 Found 607 hits with Last Name = 'dubé' and Initial = 'l'
Found 607 hits with Last Name = 'dubé' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50325178
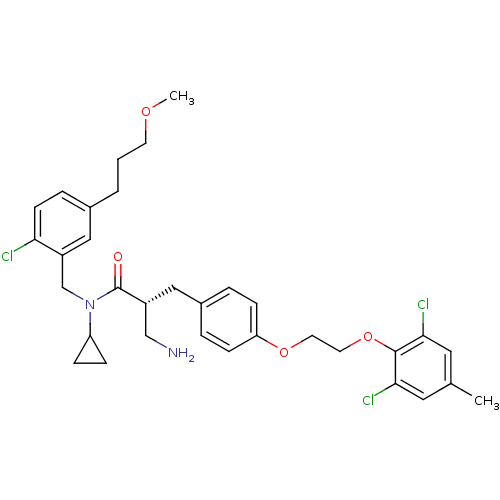
((R)-3-amino-N-(2-chloro-5-(3-methoxypropyl)benzyl)...)Show SMILES COCCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@@H](CN)Cc2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C33H39Cl3N2O4/c1-22-16-30(35)32(31(36)17-22)42-15-14-41-28-10-5-24(6-11-28)18-25(20-37)33(39)38(27-8-9-27)21-26-19-23(4-3-13-40-2)7-12-29(26)34/h5-7,10-12,16-17,19,25,27H,3-4,8-9,13-15,18,20-21,37H2,1-2H3/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 20: 5074-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.030
BindingDB Entry DOI: 10.7270/Q29P31V2 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183805
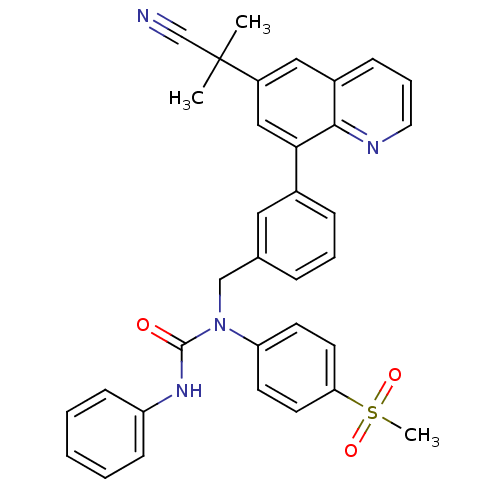
(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)Nc3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30N4O3S/c1-34(2,23-35)27-20-26-11-8-18-36-32(26)31(21-27)25-10-7-9-24(19-25)22-38(33(39)37-28-12-5-4-6-13-28)29-14-16-30(17-15-29)42(3,40)41/h4-21H,22H2,1-3H3,(H,37,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50183805

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)Nc3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30N4O3S/c1-34(2,23-35)27-20-26-11-8-18-36-32(26)31(21-27)25-10-7-9-24(19-25)22-38(33(39)37-28-12-5-4-6-13-28)29-14-16-30(17-15-29)42(3,40)41/h4-21H,22H2,1-3H3,(H,37,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4C |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314646
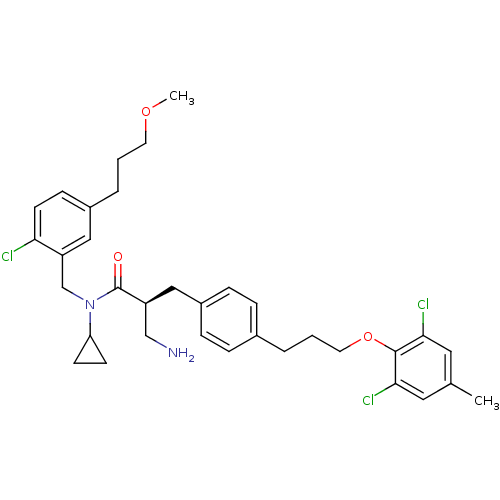
((S)-3-amino-N-(2-chloro-5-(3-methoxypropyl)benzyl)...)Show SMILES COCCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H](CN)Cc2ccc(CCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C34H41Cl3N2O3/c1-23-17-31(36)33(32(37)18-23)42-16-4-5-24-7-9-26(10-8-24)19-27(21-38)34(40)39(29-12-13-29)22-28-20-25(6-3-15-41-2)11-14-30(28)35/h7-11,14,17-18,20,27,29H,3-6,12-13,15-16,19,21-22,38H2,1-2H3/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50325178

