

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50044625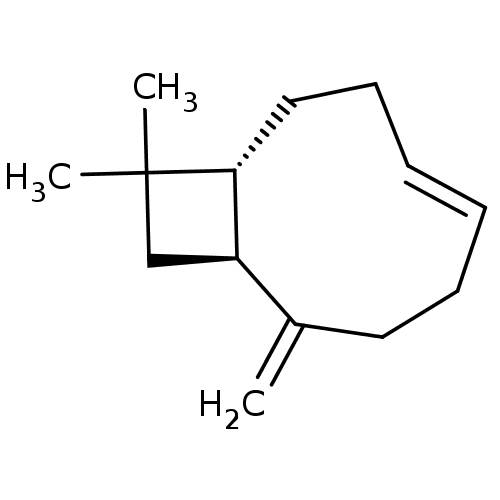 (CHEMBL3360190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of CB2 receptor (unknown origin) | Bioorg Med Chem Lett 24: 3168-74 (2014) Article DOI: 10.1016/j.bmcl.2014.04.112 BindingDB Entry DOI: 10.7270/Q2SN0BK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5244 ((5S,6S)-5-carbamimidamido-6-acetamido-1-(2-ethylbu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 1751-6 (1999) Article DOI: 10.1016/s0960-894x(99)00280-2 BindingDB Entry DOI: 10.7270/Q2ZS2TNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50144206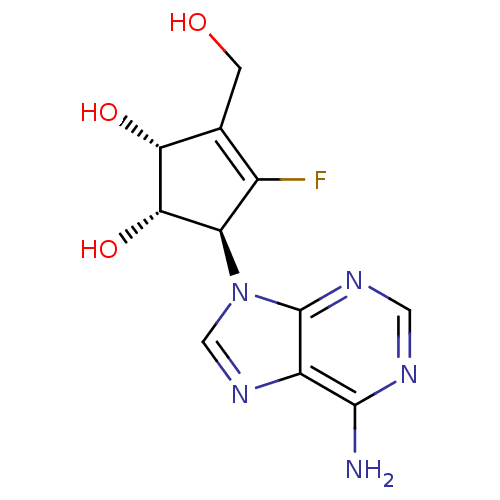 ((1S,2R,5S)-5-(6-Amino-purin-9-yl)-4-fluoro-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006215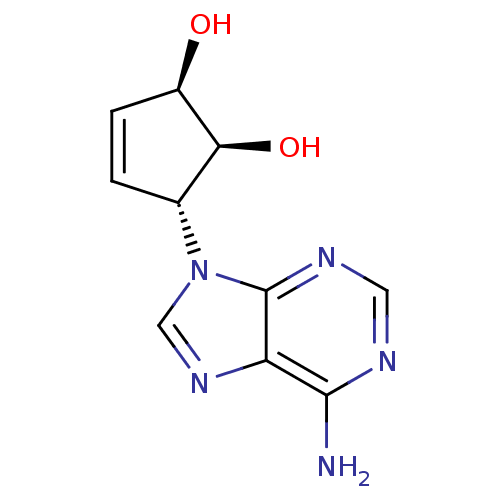 ((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5243 ((5S,6S)-5-amino-6-acetamido-1-(2-ethylbutanoyl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 1751-6 (1999) Article DOI: 10.1016/s0960-894x(99)00280-2 BindingDB Entry DOI: 10.7270/Q2ZS2TNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50144205 ((1S,2R,5S)-5-(6-Amino-purin-9-yl)-4-fluoro-cyclope...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50240464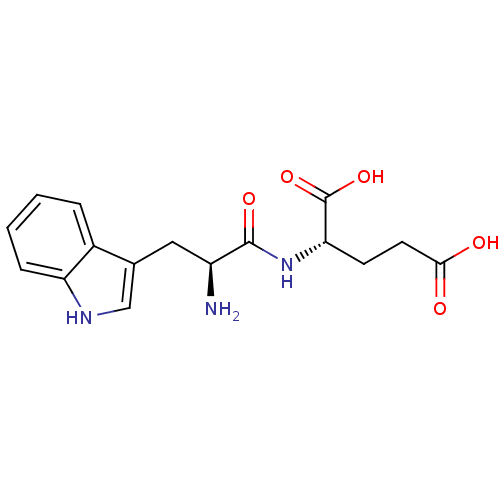 ((S)-2-((S)-2-amino-3-(1H-indol-3-yl)propanamido)pe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Antagonist activity at PPARgamma (unknown origin) | Bioorg Med Chem Lett 24: 2957-62 (2014) Article DOI: 10.1016/j.bmcl.2014.04.019 BindingDB Entry DOI: 10.7270/Q2FB54HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5244 ((5S,6S)-5-carbamimidamido-6-acetamido-1-(2-ethylbu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 1751-6 (1999) Article DOI: 10.1016/s0960-894x(99)00280-2 BindingDB Entry DOI: 10.7270/Q2ZS2TNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50336547 (5-(6-Aminopurin-9-yl)-4-chloro-3-hydroxymethylcycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50336548 (5-(6-Aminopurin-9-yl)-4-bromo-3-hydroxymethyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5243 ((5S,6S)-5-amino-6-acetamido-1-(2-ethylbutanoyl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 1751-6 (1999) Article DOI: 10.1016/s0960-894x(99)00280-2 BindingDB Entry DOI: 10.7270/Q2ZS2TNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5245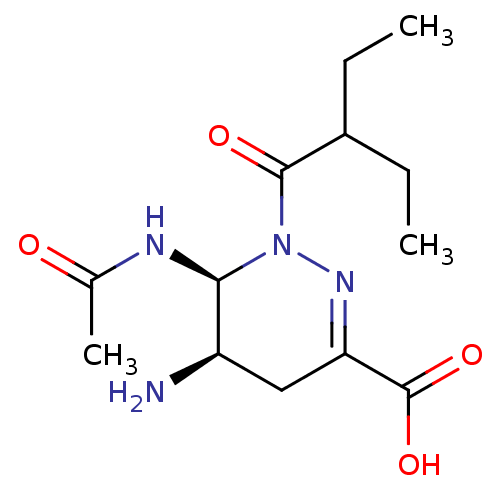 ((5R,6S)-5-amino-6-acetamido-1-(2-ethylbutanoyl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 1751-6 (1999) Article DOI: 10.1016/s0960-894x(99)00280-2 BindingDB Entry DOI: 10.7270/Q2ZS2TNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50336551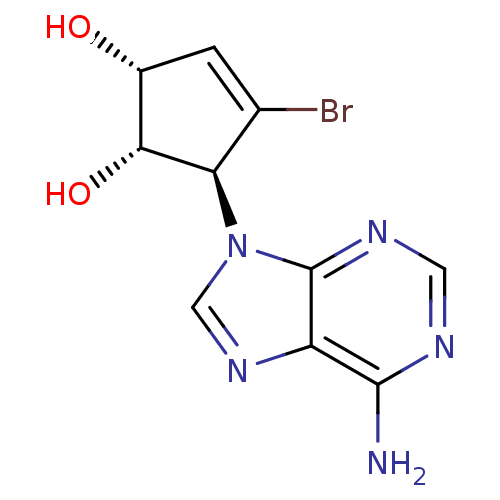 (5-(6-Aminopurin-9-yl)-4-bromocyclopent-3-ene-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50336550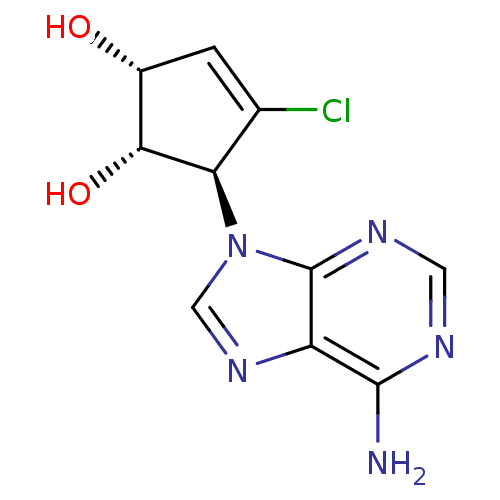 (5-(6-Aminopurin-9-yl)-4-chlorocyclopent-3-ene-1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5245 ((5R,6S)-5-amino-6-acetamido-1-(2-ethylbutanoyl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 1751-6 (1999) Article DOI: 10.1016/s0960-894x(99)00280-2 BindingDB Entry DOI: 10.7270/Q2ZS2TNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50336552 (5-(6-Aminopurin-9-yl)-4-iodocyclopent-3-ene-1,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50336549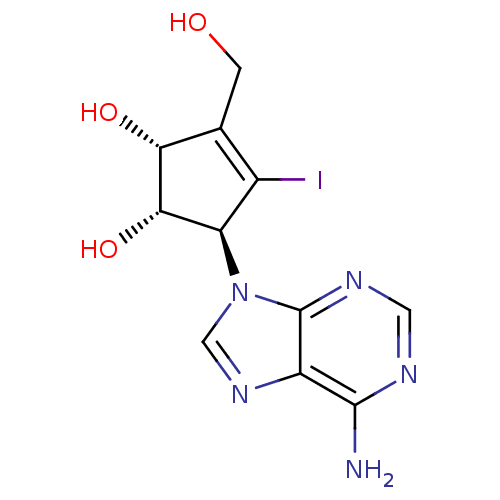 (5-(6-Aminopurin-9-yl)-4-iodo-3-hydroxymethylcyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human placental AdoHcy hydrolase expressed in Escherichia coli JM109 | J Med Chem 54: 930-8 (2011) Article DOI: 10.1021/jm1010836 BindingDB Entry DOI: 10.7270/Q2D50N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.13E+4 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at human recombinant PPARalpha expressed in HepG2 cells cotransfected with pGL3-PPRE3-TK-luc reporter assessed as beta-galactosidase... | Bioorg Med Chem Lett 21: 5876-80 (2011) Article DOI: 10.1016/j.bmcl.2011.07.095 