 Found 325 hits with Last Name = 'brickmann' and Initial = 'k'
Found 325 hits with Last Name = 'brickmann' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436963
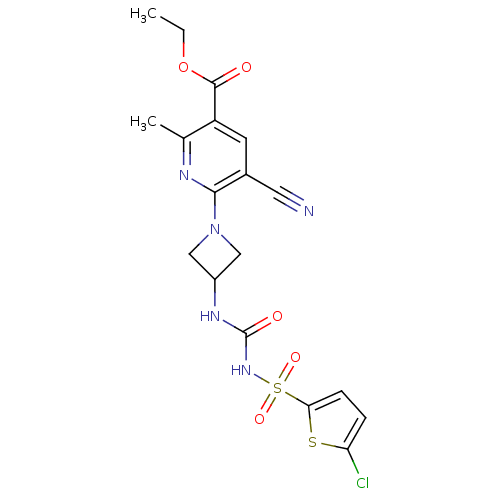
(CHEMBL2402255)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C18H18ClN5O5S2/c1-3-29-17(25)13-6-11(7-20)16(21-10(13)2)24-8-12(9-24)22-18(26)23-31(27,28)15-5-4-14(19)30-15/h4-6,12H,3,8-9H2,1-2H3,(H2,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436963

(CHEMBL2402255)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C18H18ClN5O5S2/c1-3-29-17(25)13-6-11(7-20)16(21-10(13)2)24-8-12(9-24)22-18(26)23-31(27,28)15-5-4-14(19)30-15/h4-6,12H,3,8-9H2,1-2H3,(H2,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436962
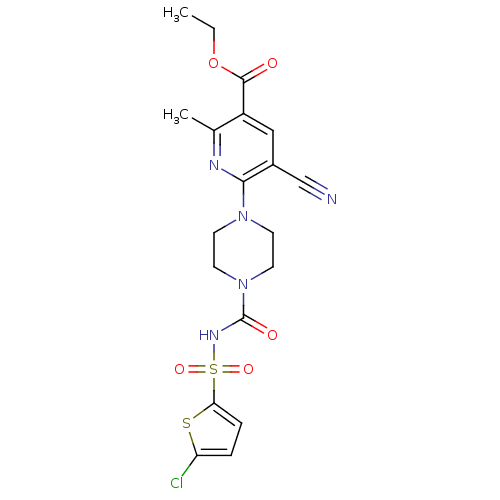
(CHEMBL2402264)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C19H20ClN5O5S2/c1-3-30-18(26)14-10-13(11-21)17(22-12(14)2)24-6-8-25(9-7-24)19(27)23-32(28,29)16-5-4-15(20)31-16/h4-5,10H,3,6-9H2,1-2H3,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436961
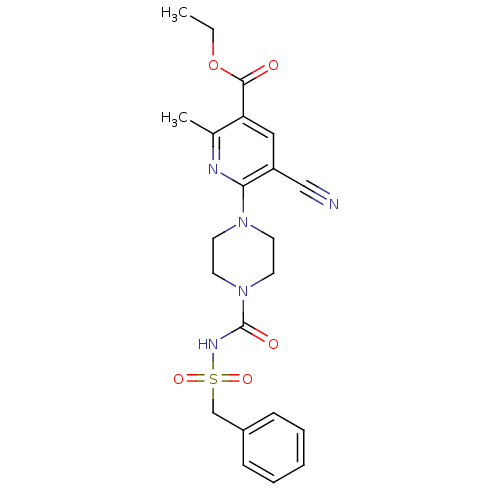
(CHEMBL2402266)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C22H25N5O5S/c1-3-32-21(28)19-13-18(14-23)20(24-16(19)2)26-9-11-27(12-10-26)22(29)25-33(30,31)15-17-7-5-4-6-8-17/h4-8,13H,3,9-12,15H2,1-2H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436963

(CHEMBL2402255)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C18H18ClN5O5S2/c1-3-29-17(25)13-6-11(7-20)16(21-10(13)2)24-8-12(9-24)22-18(26)23-31(27,28)15-5-4-14(19)30-15/h4-6,12H,3,8-9H2,1-2H3,(H2,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436960
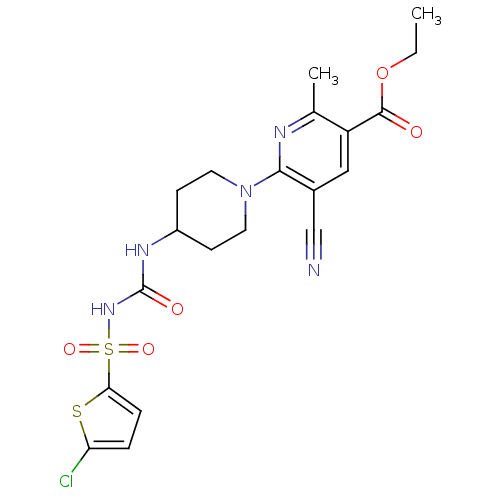
(CHEMBL2402260)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H22ClN5O5S2/c1-3-31-19(27)15-10-13(11-22)18(23-12(15)2)26-8-6-14(7-9-26)24-20(28)25-33(29,30)17-5-4-16(21)32-17/h4-5,10,14H,3,6-9H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436960