((R)-3-amino-N-(2-chloro-5-(3-methoxypropyl)benzyl)...)Show SMILES COCCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@@H](CN)Cc2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C33H39Cl3N2O4/c1-22-16-30(35)32(31(36)17-22)42-15-14-41-28-10-5-24(6-11-28)18-25(20-37)33(39)38(27-8-9-27)21-26-19-23(4-3-13-40-2)7-12-29(26)34/h5-7,10-12,16-17,19,25,27H,3-4,8-9,13-15,18,20-21,37H2,1-2H3/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in presence of pH 7.4 buffer |
Bioorg Med Chem Lett 20: 5074-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.030
BindingDB Entry DOI: 10.7270/Q29P31V2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50314654
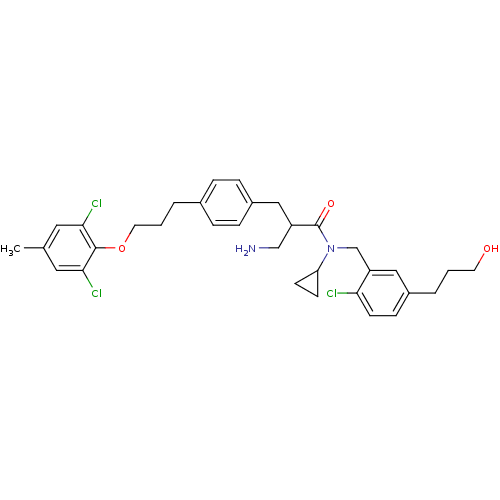
(3-amino-N-(2-chloro-5-(3-hydroxypropyl)benzyl)-N-c...)Show SMILES Cc1cc(Cl)c(OCCCc2ccc(CC(CN)C(=O)N(Cc3cc(CCCO)ccc3Cl)C3CC3)cc2)c(Cl)c1 Show InChI InChI=1S/C33H39Cl3N2O3/c1-22-16-30(35)32(31(36)17-22)41-15-3-5-23-6-8-25(9-7-23)18-26(20-37)33(40)38(28-11-12-28)21-27-19-24(4-2-14-39)10-13-29(27)34/h6-10,13,16-17,19,26,28,39H,2-5,11-12,14-15,18,20-21,37H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183792

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N4O3S/c1-21(2)34-30(36)35(26-11-13-27(14-12-26)39(5,37)38)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3,(H,34,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183794
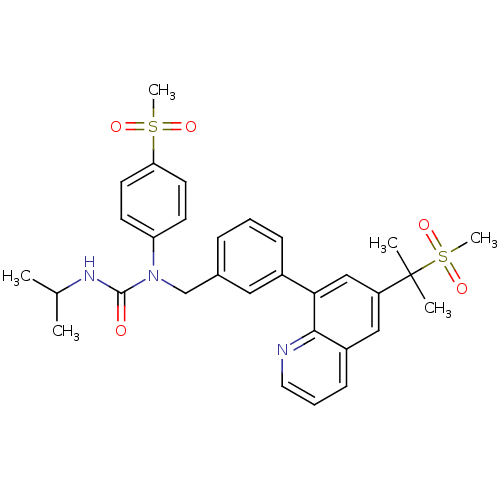
(3-isopropyl-1-(4-(methylsulfonyl)phenyl)-1-((3-(6-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H35N3O5S2/c1-21(2)33-30(35)34(26-12-14-27(15-13-26)40(5,36)37)20-22-9-7-10-23(17-22)28-19-25(31(3,4)41(6,38)39)18-24-11-8-16-32-29(24)28/h7-19,21H,20H2,1-6H3,(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183792

