

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052027 ((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053354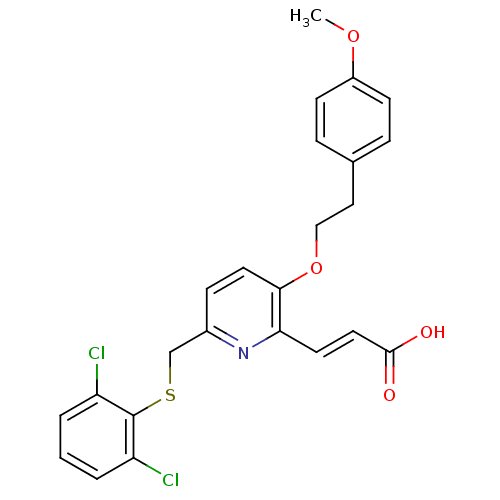 ((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053350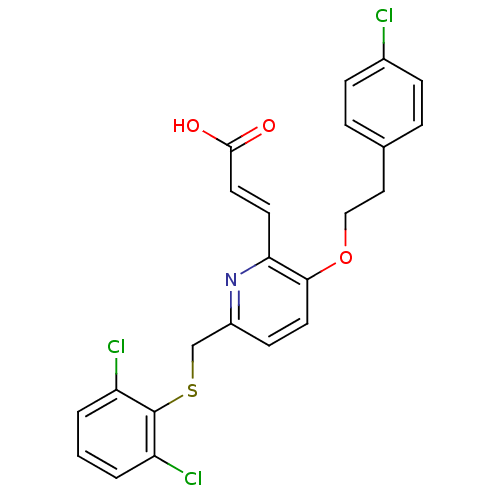 ((E)-3-[3-[2-(4-Chloro-phenyl)-ethoxy]-6-(2,6-dichl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053353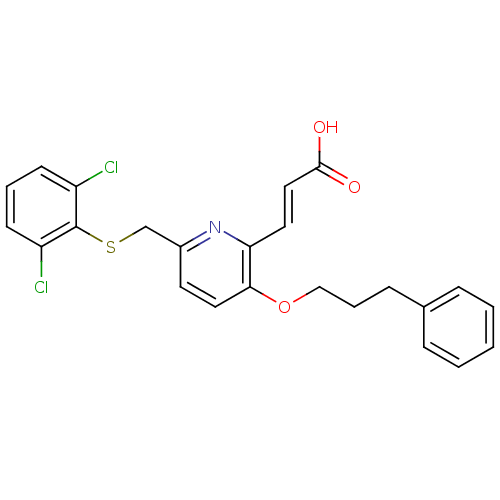 ((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-(3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053351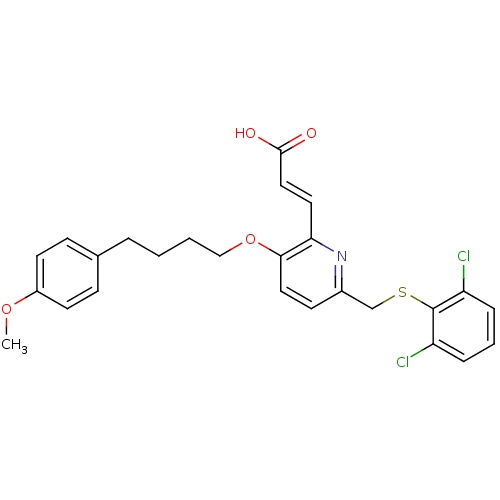 ((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053355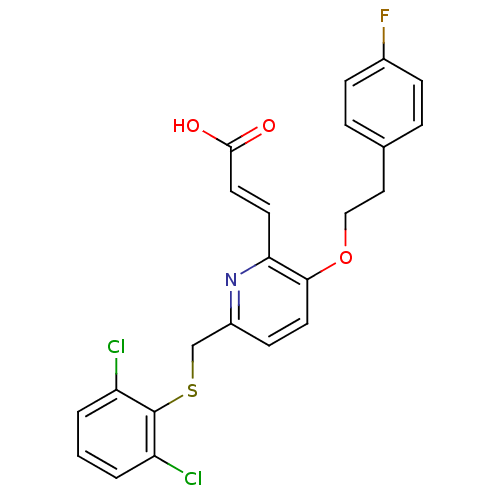 ((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053356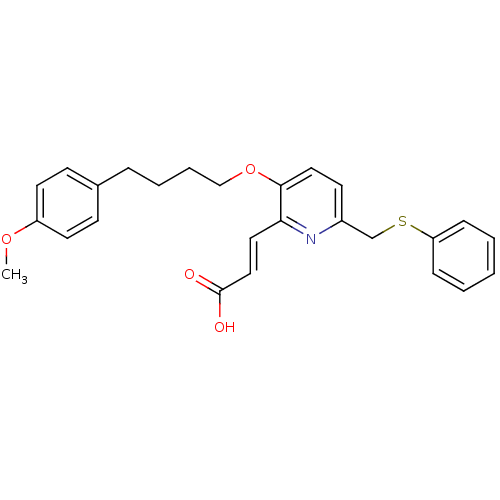 ((E)-3-{3-[4-(4-Methoxy-phenyl)-butoxy]-6-phenylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042182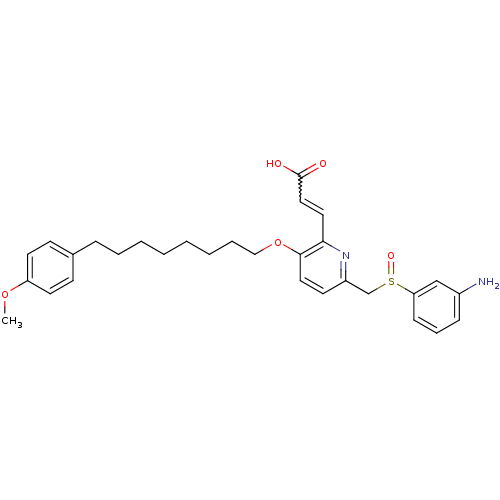 ((E)-3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037385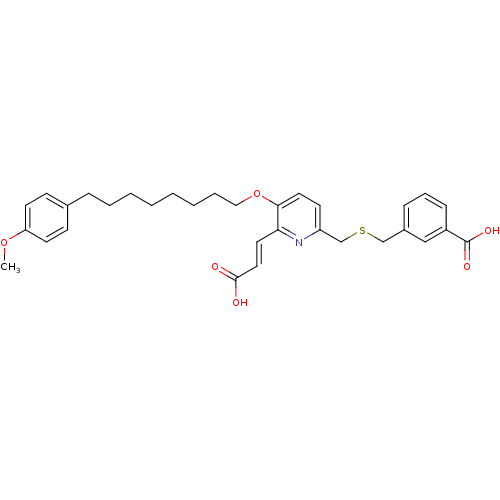 (3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053352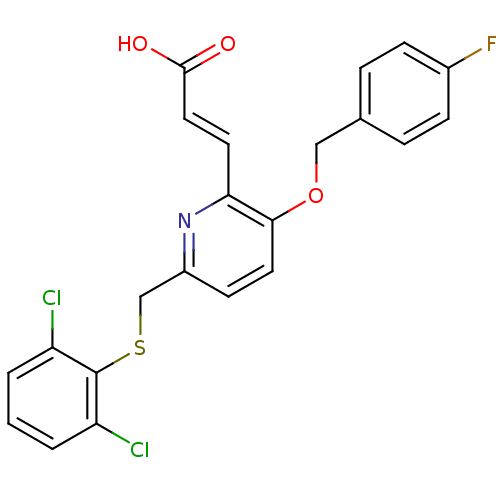 ((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144689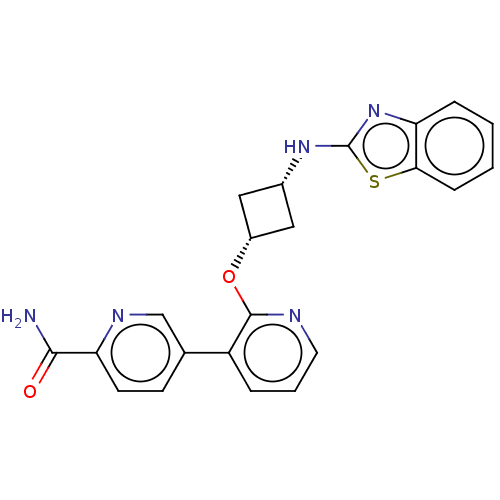 (US8952037, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM47125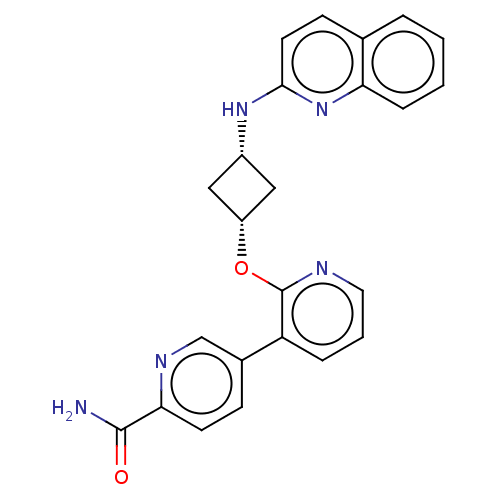 (US8952027, 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144650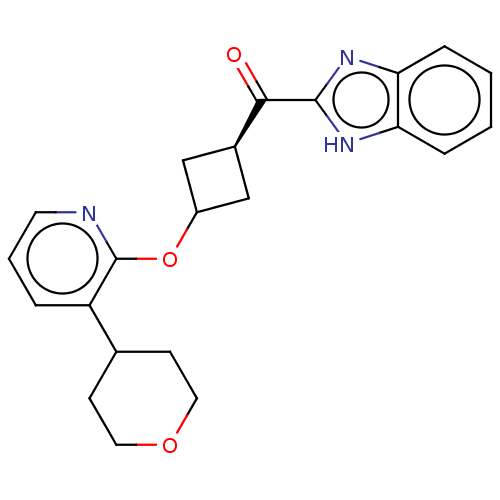 (US8952037, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.413 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144685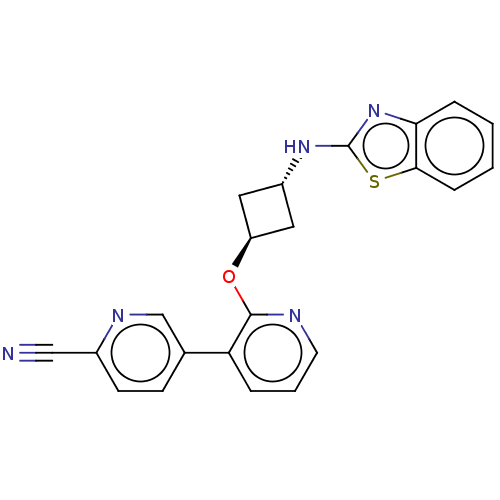 (US8952037, 58 | US8952037, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144718 (US8952037, 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.728 