(CHEMBL2402260)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H22ClN5O5S2/c1-3-31-19(27)15-10-13(11-22)18(23-12(15)2)26-8-6-14(7-9-26)24-20(28)25-33(29,30)17-5-4-16(21)32-17/h4-5,10,14H,3,6-9H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436952
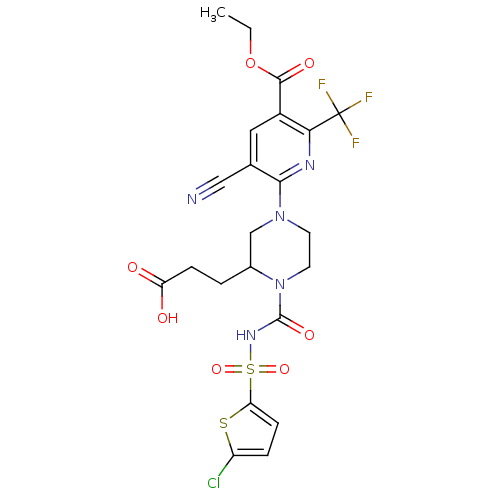
(CHEMBL2402142)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(C(CCC(O)=O)C1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C22H21ClF3N5O7S2/c1-2-38-20(34)14-9-12(10-27)19(28-18(14)22(24,25)26)30-7-8-31(13(11-30)3-5-16(32)33)21(35)29-40(36,37)17-6-4-15(23)39-17/h4,6,9,13H,2-3,5,7-8,11H2,1H3,(H,29,35)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436958
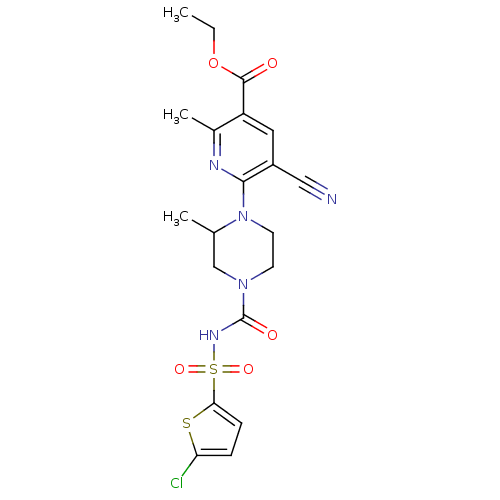
(CHEMBL2402144)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1C)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H22ClN5O5S2/c1-4-31-19(27)15-9-14(10-22)18(23-13(15)3)26-8-7-25(11-12(26)2)20(28)24-33(29,30)17-6-5-16(21)32-17/h5-6,9,12H,4,7-8,11H2,1-3H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436961

(CHEMBL2402266)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C22H25N5O5S/c1-3-32-21(28)19-13-18(14-23)20(24-16(19)2)26-9-11-27(12-10-26)22(29)25-33(30,31)15-17-7-5-4-6-8-17/h4-8,13H,3,9-12,15H2,1-2H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436962

(CHEMBL2402264)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C19H20ClN5O5S2/c1-3-30-18(26)14-10-13(11-21)17(22-12(14)2)24-6-8-25(9-7-24)19(27)23-32(28,29)16-5-4-15(20)31-16/h4-5,10H,3,6-9H2,1-2H3,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436958

(CHEMBL2402144)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1C)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H22ClN5O5S2/c1-4-31-19(27)15-9-14(10-22)18(23-13(15)3)26-8-7-25(11-12(26)2)20(28)24-33(29,30)17-6-5-16(21)32-17/h5-6,9,12H,4,7-8,11H2,1-3H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436957
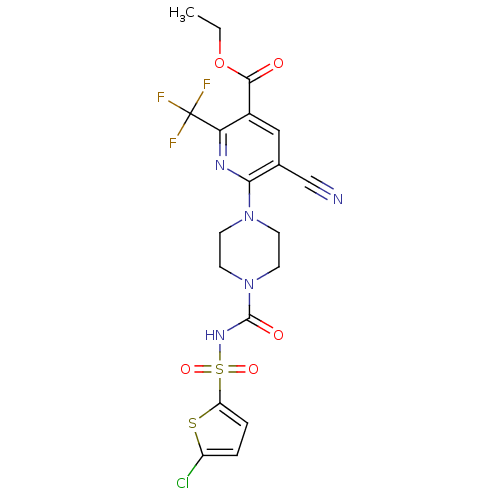
(CHEMBL2402244)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C19H17ClF3N5O5S2/c1-2-33-17(29)12-9-11(10-24)16(25-15(12)19(21,22)23)27-5-7-28(8-6-27)18(30)26-35(31,32)14-4-3-13(20)34-14/h3-4,9H,2,5-8H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436957

(CHEMBL2402244)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C19H17ClF3N5O5S2/c1-2-33-17(29)12-9-11(10-24)16(25-15(12)19(21,22)23)27-5-7-28(8-6-27)18(30)26-35(31,32)14-4-3-13(20)34-14/h3-4,9H,2,5-8H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436962

