

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disintegrin and metalloproteinase domain-containing protein 17 (Sus scrofa (pig)) | BDBM50227856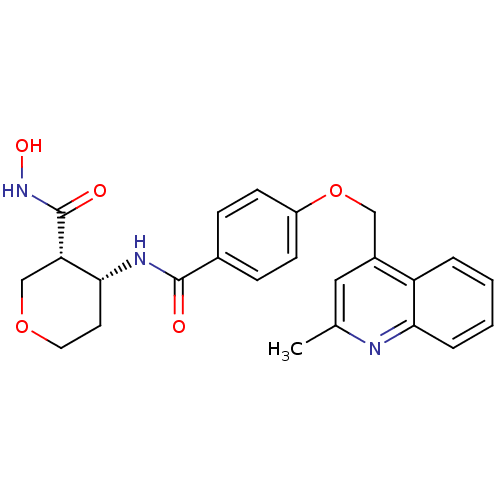 ((3R,4R)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of pig TACE | Bioorg Med Chem Lett 18: 241-6 (2008) Article DOI: 10.1016/j.bmcl.2007.10.093 BindingDB Entry DOI: 10.7270/Q2JM2BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Sus scrofa (pig)) | BDBM50183715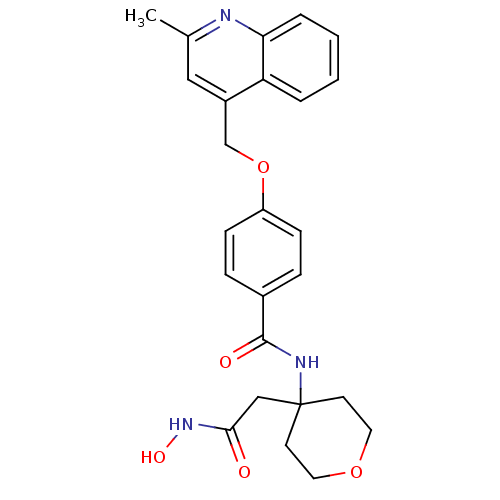 (CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of porcine TACE | Bioorg Med Chem Lett 16: 2699-704 (2006) Article DOI: 10.1016/j.bmcl.2006.02.015 BindingDB Entry DOI: 10.7270/Q2TB16H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50229322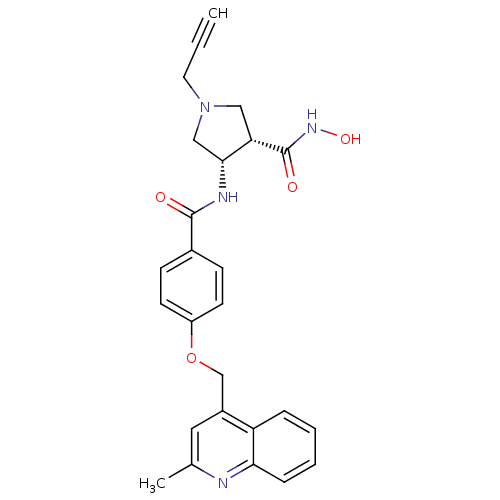 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP7 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26567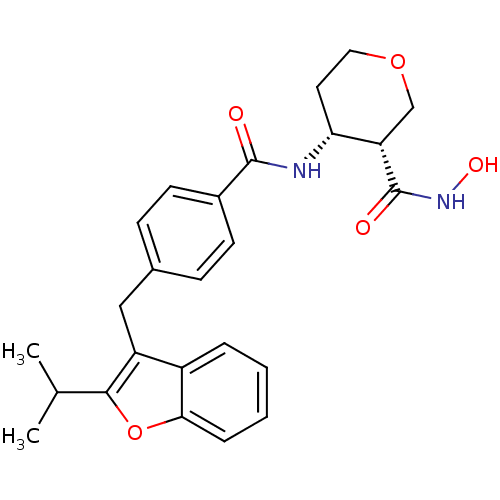 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)-1-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP12 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50183711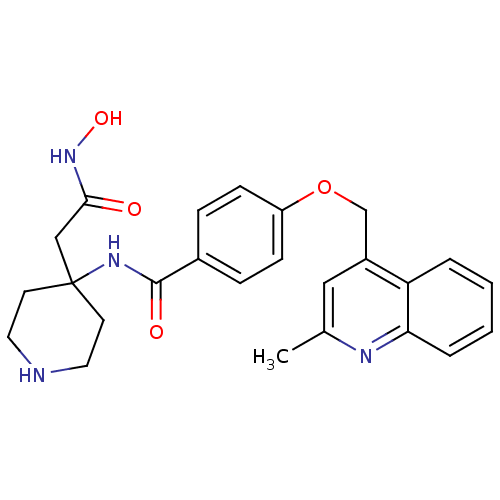 (CHEMBL208009 | N-(4-(2-(hydroxyamino)-2-oxoethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to MMP7 | Bioorg Med Chem Lett 16: 2699-704 (2006) Article DOI: 10.1016/j.bmcl.2006.02.015 BindingDB Entry DOI: 