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144659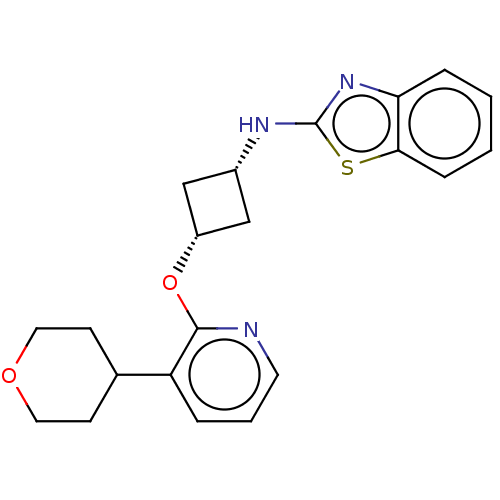 (US8952037, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.751 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144699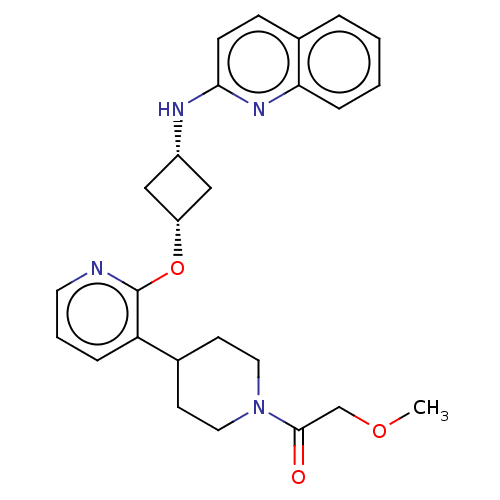 (US8952037, 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.948 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144703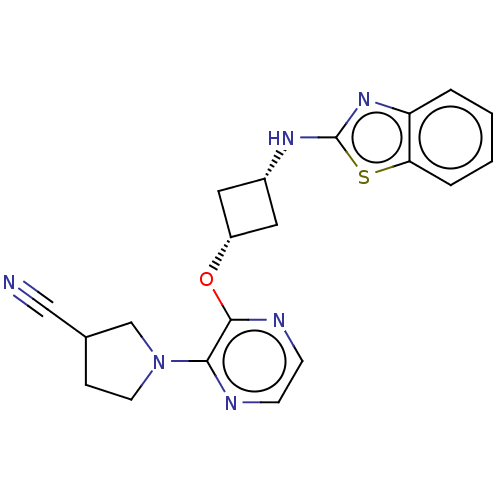 (US8952037, 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144721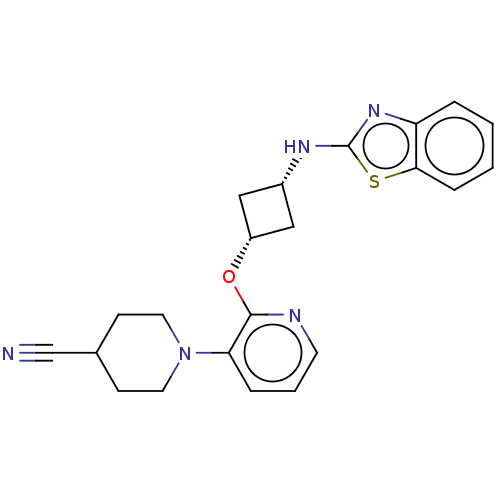 (US8952037, 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144682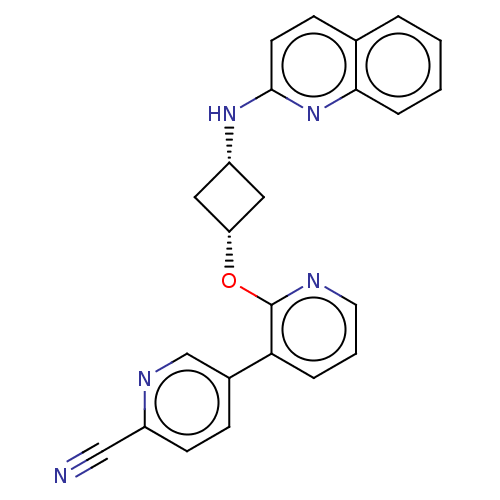 (US8952037, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144696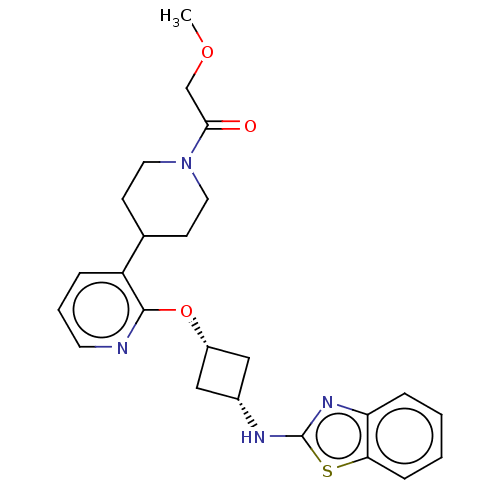 (US8952037, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144698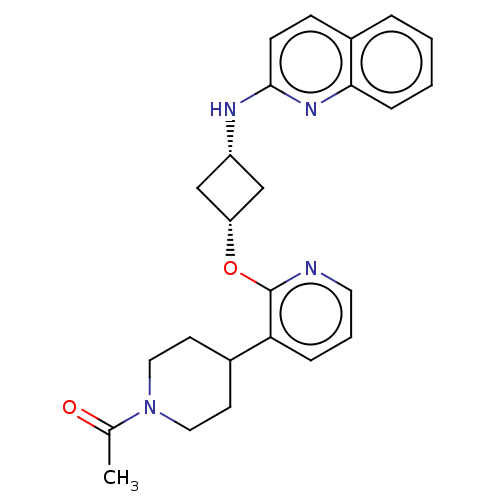 (US8952037, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144666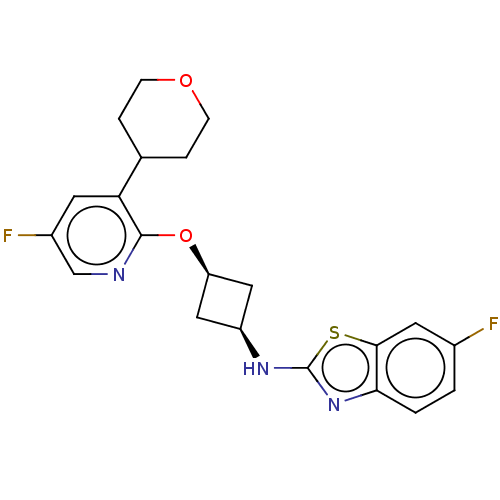 (US8952037, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144729 (US8952037, 112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144654 (US8952037, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144676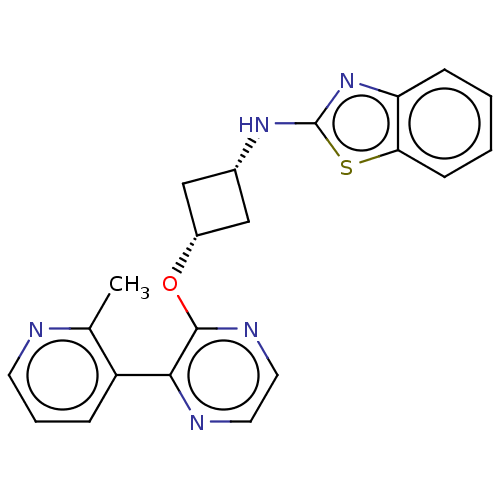 (US8952037, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.94 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144713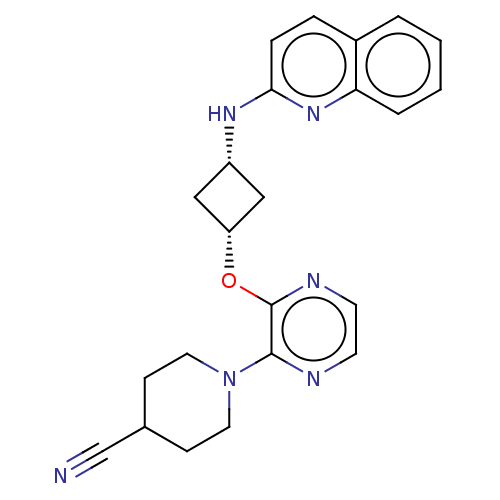 (US8952037, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.26 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144711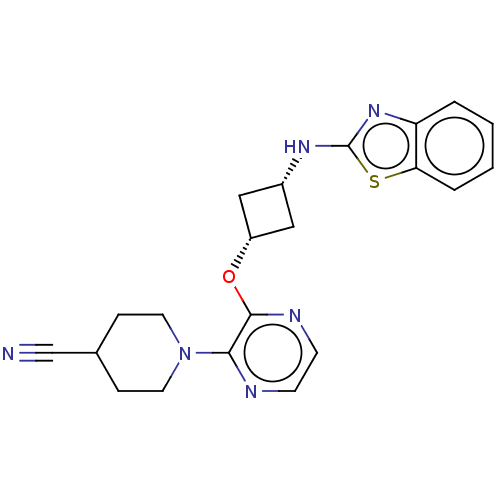 (US8952037, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.31 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144680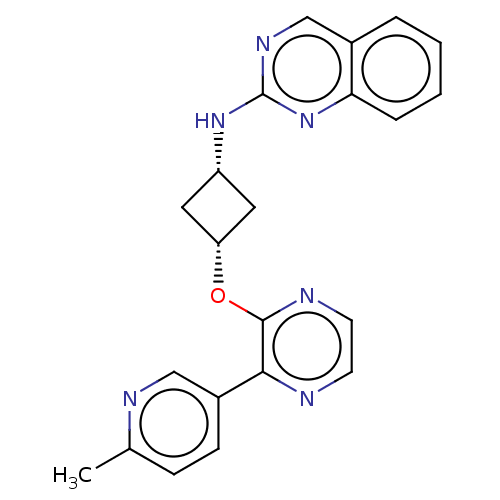 (US8952037, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.43 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144667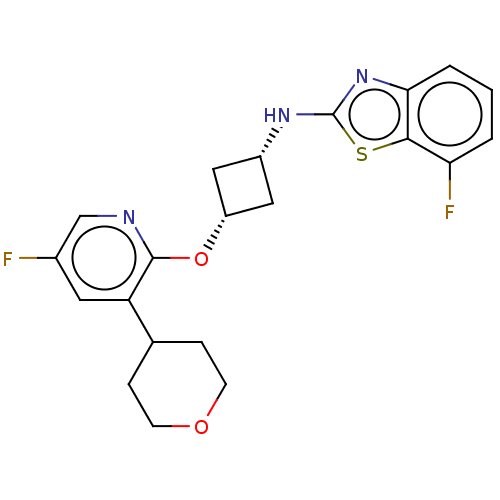 (US8952037, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144655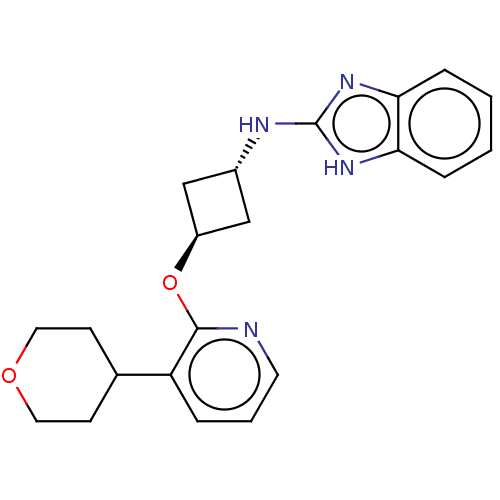 (US8952037, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144677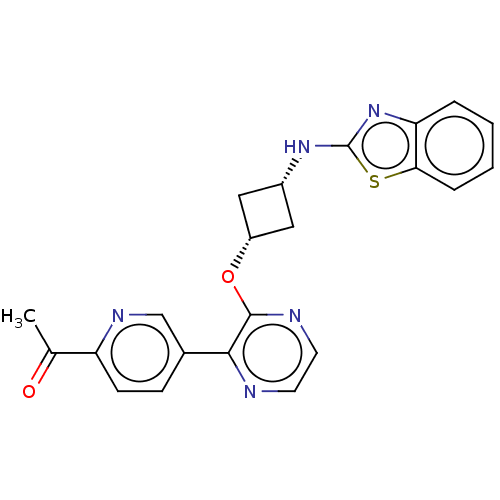 (US8952037, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.74 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144647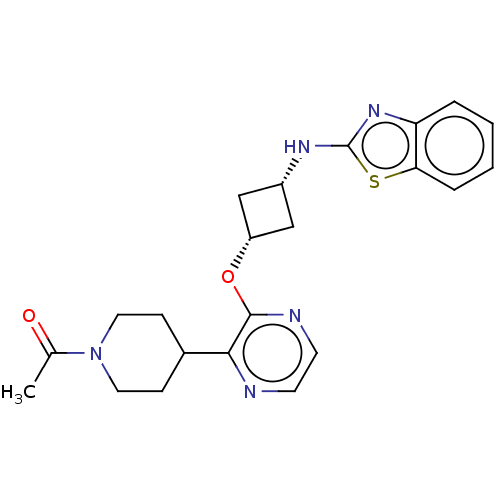 (US8952037, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.77 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144731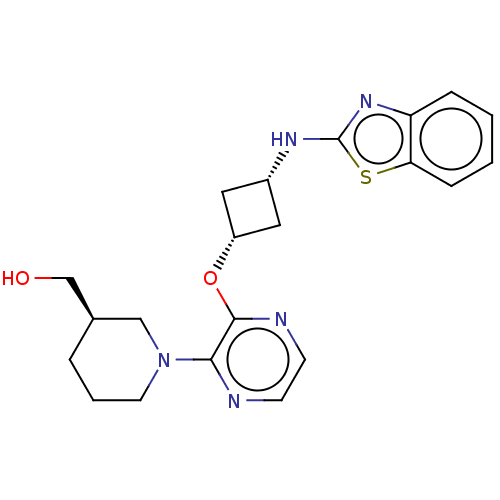 (US8952037, 92) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.81 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144687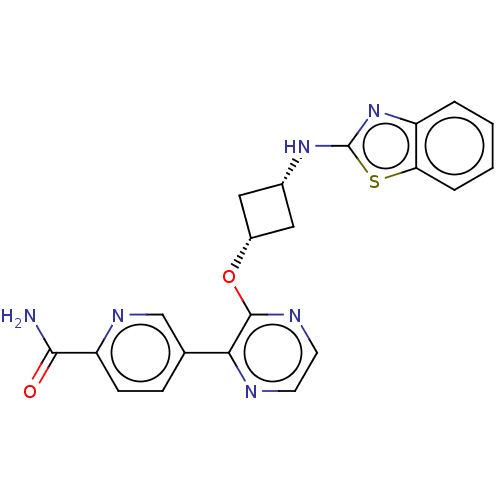 (US8952037, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.96 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144685 (US8952037, 58 | US8952037, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.97 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144700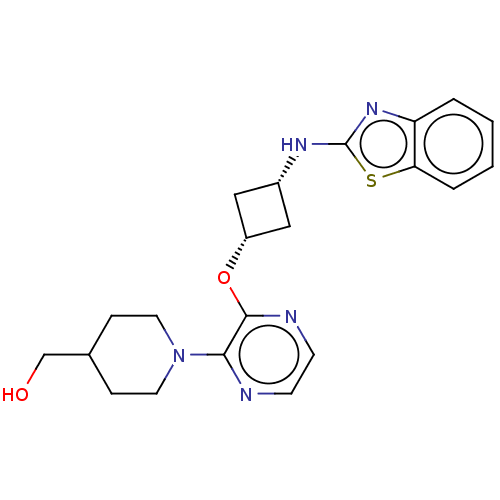 (US8952037, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144697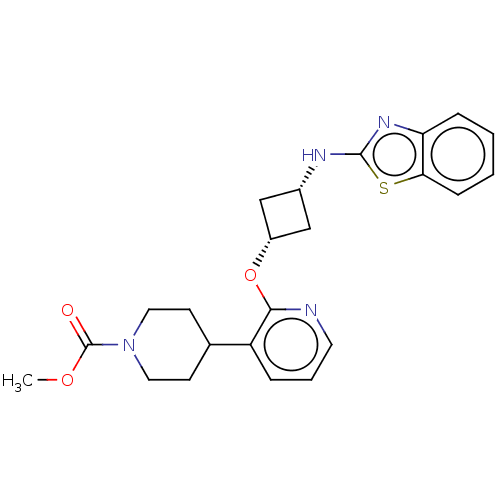 (US8952037, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.18 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144658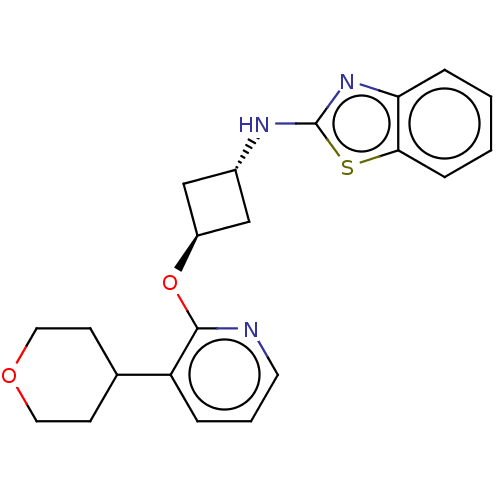 (US8952037, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.19 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144717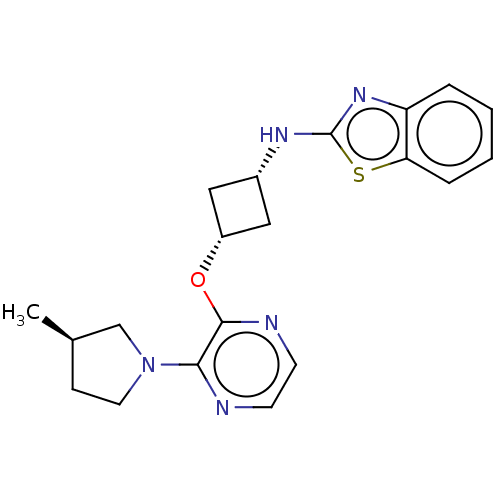 (US8952037, 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.24 