(CHEMBL2402264)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C19H20ClN5O5S2/c1-3-30-18(26)14-10-13(11-21)17(22-12(14)2)24-6-8-25(9-7-24)19(27)23-32(28,29)16-5-4-15(20)31-16/h4-5,10H,3,6-9H2,1-2H3,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50350728
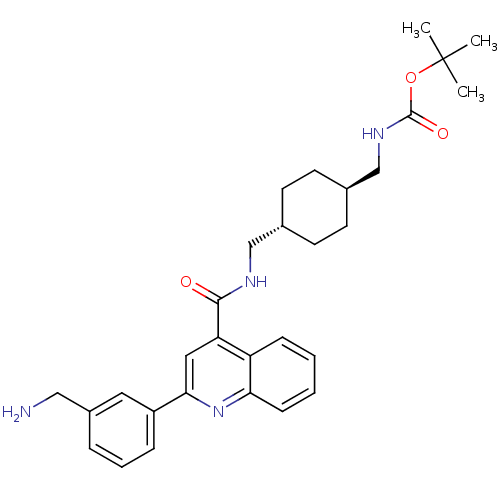
(CHEMBL1818291)Show SMILES CC(C)(C)OC(=O)NC[C@H]1CC[C@H](CNC(=O)c2cc(nc3ccccc23)-c2cccc(CN)c2)CC1 |r,wU:9.8,wD:12.12,(-10.3,5.18,;-8.97,5.96,;-8.98,7.5,;-10.31,6.73,;-7.64,5.19,;-6.3,5.97,;-6.31,7.51,;-4.97,5.2,;-4.96,3.66,;-3.63,2.89,;-2.3,3.67,;-.97,2.89,;-.97,1.35,;.37,.58,;.36,-.96,;1.7,-1.73,;3.03,-.96,;1.69,-3.27,;3.02,-4.03,;3.03,-5.57,;1.69,-6.35,;.36,-5.58,;-.97,-6.33,;-2.29,-5.57,;-2.29,-4.03,;-.96,-3.27,;.36,-4.04,;4.36,-6.34,;4.36,-7.88,;5.69,-8.65,;7.03,-7.87,;7.02,-6.33,;8.35,-5.55,;9.69,-6.31,;5.68,-5.56,;-2.3,.59,;-3.63,1.35,)| Show InChI InChI=1S/C30H38N4O3/c1-30(2,3)37-29(36)33-19-21-13-11-20(12-14-21)18-32-28(35)25-16-27(23-8-6-7-22(15-23)17-31)34-26-10-5-4-9-24(25)26/h4-10,15-16,20-21H,11-14,17-19,31H2,1-3H3,(H,32,35)(H,33,36)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... |
Bioorg Med Chem 19: 3039-53 (2011)
Article DOI: 10.1016/j.bmc.2011.04.014
BindingDB Entry DOI: 10.7270/Q2G44QN2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436961

(CHEMBL2402266)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C22H25N5O5S/c1-3-32-21(28)19-13-18(14-23)20(24-16(19)2)26-9-11-27(12-10-26)22(29)25-33(30,31)15-17-7-5-4-6-8-17/h4-8,13H,3,9-12,15H2,1-2H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436960

(CHEMBL2402260)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H22ClN5O5S2/c1-3-31-19(27)15-10-13(11-22)18(23-12(15)2)26-8-6-14(7-9-26)24-20(28)25-33(29,30)17-5-4-16(21)32-17/h4-5,10,14H,3,6-9H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436959
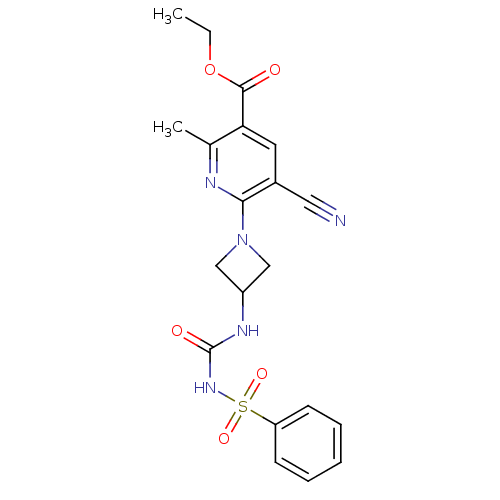
(CHEMBL2402257)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H21N5O5S/c1-3-30-19(26)17-9-14(10-21)18(22-13(17)2)25-11-15(12-25)23-20(27)24-31(28,29)16-7-5-4-6-8-16/h4-9,15H,3,11-12H2,1-2H3,(H2,23,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436958

(CHEMBL2402144)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1C)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H22ClN5O5S2/c1-4-31-19(27)15-9-14(10-22)18(23-13(15)3)26-8-7-25(11-12(26)2)20(28)24-33(29,30)17-6-5-16(21)32-17/h5-6,9,12H,4,7-8,11H2,1-3H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436934
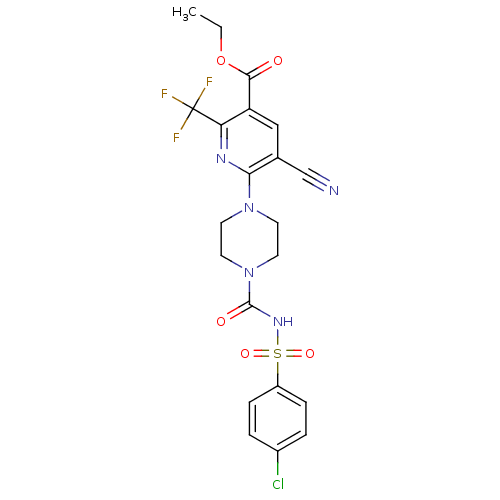
(CHEMBL2402249)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H19ClF3N5O5S/c1-2-35-19(31)16-11-13(12-26)18(27-17(16)21(23,24)25)29-7-9-30(10-8-29)20(32)28-36(33,34)15-5-3-14(22)4-6-15/h3-6,11H,2,7-10H2,1H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436953
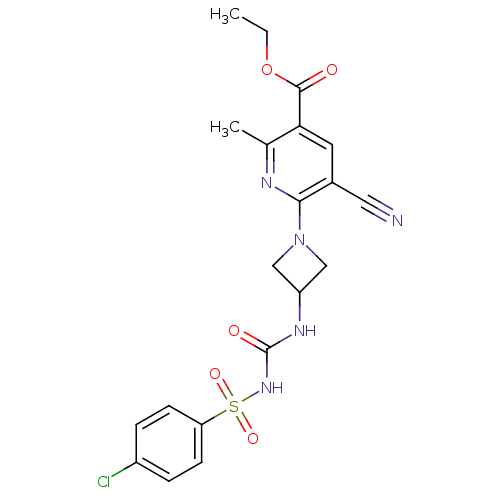
(CHEMBL2402258)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H20ClN5O5S/c1-3-31-19(27)17-8-13(9-22)18(23-12(17)2)26-10-15(11-26)24-20(28)25-32(29,30)16-6-4-14(21)5-7-16/h4-8,15H,3,10-11H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436957

