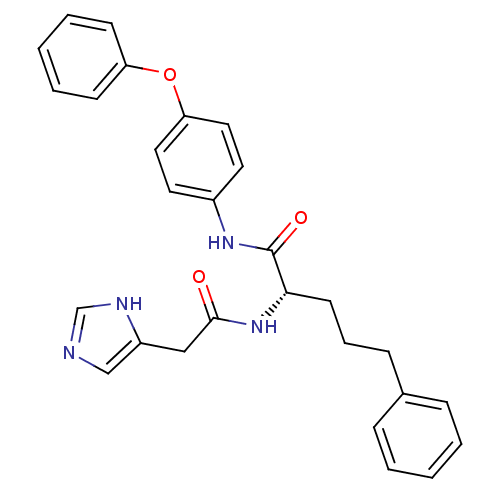
(CHEMBL2018474)Show SMILES O=C(Cc1cnc[nH]1)N[C@@H](CCCc1ccccc1)C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-27(18-23-19-29-20-30-23)32-26(13-7-10-21-8-3-1-4-9-21)28(34)31-22-14-16-25(17-15-22)35-24-11-5-2-6-12-24/h1-6,8-9,11-12,14-17,19-20,26H,7,10,13,18H2,(H,29,30)(H,31,34)(H,32,33)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393339
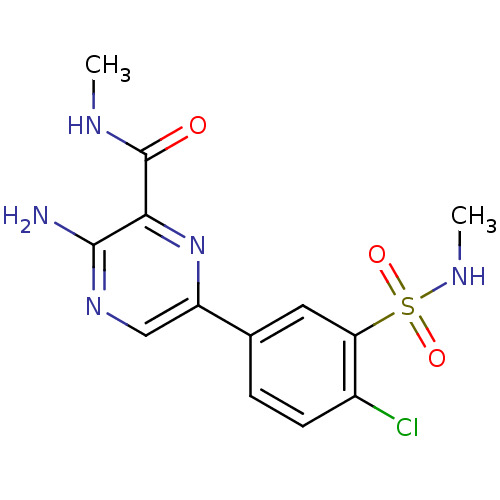
(CHEMBL2152131)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)NC Show InChI InChI=1S/C13H14ClN5O3S/c1-16-13(20)11-12(15)18-6-9(19-11)7-3-4-8(14)10(5-7)23(21,22)17-2/h3-6,17H,1-2H3,(H2,15,18)(H,16,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
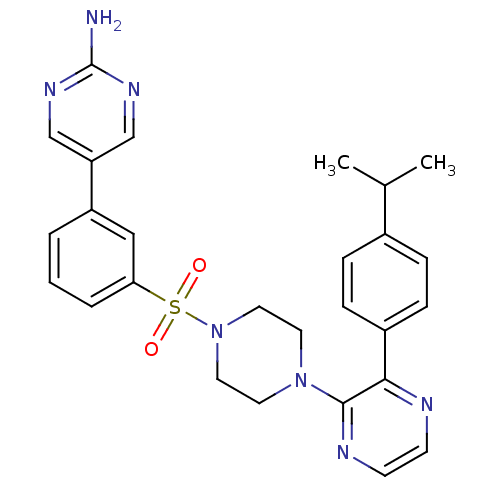
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393334
(CHEMBL2152126)Show SMILES CC(C)c1ccc(cc1)-c1nccnc1N1CCN(CC1)S(=O)(=O)c1cccc(c1)-c1cnc(N)nc1 Show InChI InChI=1S/C27H29N7O2S/c1-19(2)20-6-8-21(9-7-20)25-26(30-11-10-29-25)33-12-14-34(15-13-33)37(35,36)24-5-3-4-22(16-24)23-17-31-27(28)32-18-23/h3-11,16-19H,12-15H2,1-2H3,(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393353
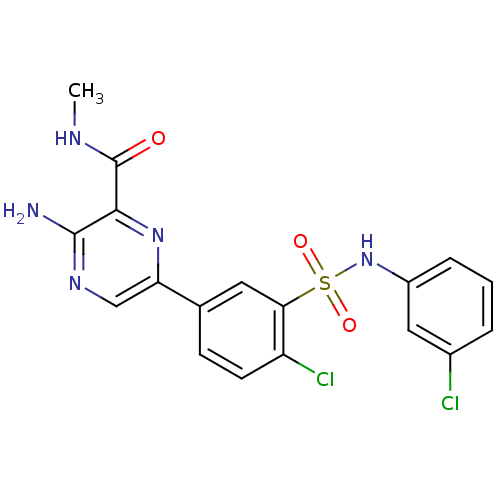
(CHEMBL2152144)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C18H15Cl2N5O3S/c1-22-18(26)16-17(21)23-9-14(24-16)10-5-6-13(20)15(7-10)29(27,28)25-12-4-2-3-11(19)8-12/h2-9,25H,1H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393335
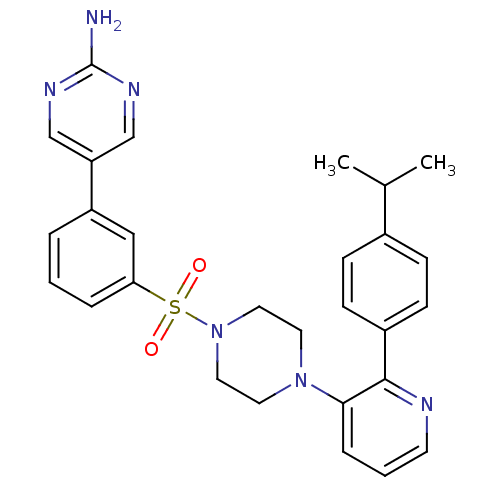