BindingDB Entry DOI: 10.7270/Q2TD9XRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491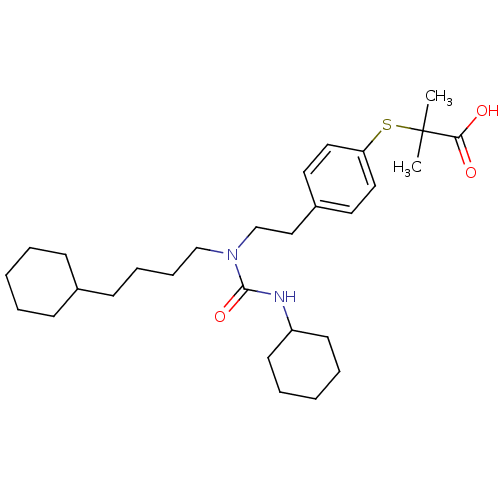 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to human PPAR-alpha LBD assessed as recruitment of fluorescein-labeled coactivator peptide by surface plasmon resonance method | Bioorg Med Chem Lett 24: 3168-74 (2014) Article DOI: 10.1016/j.bmcl.2014.04.112 BindingDB Entry DOI: 10.7270/Q2SN0BK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50044625 (CHEMBL3360190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to human PPAR-alpha LBD assessed as recruitment of fluorescein-labeled coactivator peptide by surface plasmon resonance method | Bioorg Med Chem Lett 24: 3168-74 (2014) Article DOI: 10.1016/j.bmcl.2014.04.112 BindingDB Entry DOI: 10.7270/Q2SN0BK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at PPAR-alpha (unknown origin) expressed in HEK293 cells by TR-FRET assay | Bioorg Med Chem Lett 24: 3168-74 (2014) Article DOI: 10.1016/j.bmcl.2014.04.112 BindingDB Entry DOI: 10.7270/Q2SN0BK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28700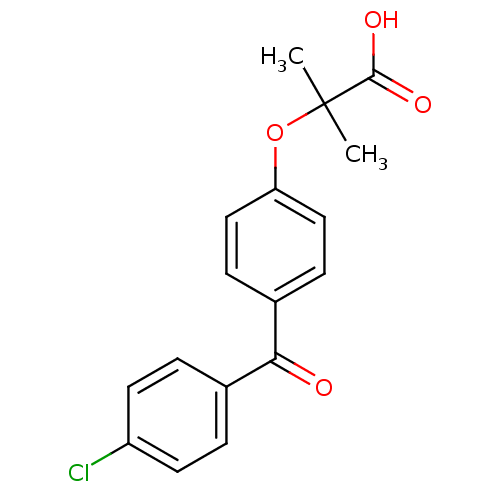 (2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropionic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to histidine-tagged human PPARalpha-LBD assessed as recruitment of co-activator peptide fluorescein-labeled PGC1alpha after 2 hrs by... | Bioorg Med Chem Lett 21: 5876-80 (2011) Article DOI: 10.1016/j.bmcl.2011.07.095 BindingDB Entry DOI: 10.7270/Q2TD9XRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at PPAR-alpha (unknown origin) expressed in HEK293 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 3168-74 (2014) Article DOI: 10.1016/j.bmcl.2014.04.112 BindingDB Entry DOI: 10.7270/Q2SN0BK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50044625 (CHEMBL3360190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.58E+3 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at