

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM254888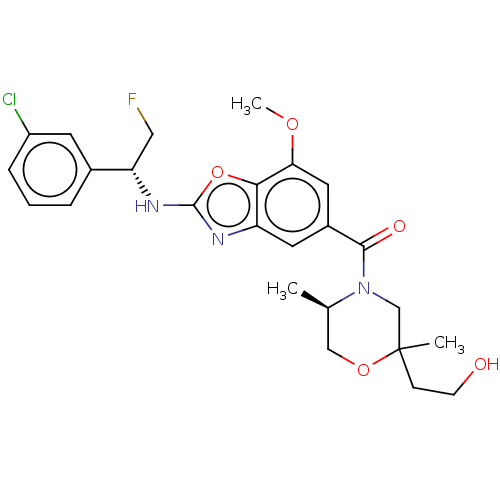 (US9493472, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254889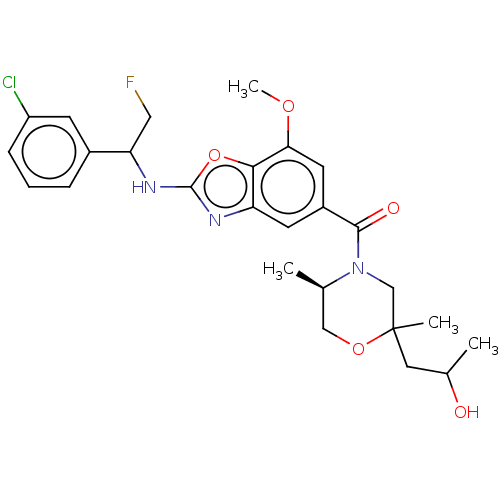 (US9493472, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254905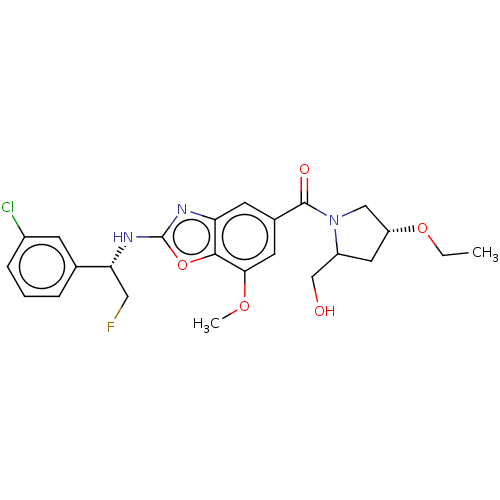 (US9493472, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254904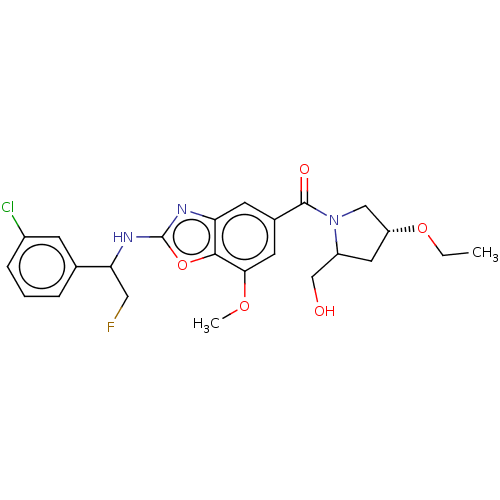 (US9493472, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254886 (US9493472, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254896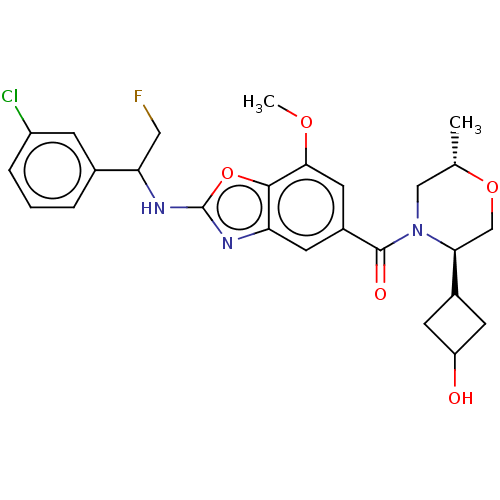 (US9493472, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254895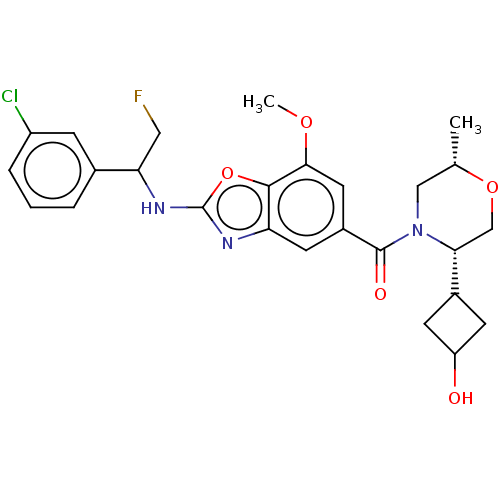 (US9493472, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254911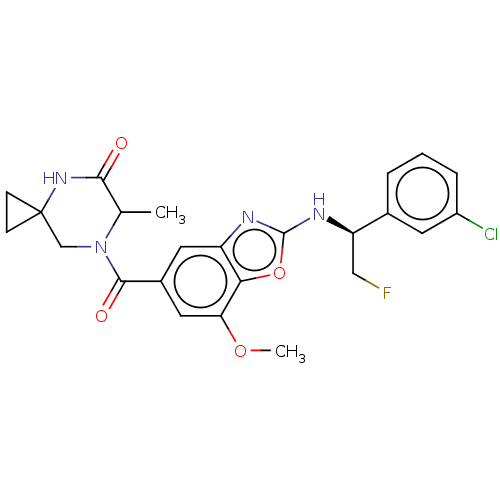 (US9493472, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254894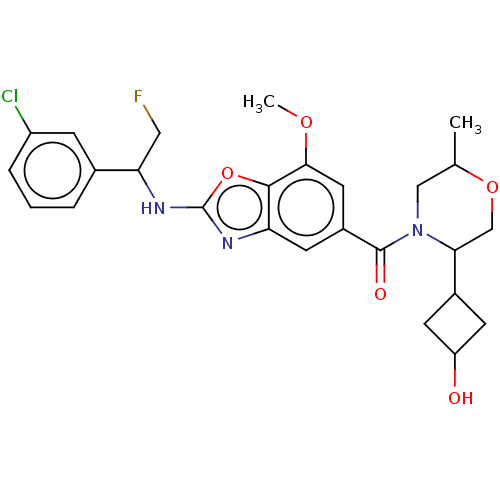 (US9493472, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907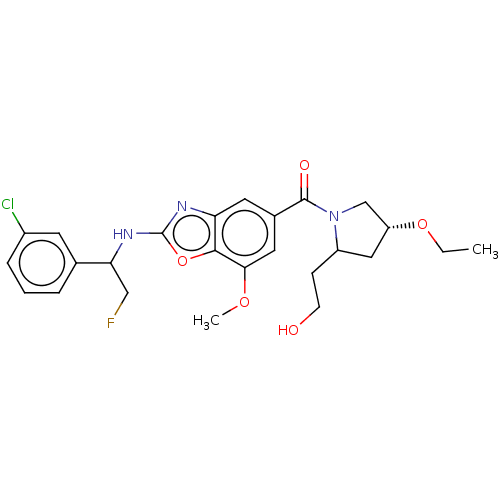 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254910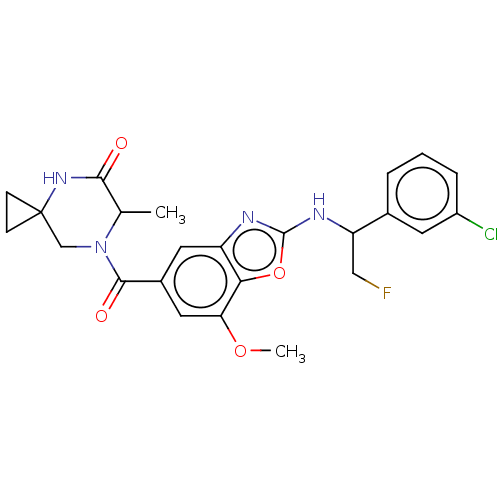 (US9493472, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254892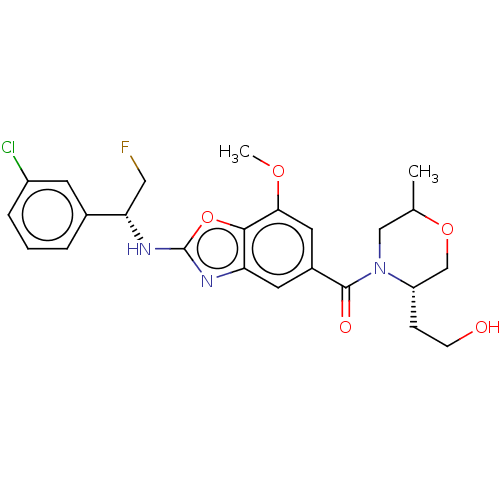 (US9493472, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254897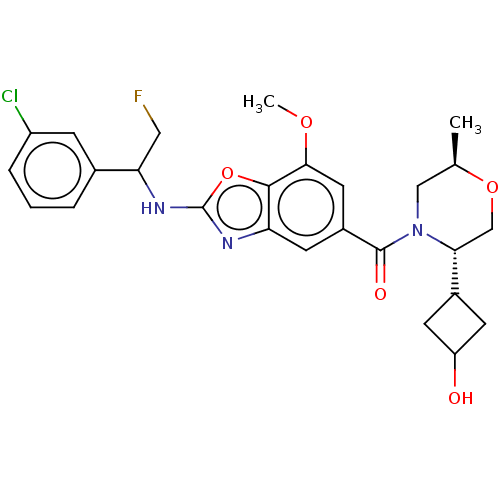 (US9493472, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [960-1390] (Homo sapiens (Human)) | BDBM240734 (US9422263, 5 | US9422263, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The N-terminally His6-tagged recombinant kinase domain of the human c-Met (amino acids 960-1390), expressed in insect cells (SF21) and purified by Ni... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM103461 (US8551989, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description Homogeneous time-resolved fluorescence assay using c-Met receptor tyrosine kinase. | US Patent US8551989 (2013) BindingDB Entry DOI: 10.7270/Q24F1PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50048422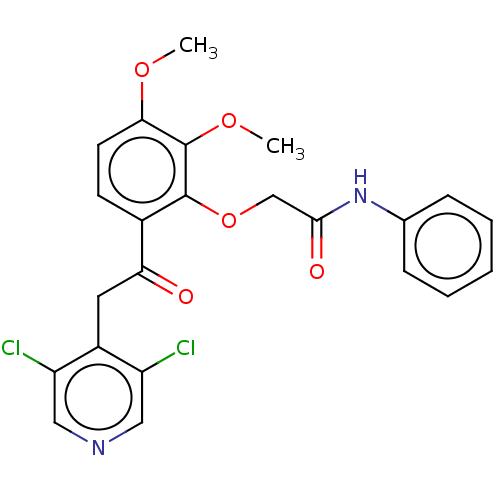 (CHEMBL3315084) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D after 1 hr by SPA in presence of radioactively labeled cAMP | J Med Chem 57: 5893-903 (2014) Article DOI: 10.1021/jm500378a BindingDB Entry DOI: 10.7270/Q2CV4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254882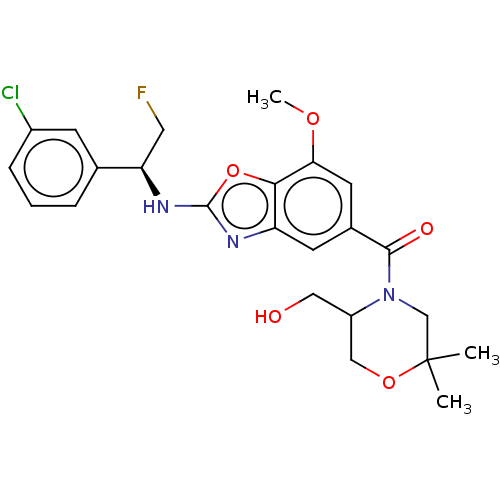 (US9493472, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254890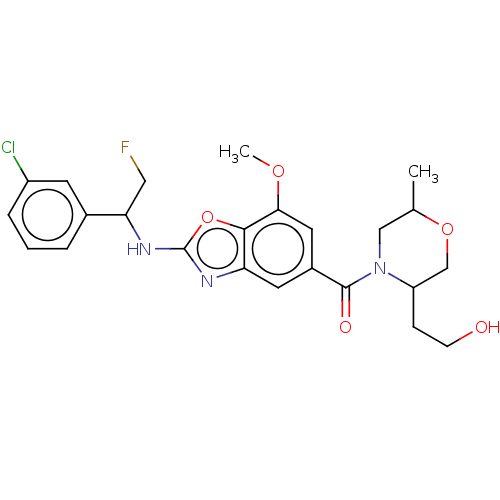 (US9493472, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254899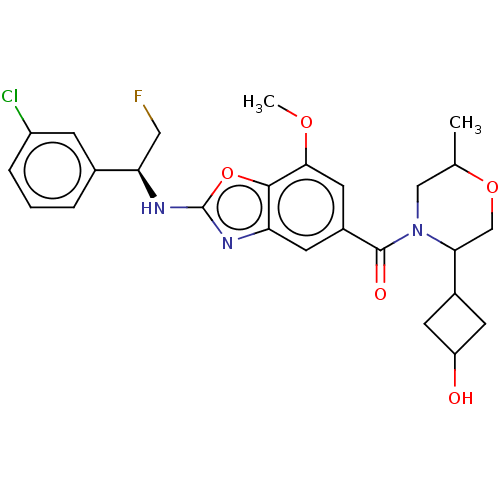 (US9493472, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254898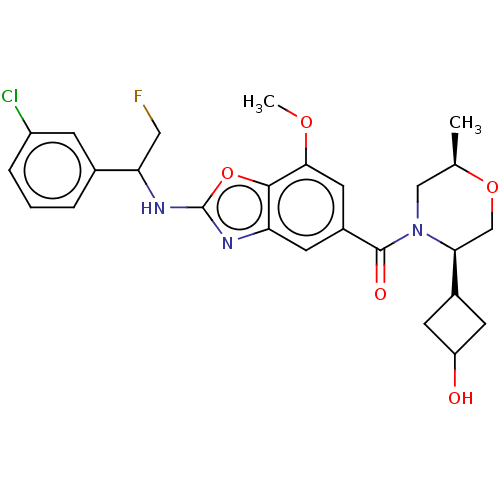 (US9493472, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254900 (US9493472, 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254881 (US9493472, 4 | US9493472, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254901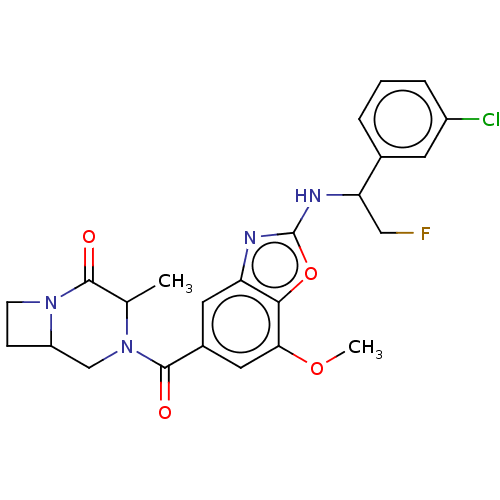 (US9493472, 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254891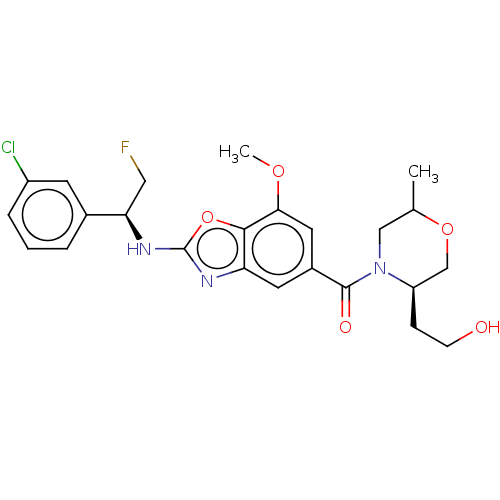 (US9493472, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [960-1390] (Homo sapiens (Human)) | BDBM62337 (Synthetic Example 4 (WO2008/071451)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The N-terminally His6-tagged recombinant kinase domain of the human c-Met (amino acids 960-1390), expressed in insect cells (SF21) and purified by Ni... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [960-1390] (Homo sapiens (Human)) | BDBM240732 (US9422263, 2 | US9422263, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The N-terminally His6-tagged recombinant kinase domain of the human c-Met (amino acids 960-1390), expressed in insect cells (SF21) and purified by Ni... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [960-1390] (Homo sapiens (Human)) | BDBM240732 (US9422263, 2 | US9422263, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The N-terminally His6-tagged recombinant kinase domain of the human c-Met (amino acids 960-1390), expressed in insect cells (SF21) and purified by Ni... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254906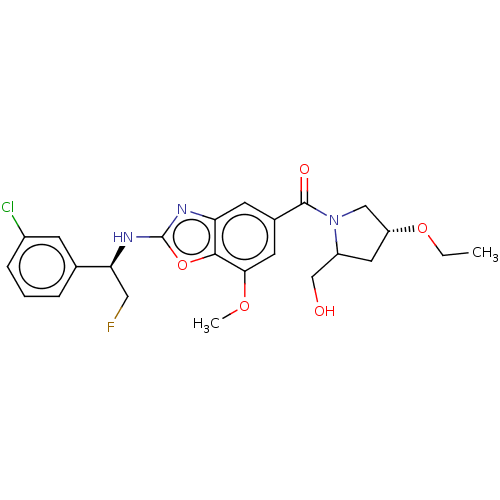 (US9493472, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254880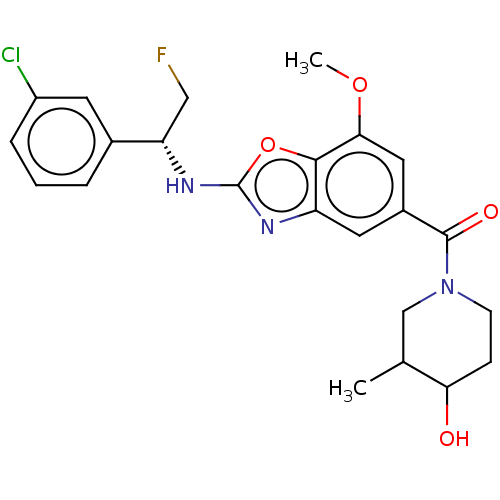 (US9493472, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [960-1390] (Homo sapiens (Human)) | BDBM240741 (US9422263, 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The N-terminally His6-tagged recombinant kinase domain of the human c-Met (amino acids 960-1390), expressed in insect cells (SF21) and purified by Ni... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [960-1390] (Homo sapiens (Human)) | BDBM240737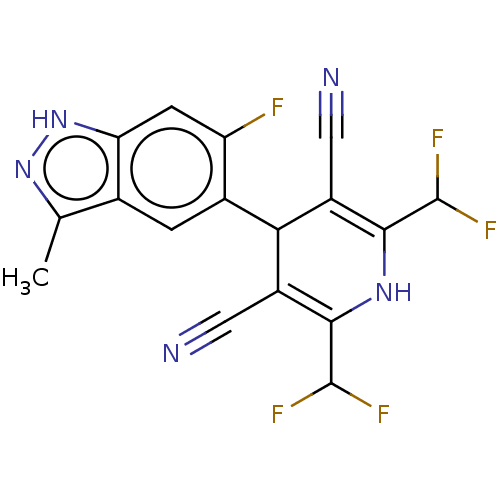 (US9422263, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The N-terminally His6-tagged recombinant kinase domain of the human c-Met (amino acids 960-1390), expressed in insect cells (SF21) and purified by Ni... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50048421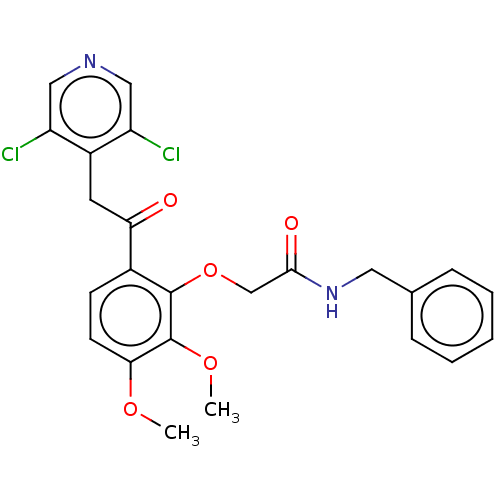 (CHEMBL3315083) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D after 1 hr by SPA in presence of radioactively labeled cAMP | J Med Chem 57: 5893-903 (2014) Article DOI: 10.1021/jm500378a BindingDB Entry DOI: 10.7270/Q2CV4KC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254878 (US9493472, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50438083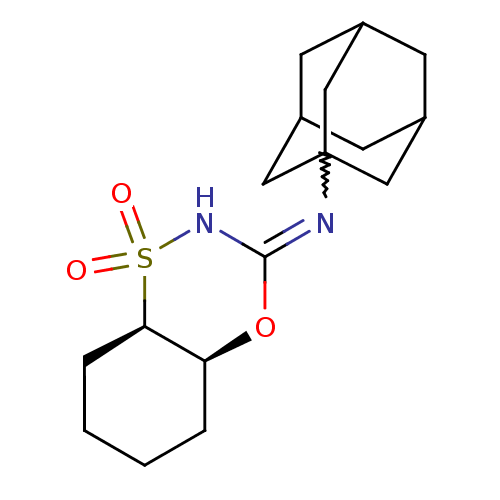 (CHEMBL2409755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 23: 4685-91 (2013) Article DOI: 10.1016/j.bmcl.2013.05.102 BindingDB Entry DOI: 10.7270/Q2BV7J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50438080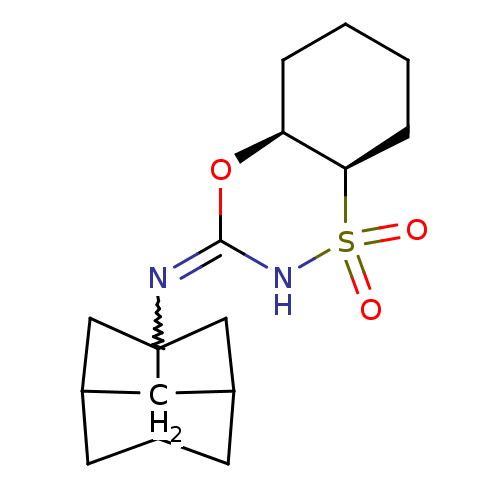 (CHEMBL2409607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 23: 4685-91 (2013) Article DOI: 10.1016/j.bmcl.2013.05.102 BindingDB Entry DOI: 10.7270/Q2BV7J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50438070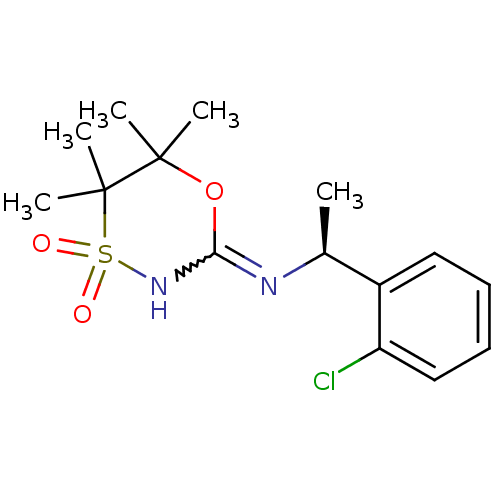 (CHEMBL2409724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 23: 4685-91 (2013) Article DOI: 10.1016/j.bmcl.2013.05.102 BindingDB Entry DOI: 10.7270/Q2BV7J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50438068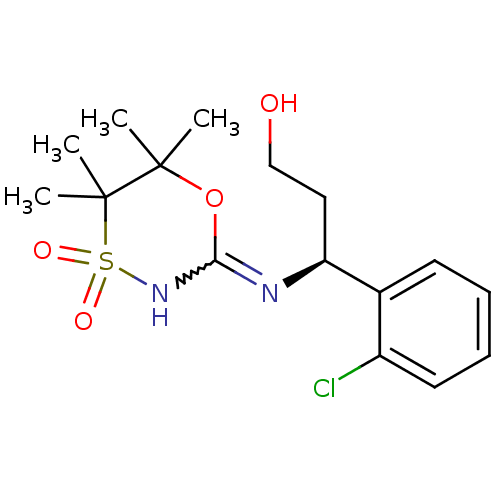 (CHEMBL2409726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 23: 4685-91 (2013) Article DOI: 10.1016/j.bmcl.2013.05.102 BindingDB Entry DOI: 10.7270/Q2BV7J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254903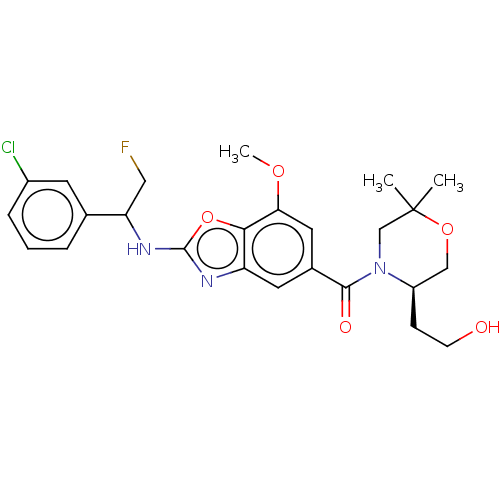 (US9493472, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254902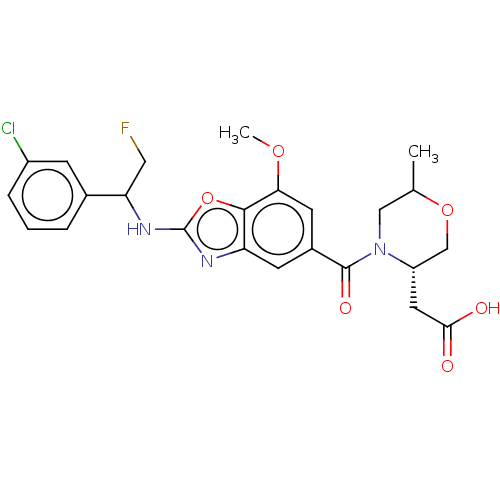 (US9493472, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM317598 (US9624199, 8 | {6-[(2,6-Difluorobenzyl)(methyl)ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To prepare cell membranes with human α1- and α2-adrenergic receptors, CHO cells stably overexpressing α1- and α2-adrenergic recep... | US Patent US9624199 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6VB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM398510 ((rac)-{2-[(1-Methoxybutan-2-yl)amino]pyrimidin-5-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Antagonism