(CHEMBL2152127)Show SMILES CC(C)c1ccc(cc1)-c1ncccc1N1CCN(CC1)S(=O)(=O)c1cccc(c1)-c1cnc(N)nc1 Show InChI InChI=1S/C28H30N6O2S/c1-20(2)21-8-10-22(11-9-21)27-26(7-4-12-30-27)33-13-15-34(16-14-33)37(35,36)25-6-3-5-23(17-25)24-18-31-28(29)32-19-24/h3-12,17-20H,13-16H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393342

(CHEMBL2152252)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(F)c1F Show InChI InChI=1S/C18H14ClF2N5O3S/c1-23-18(27)16-17(22)24-8-13(25-16)9-5-6-10(19)14(7-9)30(28,29)26-12-4-2-3-11(20)15(12)21/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393360
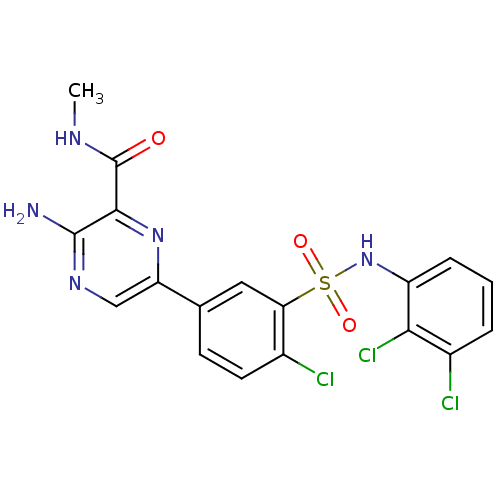
(CHEMBL2152250)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(Cl)c1Cl Show InChI InChI=1S/C18H14Cl3N5O3S/c1-23-18(27)16-17(22)24-8-13(25-16)9-5-6-10(19)14(7-9)30(28,29)26-12-4-2-3-11(20)15(12)21/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393364
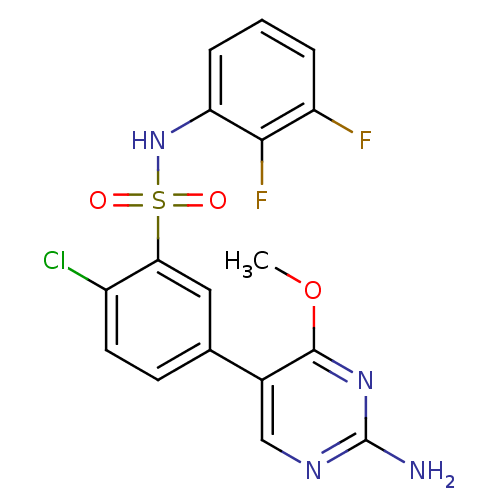
(CHEMBL2152256)Show SMILES COc1nc(N)ncc1-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(F)c1F Show InChI InChI=1S/C17H13ClF2N4O3S/c1-27-16-10(8-22-17(21)23-16)9-5-6-11(18)14(7-9)28(25,26)24-13-4-2-3-12(19)15(13)20/h2-8,24H,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393354
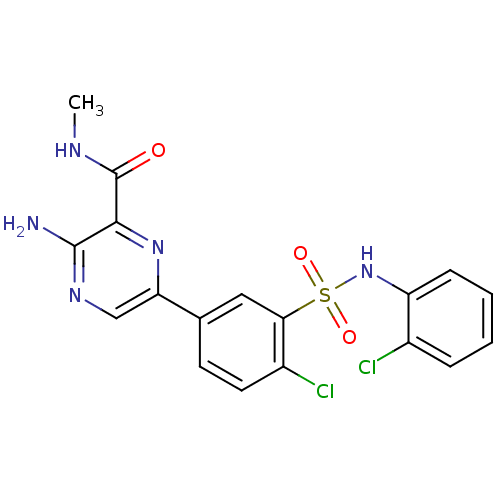
(CHEMBL2152145)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccccc1Cl Show InChI InChI=1S/C18H15Cl2N5O3S/c1-22-18(26)16-17(21)23-9-14(24-16)10-6-7-12(20)15(8-10)29(27,28)25-13-5-3-2-4-11(13)19/h2-9,25H,1H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393332