10.7270/Q2TB16H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50183715 (CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to MMP7 | Bioorg Med Chem Lett 16: 2699-704 (2006) Article DOI: 10.1016/j.bmcl.2006.02.015 BindingDB Entry DOI: 10.7270/Q2TB16H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26565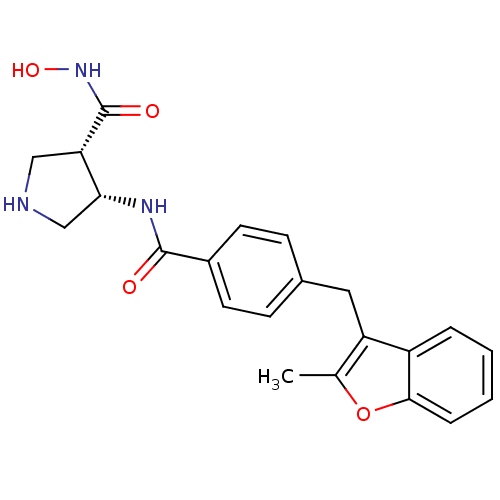 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26564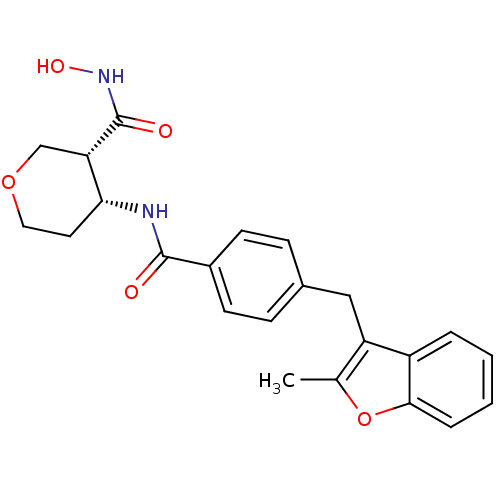 ((3R,4R)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP3 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50502498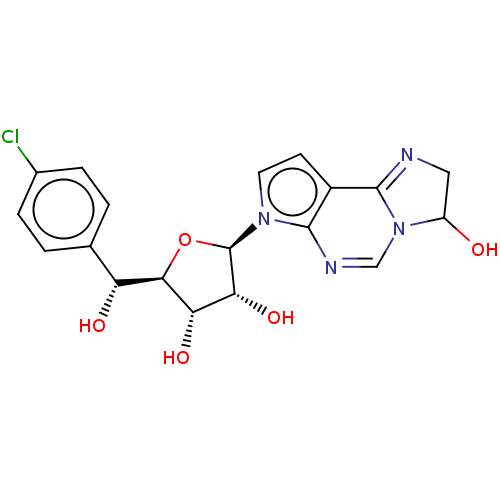 (CHEMBL4518534) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant PRMT5/MEP50 expressed in baculovirus infected High-five cells using histone 4 peptide as substrate assessed as ... | ACS Med Chem Lett 10: 1033-1038 (2019) Article DOI: 10.1021/acsmedchemlett.9b00074 BindingDB Entry DOI: 10.7270/Q29W0JRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26562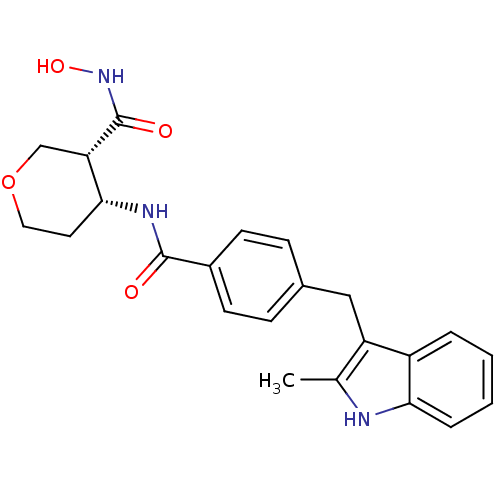 ((3R,4R)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26561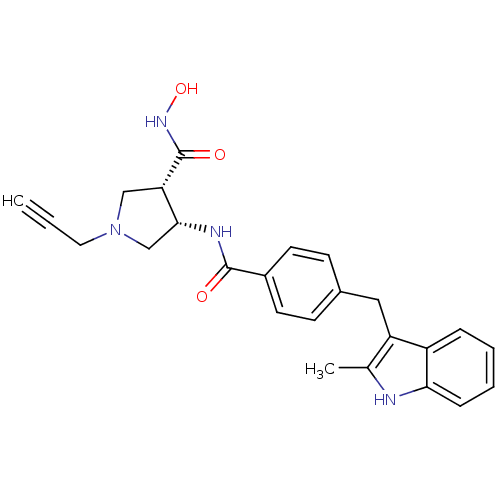 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50183715 (CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to MMP3 | Bioorg Med Chem Lett 16: 2699-704 (2006) Article DOI: 10.1016/j.bmcl.2006.02.015 BindingDB Entry DOI: 10.7270/Q2TB16H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26566 ((3S,4S)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)-1-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50183711 (CHEMBL208009 | N-(4-(2-(hydroxyamino)-2-oxoethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to MMP3 | Bioorg Med Chem Lett 16: 2699-704 (2006) Article DOI: 10.1016/j.bmcl.2006.02.015 BindingDB Entry DOI: 10.7270/Q2TB16H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50183711 (CHEMBL208009 | N-(4-(2-(hydroxyamino)-2-oxoethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to ADAMTS5 | Bioorg Med Chem Lett 16: 2699-704 (2006) Article DOI: 10.1016/j.bmcl.2006.02.015 BindingDB Entry DOI: 10.7270/Q2TB16H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26563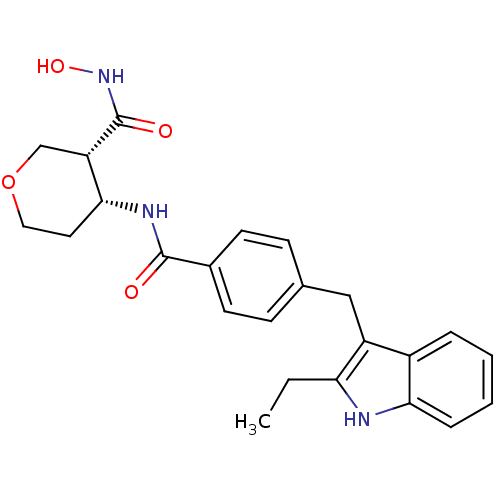 ((3R,4R)-4-N-{4-[(2-ethyl-1H-indol-3-yl)methyl]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26573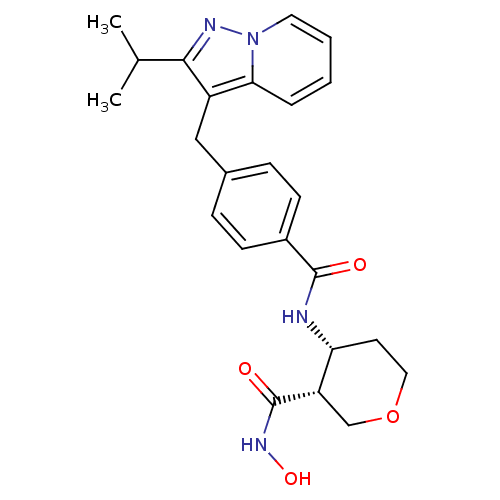 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26560 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136288 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50183711 (CHEMBL208009 | N-(4-(2-(hydroxyamino)-2-oxoethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to MMP8 | Bioorg Med Chem Lett 16: 2699-704 (2006) Article DOI: 10.1016/j.bmcl.2006.02.015 BindingDB Entry DOI: 10.7270/Q2TB16H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP10 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136279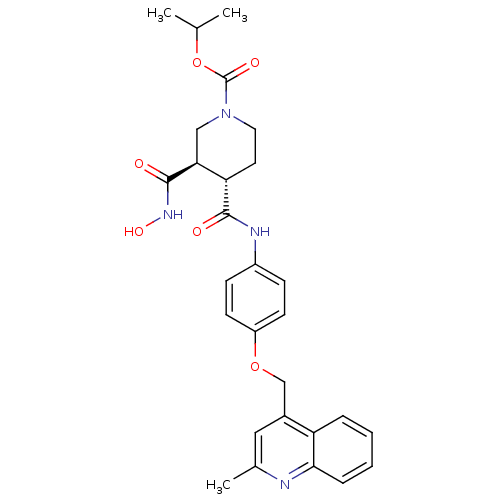 