(CHEMBL2402244)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C19H17ClF3N5O5S2/c1-2-33-17(29)12-9-11(10-24)16(25-15(12)19(21,22)23)27-5-7-28(8-6-27)18(30)26-35(31,32)14-4-3-13(20)34-14/h3-4,9H,2,5-8H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436949
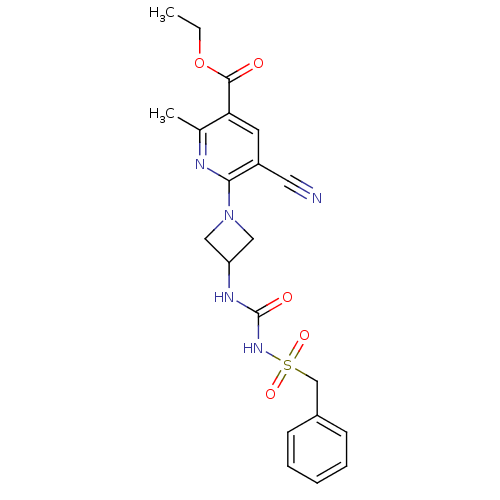
(CHEMBL2402259)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C21H23N5O5S/c1-3-31-20(27)18-9-16(10-22)19(23-14(18)2)26-11-17(12-26)24-21(28)25-32(29,30)13-15-7-5-4-6-8-15/h4-9,17H,3,11-13H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436949

(CHEMBL2402259)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C21H23N5O5S/c1-3-31-20(27)18-9-16(10-22)19(23-14(18)2)26-11-17(12-26)24-21(28)25-32(29,30)13-15-7-5-4-6-8-15/h4-9,17H,3,11-13H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50345312
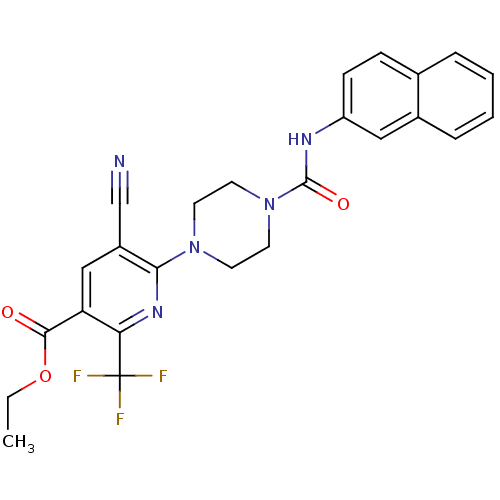
(CHEMBL1784198 | Ethyl 5-cyano-6-{4-[(2-naphthylami...)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C25H22F3N5O3/c1-2-36-23(34)20-14-18(15-29)22(31-21(20)25(26,27)28)32-9-11-33(12-10-32)24(35)30-19-8-7-16-5-3-4-6-17(16)13-19/h3-8,13-14H,2,9-12H2,1H3,(H,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 21: 2877-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.088
BindingDB Entry DOI: 10.7270/Q2TX3FQ9 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436953