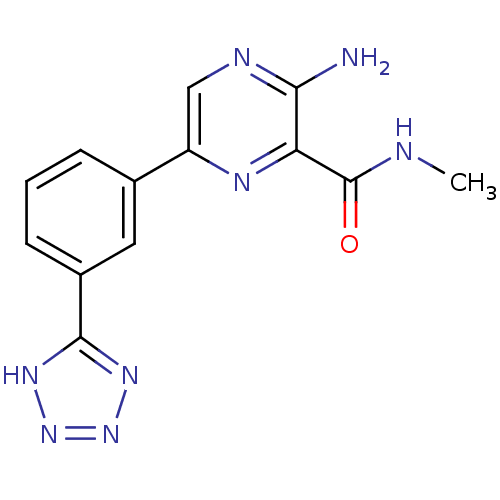
(CHEMBL2152125)Show InChI InChI=1S/C13H12N8O/c1-15-13(22)10-11(14)16-6-9(17-10)7-3-2-4-8(5-7)12-18-20-21-19-12/h2-6H,1H3,(H2,14,16)(H,15,22)(H,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393358
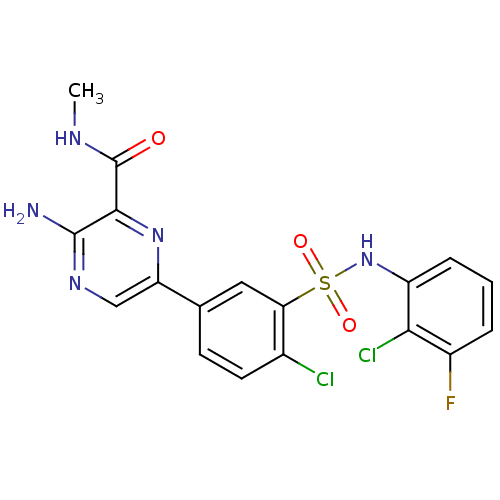
(CHEMBL2152149)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(F)c1Cl Show InChI InChI=1S/C18H14Cl2FN5O3S/c1-23-18(27)16-17(22)24-8-13(25-16)9-5-6-10(19)14(7-9)30(28,29)26-12-4-2-3-11(21)15(12)20/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380413

(CHEMBL2018573)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2nccn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(34(17-20)27(36)18-35-31-13-14-32-35)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for... |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393340

(CHEMBL2152132)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(N)(=O)=O Show InChI InChI=1S/C12H12ClN5O3S/c1-16-12(19)10-11(14)17-5-8(18-10)6-2-3-7(13)9(4-6)22(15,20)21/h2-5H,1H3,(H2,14,17)(H,16,19)(H2,15,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380418

(CHEMBL2018571)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2ccnn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(35(17-20)27(36)18-34-14-13-31-33-34)28(37)32-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,32,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for... |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393355