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136261 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136268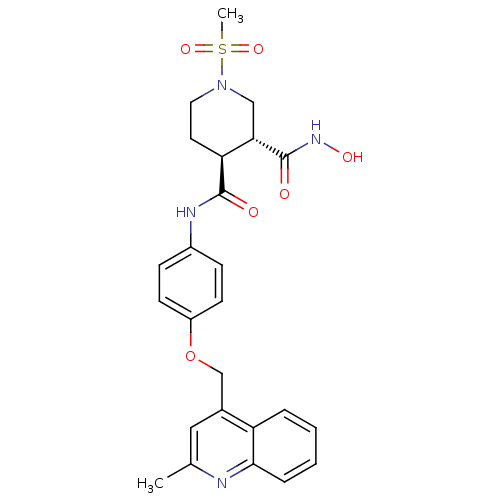 ((3R,4S)-1-Methanesulfonyl-piperidine-3,4-dicarboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 306 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM26561 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26572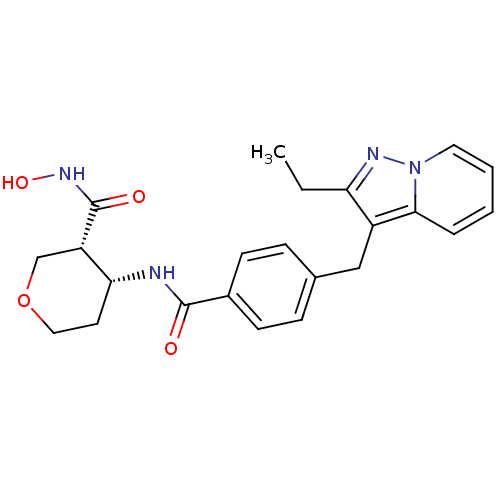 ((3R,4R)-4-N-[4-({2-ethylpyrazolo[1,5-a]pyridin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 327 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136262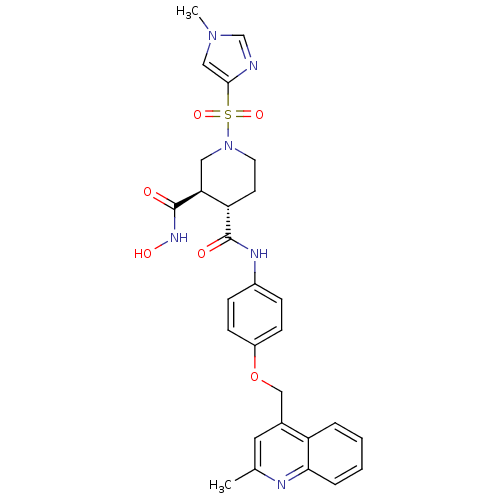 ((3R,4S)-1-(1-Methyl-1H-imidazole-4-sulfonyl)-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 366 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50152914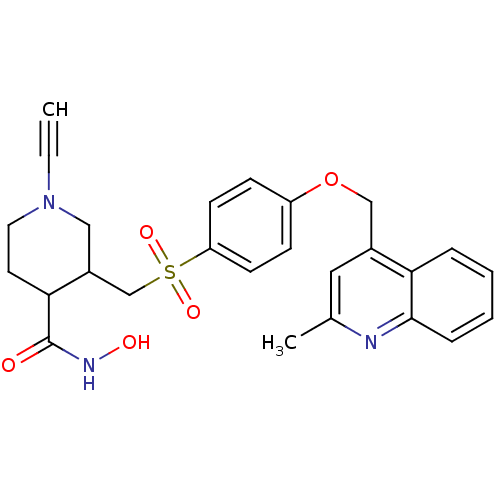 (1-Ethynyl-3-[4-(2-methyl-quinolin-4-ylmethoxy)-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 (MMP-13) | Bioorg Med Chem Lett 14: 4453-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.049 BindingDB Entry DOI: 10.7270/Q2NV9HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136286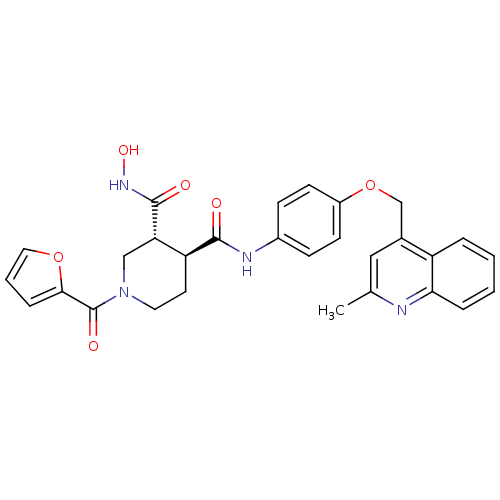 ((3R,4S)-1-(Furan-2-carbonyl)-piperidine-3,4-dicarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50136261 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-2 (MMP-2) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to dopamine transporter | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP13 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50152909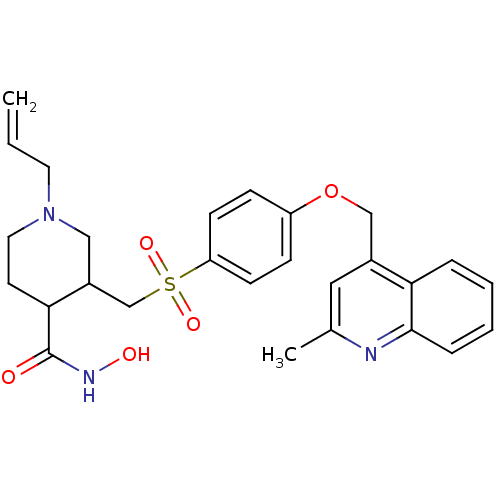 (1-Allyl-3-[4-(2-methyl-quinolin-4-ylmethoxy)-benze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 503 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 (MMP-13) | Bioorg Med Chem Lett 14: 4453-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.049 BindingDB Entry DOI: 10.7270/Q2NV9HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26570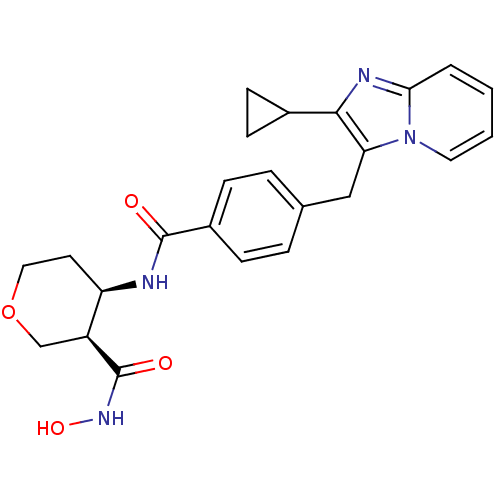 ((3R,4R)-4-N-[4-({2-cyclopropylimidazo[1,2-a]pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26569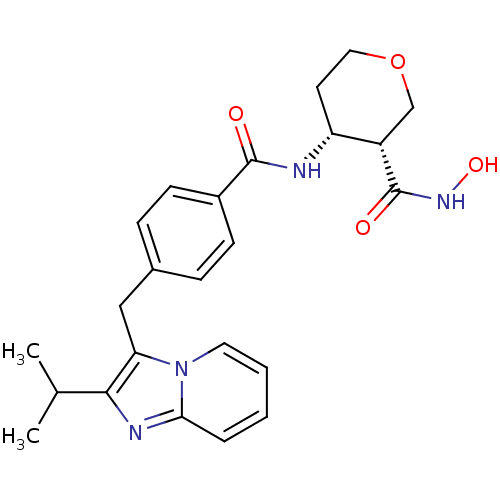 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50152930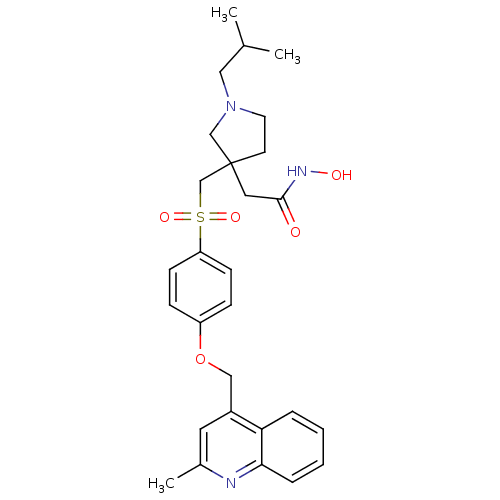 (CHEMBL359569 | N-Hydroxy-2-{1-isobutyl-3-[4-(2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 (MMP-13) | Bioorg Med Chem Lett 14: 4453-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.049 BindingDB Entry DOI: 10.7270/Q2NV9HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM26565 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50136288 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 642 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-2 (MMP-2) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50152924 (CHEMBL184553 | N-Hydroxy-2-{1-isopropyl-3-[4-(2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 653 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 (MMP-13) | Bioorg Med Chem Lett 14: 4453-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.049 BindingDB Entry DOI: 10.7270/Q2NV9HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50136268 ((3R,4S)-1-Methanesulfonyl-piperidine-3,4-dicarboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 656 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-2 (MMP-2) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26574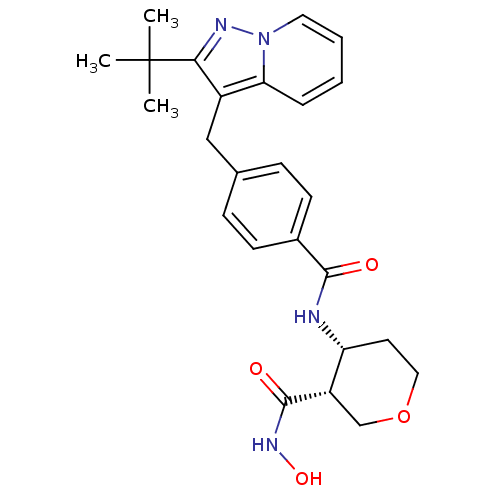 ((3R,4R)-4-N-[4-({2-tert-butylpyrazolo[1,5-a]pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 726 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50152915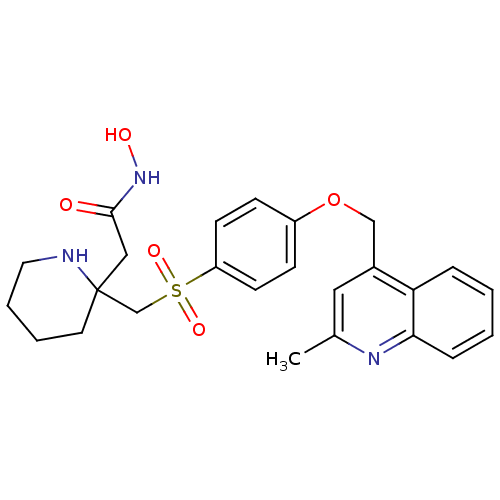 (CHEMBL185321 | N-Hydroxy-2-{2-[4-(2-methyl-quinoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 753 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 (MMP-13) | Bioorg Med Chem Lett 14: 4453-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.049 BindingDB Entry DOI: 10.7270/Q2NV9HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50152933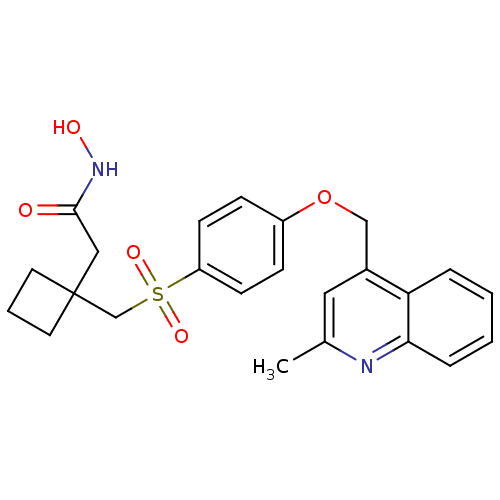 (CHEMBL364875 | N-Hydroxy-2-{1-[4-(2-methyl-quinoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 753 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 (MMP-13) | Bioorg Med Chem Lett 14: 