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144673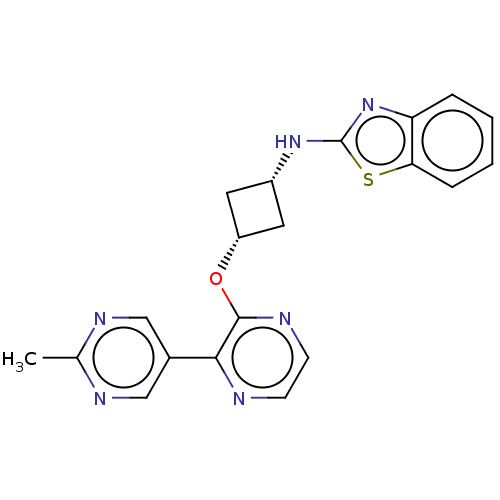 (US8952037, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144671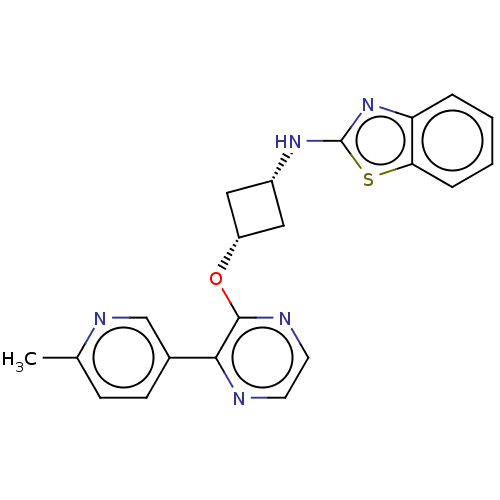 (US8952037, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.39 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144665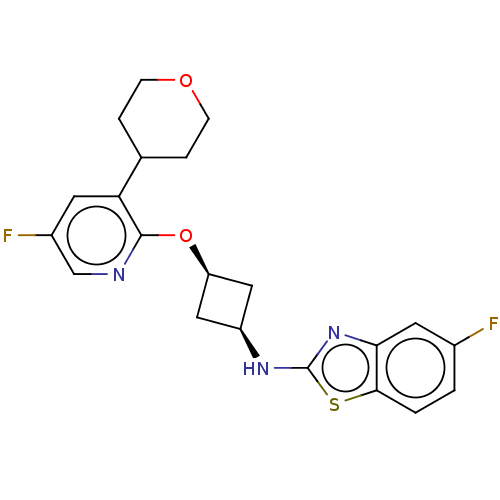 (US8952037, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.49 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144668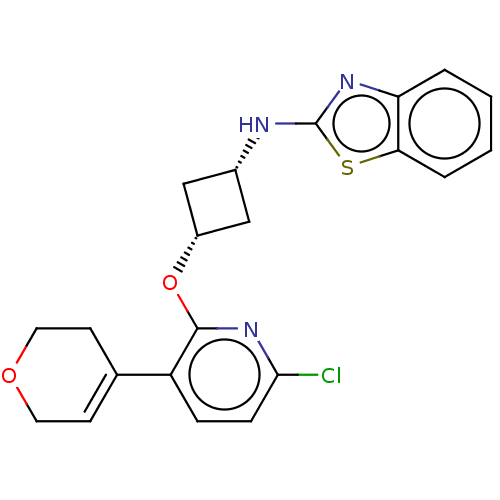 (US8952037, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144705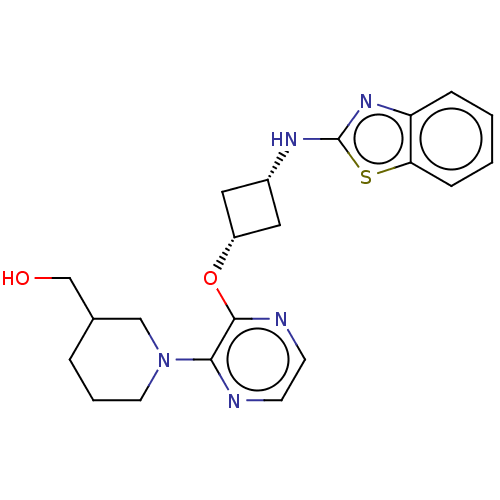 (US8952037, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.62 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144688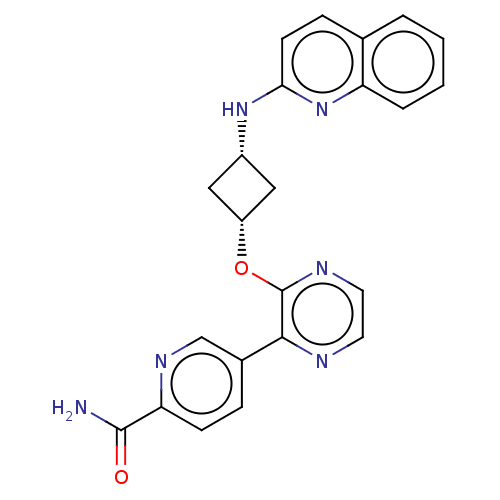 (US8952037, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144672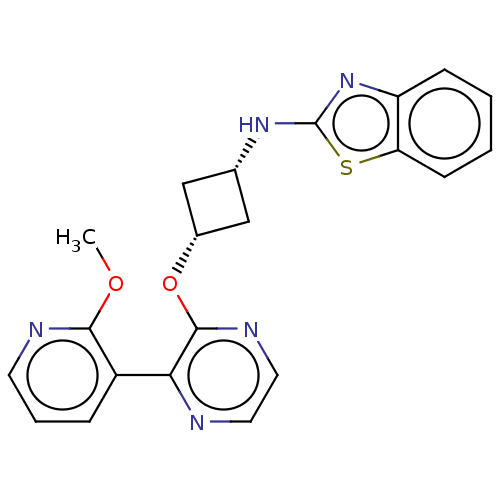 (US8952037, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.11 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144669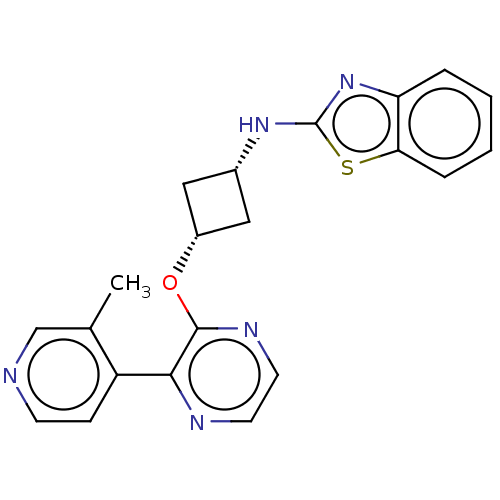 (US8952037, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.23 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM47126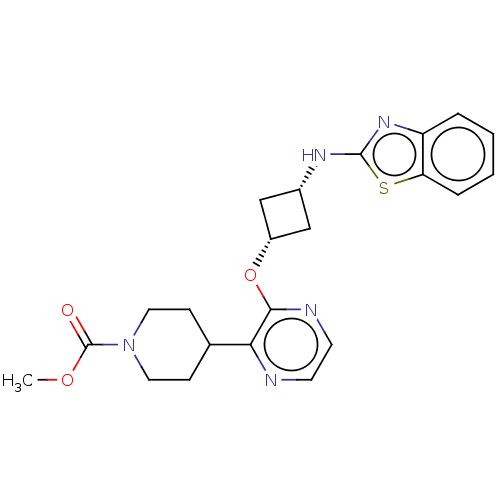 (US8952027, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.24 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144694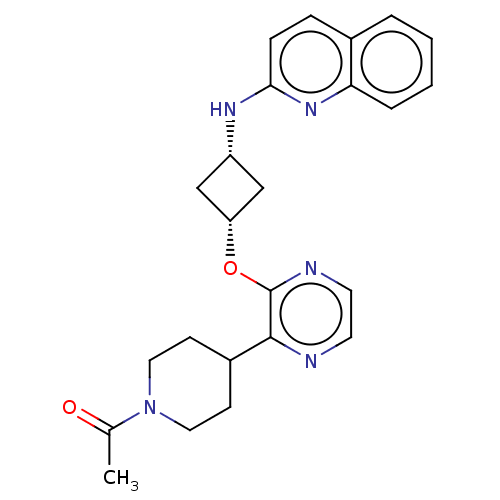 (US8952037, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.93 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description Enzyme Activity. An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 uL, of serial diluted P... | US Patent US8952037 (2015) BindingDB Entry DOI: 10.7270/Q23X85CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 266 total ) | Next | Last >> |