(CHEMBL2402258)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H20ClN5O5S/c1-3-31-19(27)17-8-13(9-22)18(23-12(17)2)26-10-15(11-26)24-20(28)25-32(29,30)16-6-4-14(21)5-7-16/h4-8,15H,3,10-11H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436956
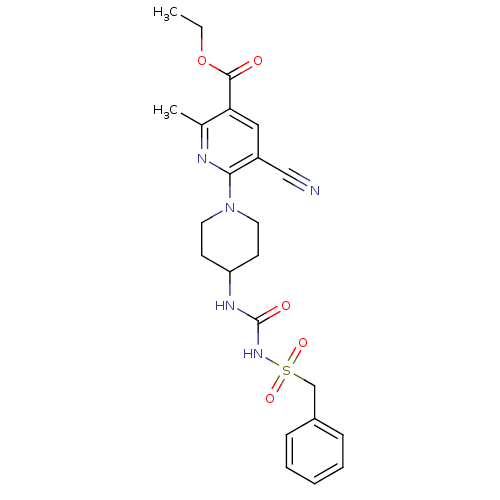
(CHEMBL2402263)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)NC(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H27N5O5S/c1-3-33-22(29)20-13-18(14-24)21(25-16(20)2)28-11-9-19(10-12-28)26-23(30)27-34(31,32)15-17-7-5-4-6-8-17/h4-8,13,19H,3,9-12,15H2,1-2H3,(H2,26,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50350748
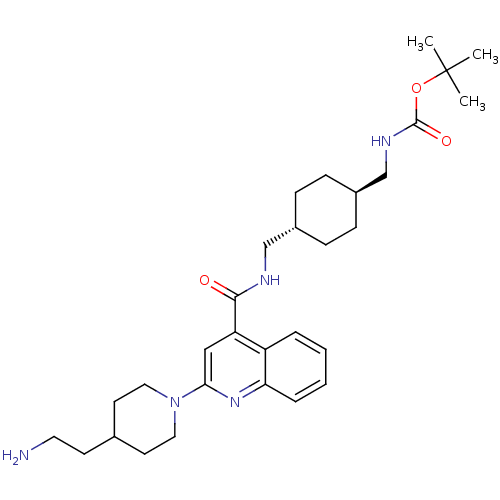
(CHEMBL1818297)Show SMILES CC(C)(C)OC(=O)NC[C@H]1CC[C@H](CNC(=O)c2cc(nc3ccccc23)N2CCC(CCN)CC2)CC1 |r,wU:9.8,wD:12.12,(42.97,-14.06,;44.3,-13.29,;44.3,-11.75,;42.96,-12.52,;45.64,-14.05,;46.97,-13.28,;46.97,-11.74,;48.31,-14.05,;48.31,-15.59,;49.65,-16.35,;50.98,-15.58,;52.31,-16.35,;52.31,-17.89,;53.64,-18.67,;53.64,-20.21,;54.97,-20.98,;56.31,-20.21,;54.97,-22.52,;56.3,-23.28,;56.31,-24.82,;54.97,-25.6,;53.63,-24.82,;52.31,-25.58,;50.99,-24.82,;50.99,-23.28,;52.32,-22.52,;53.64,-23.29,;57.63,-25.58,;57.64,-27.13,;58.97,-27.89,;60.3,-27.12,;61.64,-27.89,;61.65,-29.43,;62.98,-30.19,;60.3,-25.57,;58.96,-24.81,;50.98,-18.66,;49.65,-17.89,)| Show InChI InChI=1S/C30H45N5O3/c1-30(2,3)38-29(37)33-20-23-10-8-22(9-11-23)19-32-28(36)25-18-27(34-26-7-5-4-6-24(25)26)35-16-13-21(12-15-31)14-17-35/h4-7,18,21-23H,8-17,19-20,31H2,1-3H3,(H,32,36)(H,33,37)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... |
Bioorg Med Chem 19: 3039-53 (2011)
Article DOI: 10.1016/j.bmc.2011.04.014
BindingDB Entry DOI: 10.7270/Q2G44QN2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436954
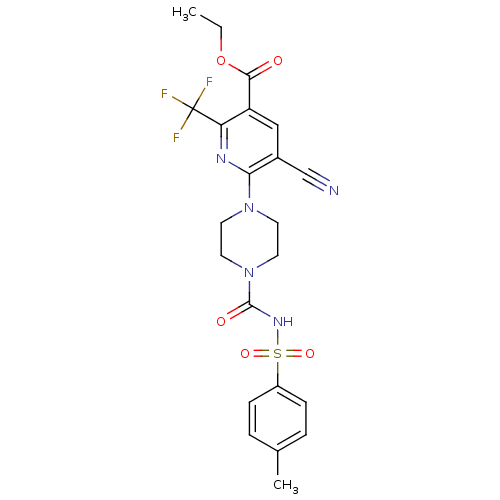
(CHEMBL2402248)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C22H22F3N5O5S/c1-3-35-20(31)17-12-15(13-26)19(27-18(17)22(23,24)25)29-8-10-30(11-9-29)21(32)28-36(33,34)16-6-4-14(2)5-7-16/h4-7,12H,3,8-11H2,1-2H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436955
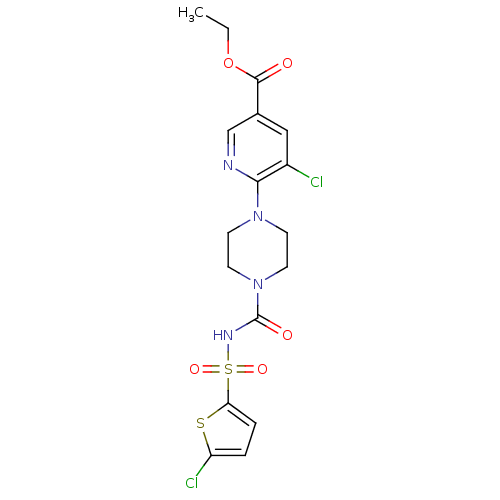
(CHEMBL2402251)Show SMILES CCOC(=O)c1cnc(N2CCN(CC2)C(=O)NS(=O)(=O)c2ccc(Cl)s2)c(Cl)c1 Show InChI InChI=1S/C17H18Cl2N4O5S2/c1-2-28-16(24)11-9-12(18)15(20-10-11)22-5-7-23(8-6-22)17(25)21-30(26,27)14-4-3-13(19)29-14/h3-4,9-10H,2,5-8H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436953

(CHEMBL2402258)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H20ClN5O5S/c1-3-31-19(27)17-8-13(9-22)18(23-12(17)2)26-10-15(11-26)24-20(28)25-32(29,30)16-6-4-14(21)5-7-16/h4-8,15H,3,10-11H2,1-2H3,(H2,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436952