4453-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.049 BindingDB Entry DOI: 10.7270/Q2NV9HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50152907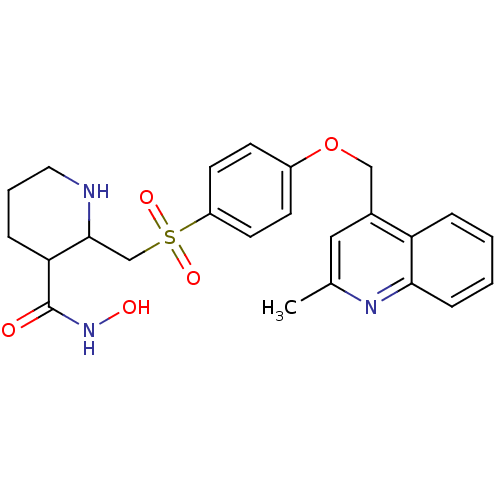 (2-[4-(2-Methyl-quinolin-4-ylmethoxy)-benzenesulfon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 754 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 (MMP-13) | Bioorg Med Chem Lett 14: 4453-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.049 BindingDB Entry DOI: 10.7270/Q2NV9HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM26567 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)-1-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 766 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136285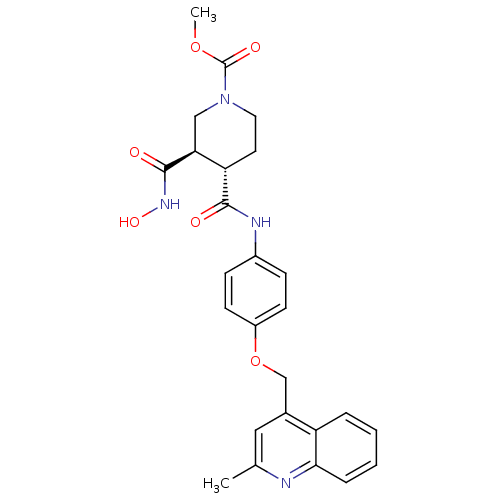 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 767 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50136275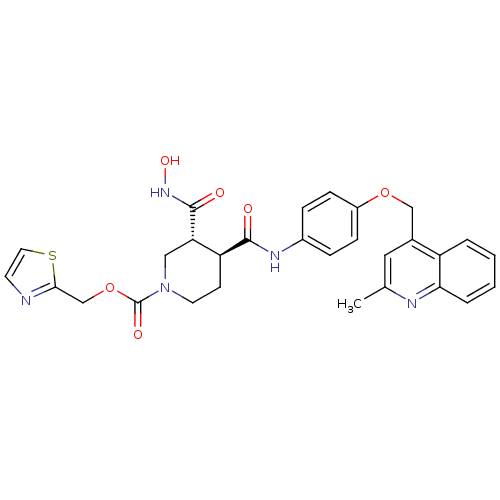 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 768 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-9 (MMP-9) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50136279 ((3R,4S)-3-Hydroxycarbamoyl-4-[4-(2-methyl-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 771 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloproteinase-2 (MMP-2) | Bioorg Med Chem Lett 13: 4299-304 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1835 total ) | Next | Last >> |