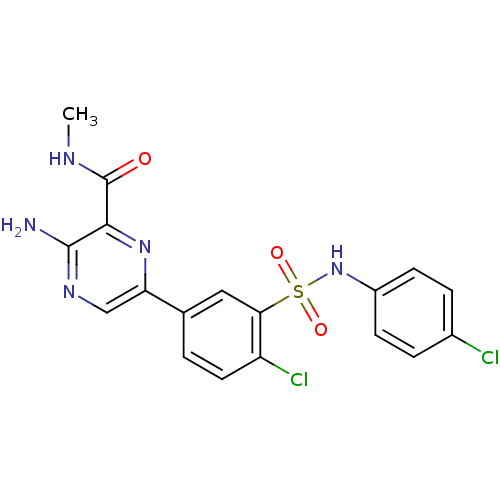
(CHEMBL2152146)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C18H15Cl2N5O3S/c1-22-18(26)16-17(21)23-9-14(24-16)10-2-7-13(20)15(8-10)29(27,28)25-12-5-3-11(19)4-6-12/h2-9,25H,1H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393351
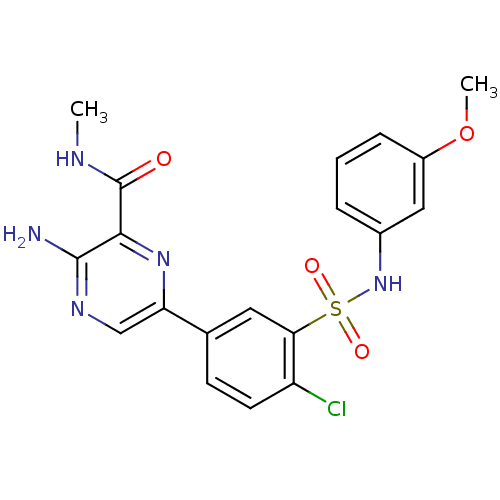
(CHEMBL2152142)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(OC)c1 Show InChI InChI=1S/C19H18ClN5O4S/c1-22-19(26)17-18(21)23-10-15(24-17)11-6-7-14(20)16(8-11)30(27,28)25-12-4-3-5-13(9-12)29-2/h3-10,25H,1-2H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380411
(CHEMBL2018465)Show SMILES Clc1ccc(Oc2ccc(NC(=O)[C@H](COCc3ccccc3)NC(=O)Cc3cnc[nH]3)cc2)cc1 |r| Show InChI InChI=1S/C27H25ClN4O4/c28-20-6-10-23(11-7-20)36-24-12-8-21(9-13-24)31-27(34)25(17-35-16-19-4-2-1-3-5-19)32-26(33)14-22-15-29-18-30-22/h1-13,15,18,25H,14,16-17H2,(H,29,30)(H,31,34)(H,32,33)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380425

(CHEMBL2018460)Show SMILES O=C(Cc1cnc[nH]1)N[C@@H](COCc1ccccc1)C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H26N4O4/c32-26(15-22-16-28-19-29-22)31-25(18-34-17-20-7-3-1-4-8-20)27(33)30-21-11-13-24(14-12-21)35-23-9-5-2-6-10-23/h1-14,16,19,25H,15,17-18H2,(H,28,29)(H,30,33)(H,31,32)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393329
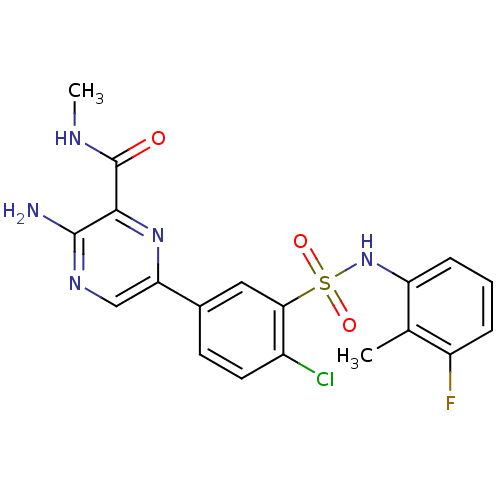