(CHEMBL2402142)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(C(CCC(O)=O)C1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C22H21ClF3N5O7S2/c1-2-38-20(34)14-9-12(10-27)19(28-18(14)22(24,25)26)30-7-8-31(13(11-30)3-5-16(32)33)21(35)29-40(36,37)17-6-4-15(23)39-17/h4,6,9,13H,2-3,5,7-8,11H2,1H3,(H,29,35)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436951
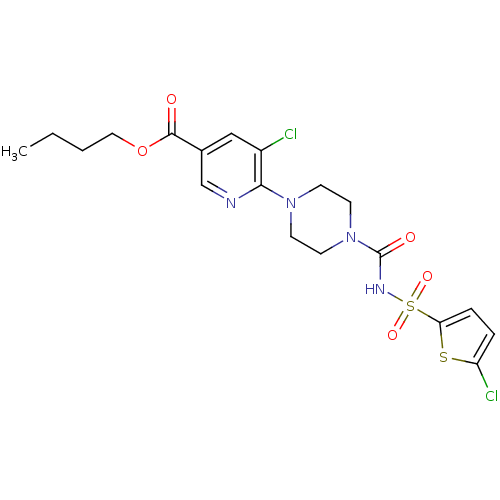
(CHEMBL2402149)Show SMILES CCCCOC(=O)c1cnc(N2CCN(CC2)C(=O)NS(=O)(=O)c2ccc(Cl)s2)c(Cl)c1 Show InChI InChI=1S/C19H22Cl2N4O5S2/c1-2-3-10-30-18(26)13-11-14(20)17(22-12-13)24-6-8-25(9-7-24)19(27)23-32(28,29)16-5-4-15(21)31-16/h4-5,11-12H,2-3,6-10H2,1H3,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436934

(CHEMBL2402249)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H19ClF3N5O5S/c1-2-35-19(31)16-11-13(12-26)18(27-17(16)21(23,24)25)29-7-9-30(10-8-29)20(32)28-36(33,34)15-5-3-14(22)4-6-15/h3-6,11H,2,7-10H2,1H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50350750

(CHEMBL1818300)Show SMILES CC(C)(C)OC(=O)NC[C@H]1CC[C@H](CNC(=O)c2cc(nc3ccccc23)N2CCC(CCN3CC(O)C3)CC2)CC1 |r,wU:9.8,wD:12.12,(30.79,-33.91,;32.12,-33.14,;32.12,-31.6,;30.78,-32.37,;33.46,-33.9,;34.79,-33.13,;34.79,-31.59,;36.13,-33.9,;36.13,-35.44,;37.47,-36.2,;38.8,-35.43,;40.12,-36.2,;40.12,-37.74,;41.46,-38.51,;41.46,-40.05,;42.79,-40.83,;44.12,-40.06,;42.79,-42.37,;44.12,-43.13,;44.12,-44.66,;42.78,-45.44,;41.45,-44.67,;40.12,-45.43,;38.8,-44.66,;38.8,-43.12,;40.13,-42.36,;41.46,-43.13,;45.45,-45.43,;45.45,-46.97,;46.78,-47.74,;48.12,-46.97,;49.45,-47.73,;50.78,-46.96,;52.12,-47.72,;52.52,-49.2,;54.01,-48.8,;55.35,-49.57,;53.61,-47.31,;48.11,-45.42,;46.77,-44.66,;38.8,-38.51,;37.47,-37.74,)| Show InChI InChI=1S/C33H49N5O4/c1-33(2,3)42-32(41)35-20-25-10-8-24(9-11-25)19-34-31(40)28-18-30(36-29-7-5-4-6-27(28)29)38-16-13-23(14-17-38)12-15-37-21-26(39)22-37/h4-7,18,23-26,39H,8-17,19-22H2,1-3H3,(H,34,40)(H,35,41)/t24-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... |
Bioorg Med Chem 19: 3039-53 (2011)
Article DOI: 10.1016/j.bmc.2011.04.014
BindingDB Entry DOI: 10.7270/Q2G44QN2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50345326

(CHEMBL1784212 | Ethyl 5-chloro-6-{4-[(1-naphthylam...)Show SMILES CCOC(=O)c1cnc(N2CCN(CC2)C(=O)Nc2cccc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C23H23ClN4O3/c1-2-31-22(29)17-14-19(24)21(25-15-17)27-10-12-28(13-11-27)23(30)26-20-9-5-7-16-6-3-4-8-18(16)20/h3-9,14-15H,2,10-13H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 21: 2877-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.088
BindingDB Entry DOI: 10.7270/Q2TX3FQ9 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436955