against the adrenoreceptor α1A was tested using a recombinant human α1A receptor CHO cell line which additionally also recombina... | Bioorg Med Chem Lett 19: 3344-7 (2009) BindingDB Entry DOI: 10.7270/Q2PN9803 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50048446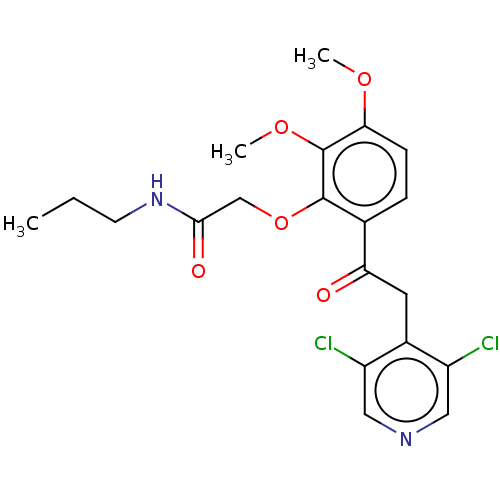 (CHEMBL3315088) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D after 1 hr by SPA in presence of radioactively labeled cAMP | J Med Chem 57: 5893-903 (2014) Article DOI: 10.1021/jm500378a BindingDB Entry DOI: 10.7270/Q2CV4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50048447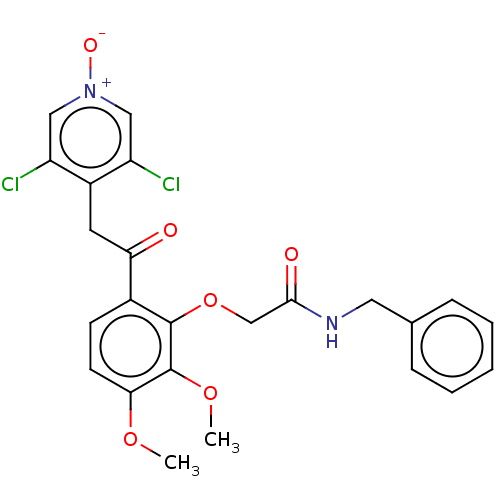 (CHEMBL3315089) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D after 1 hr by SPA in presence of radioactively labeled cAMP | J Med Chem 57: 5893-903 (2014) Article DOI: 10.1021/jm500378a BindingDB Entry DOI: 10.7270/Q2CV4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254884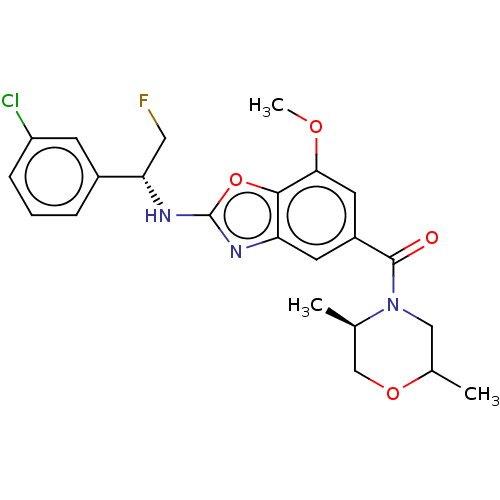 (US9493472, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM240732 (US9422263, 2 | US9422263, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.0 | 32 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant human c-Met protein (Invitrogen, Carlsbad, Calif., USA) is used. As substrate for the kinase reaction the peptide KKKSPGEYVNIEFG (JPT, Ge... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50438067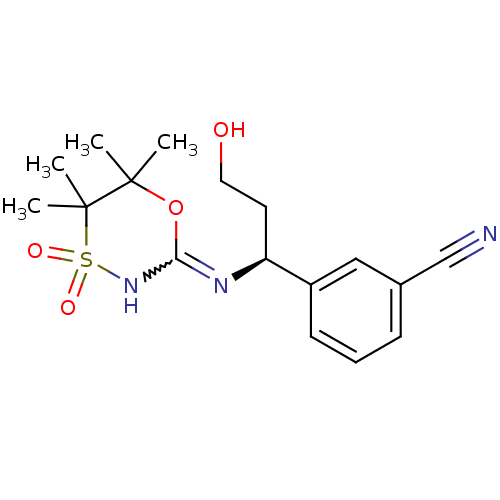 (CHEMBL2409727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 23: 4685-91 (2013) Article DOI: 10.1016/j.bmcl.2013.05.102 BindingDB Entry DOI: 10.7270/Q2BV7J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM240732 (US9422263, 2 | US9422263, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.0 | 32 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant human c-Met protein (Invitrogen, Carlsbad, Calif., USA) is used. As substrate for the kinase reaction the peptide KKKSPGEYVNIEFG (JPT, Ge... | US Patent US9422263 (2016) BindingDB Entry DOI: 10.7270/Q2JQ0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM103457 (US8551989, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.0 | 32 |
Bayer Intellectual Property GmbH US Patent | Assay Description Tyrosine kinase activity assay (NADH Read-Out) using c-Met receptor. | US Patent US8551989 (2013) BindingDB Entry DOI: 10.7270/Q24F1PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 260 total ) | Next | Last >> |