PPAR-alpha (unknown origin) expressed in HEK293 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 3168-74 (2014) Article DOI: 10.1016/j.bmcl.2014.04.112 BindingDB Entry DOI: 10.7270/Q2SN0BK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 9.64E+4 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to PPARalpha LBD (unknown origin) by surface plasmon resonance assay | Bioorg Med Chem Lett 24: 2957-62 (2014) Article DOI: 10.1016/j.bmcl.2014.04.019 BindingDB Entry DOI: 10.7270/Q2FB54HC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50240464 ((S)-2-((S)-2-amino-3-(1H-indol-3-yl)propanamido)pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to PPARalpha LBD (unknown origin) by surface plasmon resonance assay | Bioorg Med Chem Lett 24: 2957-62 (2014) Article DOI: 10.1016/j.bmcl.2014.04.019 BindingDB Entry DOI: 10.7270/Q2FB54HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at PPARalpha LBD (unknown origin) assessed as induction of PGC1alpha co-activator activity by TR-FRET analysis | Bioorg Med Chem Lett 24: 2957-62 (2014) Article DOI: 10.1016/j.bmcl.2014.04.019 BindingDB Entry DOI: 10.7270/Q2FB54HC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50240464 ((S)-2-((S)-2-amino-3-(1H-indol-3-yl)propanamido)pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.34E+4 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at PPARalpha LBD (unknown origin) assessed as induction of PGC1alpha co-activator activity by TR-FRET analysis | Bioorg Med Chem Lett 24: 2957-62 (2014) Article DOI: 10.1016/j.bmcl.2014.04.019 BindingDB Entry DOI: 10.7270/Q2FB54HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50044625 (CHEMBL3360190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at PPAR-alpha (unknown origin) expressed in HEK293 cells by TR-FRET assay | Bioorg Med Chem Lett 24: 3168-74 (2014) Article DOI: 10.1016/j.bmcl.2014.04.112 BindingDB Entry DOI: 10.7270/Q2SN0BK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||