(CHEMBL2402251)Show SMILES CCOC(=O)c1cnc(N2CCN(CC2)C(=O)NS(=O)(=O)c2ccc(Cl)s2)c(Cl)c1 Show InChI InChI=1S/C17H18Cl2N4O5S2/c1-2-28-16(24)11-9-12(18)15(20-10-11)22-5-7-23(8-6-22)17(25)21-30(26,27)14-4-3-13(19)29-14/h3-4,9-10H,2,5-8H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50350747
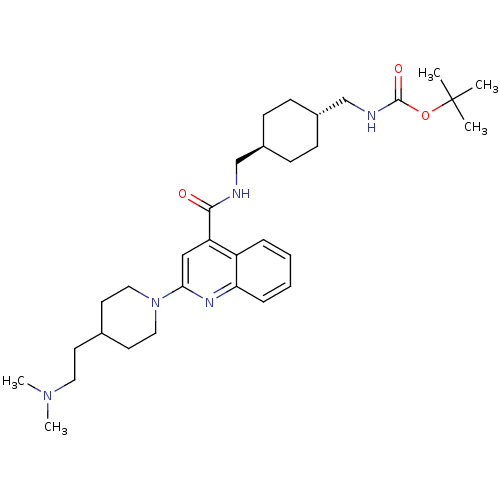
(CHEMBL1818296)Show SMILES CN(C)CCC1CCN(CC1)c1cc(C(=O)NC[C@H]2CC[C@H](CNC(=O)OC(C)(C)C)CC2)c2ccccc2n1 |r,wU:21.22,wD:18.18,(43.4,-32.84,;43.39,-31.3,;44.72,-30.52,;42.06,-30.53,;42.05,-28.99,;40.71,-28.23,;39.38,-29,;38.05,-28.23,;38.04,-26.69,;39.37,-25.92,;40.71,-26.68,;36.72,-25.92,;36.71,-24.39,;35.38,-23.63,;35.38,-22.09,;36.72,-21.32,;34.05,-21.31,;34.05,-19.77,;32.72,-19,;32.72,-17.46,;31.39,-16.68,;30.06,-17.46,;28.73,-16.7,;28.72,-15.15,;27.38,-14.39,;27.38,-12.85,;26.05,-15.16,;24.72,-14.4,;23.38,-15.17,;24.71,-12.86,;23.38,-13.62,;30.06,-19,;31.39,-19.77,;34.05,-24.39,;32.73,-23.62,;31.4,-24.38,;31.4,-25.92,;32.72,-26.69,;34.04,-25.93,;35.38,-26.7,)| Show InChI InChI=1S/C32H49N5O3/c1-32(2,3)40-31(39)34-22-25-12-10-24(11-13-25)21-33-30(38)27-20-29(35-28-9-7-6-8-26(27)28)37-18-15-23(16-19-37)14-17-36(4)5/h6-9,20,23-25H,10-19,21-22H2,1-5H3,(H,33,38)(H,34,39)/t24-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... |
Bioorg Med Chem 19: 3039-53 (2011)
Article DOI: 10.1016/j.bmc.2011.04.014
BindingDB Entry DOI: 10.7270/Q2G44QN2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50345326

(CHEMBL1784212 | Ethyl 5-chloro-6-{4-[(1-naphthylam...)Show SMILES CCOC(=O)c1cnc(N2CCN(CC2)C(=O)Nc2cccc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C23H23ClN4O3/c1-2-31-22(29)17-14-19(24)21(25-15-17)27-10-12-28(13-11-27)23(30)26-20-9-5-7-16-6-3-4-8-18(16)20/h3-9,14-15H,2,10-13H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from human recombinant P2Y12 receptor expressed in platelet cell membrane after 1 hr by scintillation counting |
Bioorg Med Chem Lett 21: 2877-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.088
BindingDB Entry DOI: 10.7270/Q2TX3FQ9 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436971

(CHEMBL2402151)Show SMILES CC(C)CCOC(=O)c1cnc(N2CCN(CC2)C(=O)NS(=O)(=O)c2ccc(Cl)s2)c(Cl)c1 Show InChI InChI=1S/C20H24Cl2N4O5S2/c1-13(2)5-10-31-19(27)14-11-15(21)18(23-12-14)25-6-8-26(9-7-25)20(28)24-33(29,30)17-4-3-16(22)32-17/h3-4,11-13H,5-10H2,1-2H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436952

(CHEMBL2402142)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(C(CCC(O)=O)C1)C(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C22H21ClF3N5O7S2/c1-2-38-20(34)14-9-12(10-27)19(28-18(14)22(24,25)26)30-7-8-31(13(11-30)3-5-16(32)33)21(35)29-40(36,37)17-6-4-15(23)39-17/h4,6,9,13H,2-3,5,7-8,11H2,1H3,(H,29,35)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50345265
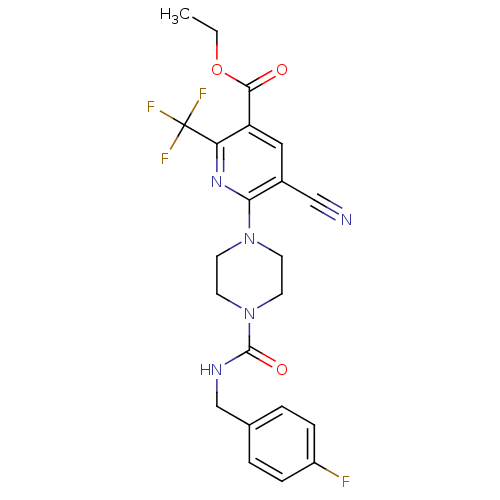
(CHEMBL1784220 | Ethyl 5-cyano-6-(4-{[(4-fluorobenz...)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NCc1ccc(F)cc1 Show InChI InChI=1S/C22H21F4N5O3/c1-2-34-20(32)17-11-15(12-27)19(29-18(17)22(24,25)26)30-7-9-31(10-8-30)21(33)28-13-14-3-5-16(23)6-4-14/h3-6,11H,2,7-10,13H2,1H3,(H,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50345265

(CHEMBL1784220 | Ethyl 5-cyano-6-(4-{[(4-fluorobenz...)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NCc1ccc(F)cc1 Show InChI InChI=1S/C22H21F4N5O3/c1-2-34-20(32)17-11-15(12-27)19(29-18(17)22(24,25)26)30-7-9-31(10-8-30)21(33)28-13-14-3-5-16(23)6-4-14/h3-6,11H,2,7-10,13H2,1H3,(H,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 21: 2877-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.088
BindingDB Entry DOI: 10.7270/Q2TX3FQ9 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436954