(CHEMBL2152253)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(F)c1C Show InChI InChI=1S/C19H17ClFN5O3S/c1-10-13(21)4-3-5-14(10)26-30(28,29)16-8-11(6-7-12(16)20)15-9-24-18(22)17(25-15)19(27)23-2/h3-9,26H,1-2H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50393333

(CHEMBL2151050)Show SMILES COc1ccc(cc1)-c1nccnc1N1CCN(CC1)S(=O)(=O)c1cccc(c1)-c1cnc(N)nc1 Show InChI InChI=1S/C25H25N7O3S/c1-35-21-7-5-18(6-8-21)23-24(28-10-9-27-23)31-11-13-32(14-12-31)36(33,34)22-4-2-3-19(15-22)20-16-29-25(26)30-17-20/h2-10,15-17H,11-14H2,1H3,(H2,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in sf9 cells coexpressing p85alpha assessed as amount of ATP consumed by luciferase-luciferin chemiluminescen... |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50393365

(CHEMBL2152257)Show SMILES Nc1ncc(-c2ccc(Cl)c(c2)S(=O)(=O)Nc2cccc(F)c2F)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C18H12ClF5N4O3S/c19-11-5-4-9(10-7-26-17(25)27-16(10)31-8-18(22,23)24)6-14(11)32(29,30)28-13-3-1-2-12(20)15(13)21/h1-7,28H,8H2,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kbeta expressed in sf9 cells coexpressing p85alpha assessed as amount of ATP consumed by luciferase-luciferin chemiluminescenc... |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393338
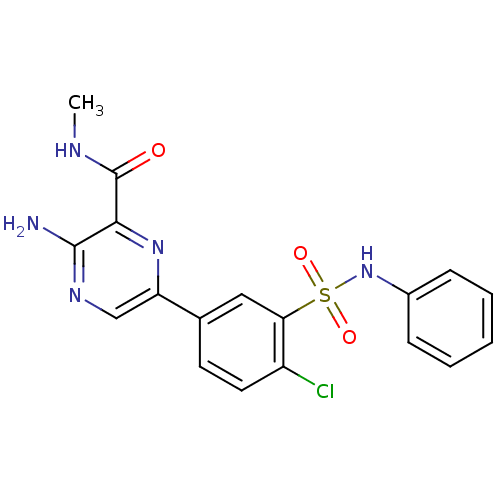
(CHEMBL2152130)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccccc1 Show InChI InChI=1S/C18H16ClN5O3S/c1-21-18(25)16-17(20)22-10-14(23-16)11-7-8-13(19)15(9-11)28(26,27)24-12-5-3-2-4-6-12/h2-10,24H,1H3,(H2,20,22)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data