(CHEMBL2402248)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C22H22F3N5O5S/c1-3-35-20(31)17-12-15(13-26)19(27-18(17)22(23,24)25)29-8-10-30(11-9-29)21(32)28-36(33,34)16-6-4-14(2)5-7-16/h4-7,12H,3,8-11H2,1-2H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436970
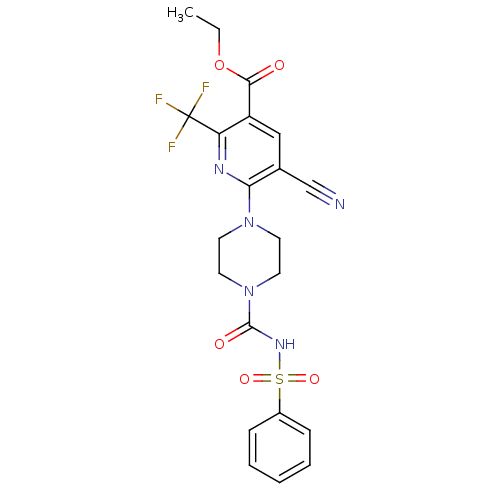
(CHEMBL2402245)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C(F)(F)F)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H20F3N5O5S/c1-2-34-19(30)16-12-14(13-25)18(26-17(16)21(22,23)24)28-8-10-29(11-9-28)20(31)27-35(32,33)15-6-4-3-5-7-15/h3-7,12H,2,8-11H2,1H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436972
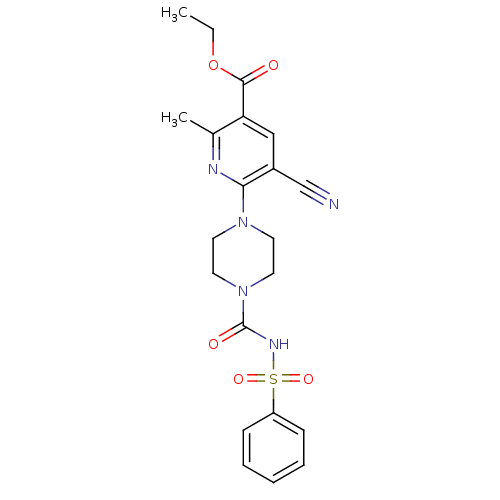
(CHEMBL2402265)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H23N5O5S/c1-3-31-20(27)18-13-16(14-22)19(23-15(18)2)25-9-11-26(12-10-25)21(28)24-32(29,30)17-7-5-4-6-8-17/h4-8,13H,3,9-12H2,1-2H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50350753

(CHEMBL1818303)Show SMILES CN(C)CCC1CCN(CC1)c1cc(C(=O)NC[C@H]2CC[C@H](CNC(=O)OCC(C)(C)C)CC2)c2ccccc2n1 |r,wU:21.22,wD:18.18,(58.23,-15.48,;58.23,-13.94,;59.57,-13.17,;56.9,-13.17,;56.9,-11.63,;55.57,-10.86,;54.23,-11.62,;52.9,-10.86,;52.91,-9.33,;54.23,-8.54,;55.57,-9.31,;51.57,-8.56,;51.56,-7.01,;50.23,-6.25,;50.22,-4.71,;51.55,-3.94,;48.88,-3.95,;48.87,-2.41,;47.54,-1.65,;47.53,-.1,;46.2,.66,;44.86,-.11,;43.53,.66,;43.53,2.2,;42.19,2.97,;42.19,4.51,;40.86,2.2,;39.52,2.96,;38.19,2.19,;36.86,2.96,;38.19,.65,;36.85,1.43,;44.87,-1.65,;46.2,-2.42,;48.9,-7.03,;47.57,-6.26,;46.24,-7.03,;46.24,-8.58,;47.57,-9.35,;48.9,-8.57,;50.24,-9.33,)| Show InChI InChI=1S/C33H51N5O3/c1-33(2,3)23-41-32(40)35-22-26-12-10-25(11-13-26)21-34-31(39)28-20-30(36-29-9-7-6-8-27(28)29)38-18-15-24(16-19-38)14-17-37(4)5/h6-9,20,24-26H,10-19,21-23H2,1-5H3,(H,34,39)(H,35,40)/t25-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... |
Bioorg Med Chem 19: 3039-53 (2011)
Article DOI: 10.1016/j.bmc.2011.04.014
BindingDB Entry DOI: 10.7270/Q2G44QN2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436972

(CHEMBL2402265)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCN(CC1)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H23N5O5S/c1-3-31-20(27)18-13-16(14-22)19(23-15(18)2)25-9-11-26(12-10-25)21(28)24-32(29,30)17-7-5-4-6-8-17/h4-8,13H,3,9-12H2,1-2H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436971

(CHEMBL2402151)Show SMILES CC(C)CCOC(=O)c1cnc(N2CCN(CC2)C(=O)NS(=O)(=O)c2ccc(Cl)s2)c(Cl)c1 Show InChI InChI=1S/C20H24Cl2N4O5S2/c1-13(2)5-10-31-19(27)14-11-15(21)18(23-12-14)25-6-8-26(9-7-25)20(28)24-33(29,30)17-4-3-16(22)32-17/h3-4,11-13H,5-10H2,1-2H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data