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N4O3S/c1-21(2)34-30(36)35(26-11-13-27(14-12-26)39(5,37)38)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3,(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183795
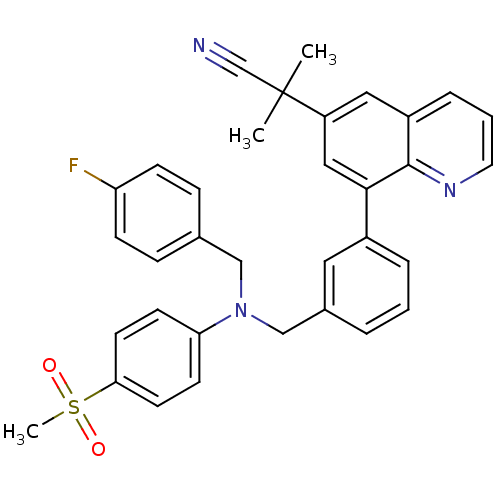
(2-(8-(3-(((4-fluorobenzyl)(4-(methylsulfonyl)pheny...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(Cc3ccc(F)cc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30FN3O2S/c1-34(2,23-36)28-19-27-8-5-17-37-33(27)32(20-28)26-7-4-6-25(18-26)22-38(21-24-9-11-29(35)12-10-24)30-13-15-31(16-14-30)41(3,39)40/h4-20H,21-22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183794

(3-isopropyl-1-(4-(methylsulfonyl)phenyl)-1-((3-(6-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H35N3O5S2/c1-21(2)33-30(35)34(26-12-14-27(15-13-26)40(5,36)37)20-22-9-7-10-23(17-22)28-19-25(31(3,4)41(6,38)39)18-24-11-8-16-32-29(24)28/h7-19,21H,20H2,1-6H3,(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183792

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N4O3S/c1-21(2)34-30(36)35(26-11-13-27(14-12-26)39(5,37)38)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3,(H,34,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183805

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)Nc3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30N4O3S/c1-34(2,23-35)27-20-26-11-8-18-36-32(26)31(21-27)25-10-7-9-24(19-25)22-38(33(39)37-28-12-5-4-6-13-28)29-14-16-30(17-15-29)42(3,40)41/h4-21H,22H2,1-3H3,(H,37,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183803

(CHEMBL206968 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES Cc1cc(no1)C(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H28N4O4S/c1-21-15-29(35-40-21)31(37)36(26-10-12-27(13-11-26)41(4,38)39)19-22-7-5-8-23(16-22)28-18-25(32(2,3)20-33)17-24-9-6-14-34-30(24)28/h5-18H,19H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183808

(CHEMBL383225 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)c3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H29N3O3S/c1-34(2,23-35)28-20-27-13-8-18-36-32(27)31(21-28)26-12-7-9-24(19-26)22-37(33(38)25-10-5-4-6-11-25)29-14-16-30(17-15-29)41(3,39)40/h4-21H,22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183805

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)Nc3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30N4O3S/c1-34(2,23-35)27-20-26-11-8-18-36-32(26)31(21-27)25-10-7-9-24(19-25)22-38(33(39)37-28-12-5-4-6-13-28)29-14-16-30(17-15-29)42(3,40)41/h4-21H,22H2,1-3H3,(H,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183794

(3-isopropyl-1-(4-(methylsulfonyl)phenyl)-1-((3-(6-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H35N3O5S2/c1-21(2)33-30(35)34(26-12-14-27(15-13-26)40(5,36)37)20-22-9-7-10-23(17-22)28-19-25(31(3,4)41(6,38)39)18-24-11-8-16-32-29(24)28/h7-19,21H,20H2,1-6H3,(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183803

(CHEMBL206968 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES Cc1cc(no1)C(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H28N4O4S/c1-21-15-29(35-40-21)31(37)36(26-10-12-27(13-11-26)41(4,38)39)19-22-7-5-8-23(16-22)28-18-25(32(2,3)20-33)17-24-9-6-14-34-30(24)28/h5-18H,19H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314653

((S)-3-amino-N-(2-chloro-5-(3-cyanopropyl)benzyl)-N...)Show SMILES Cc1cc(Cl)c(OCCCc2ccc(C[C@@H](CN)C(=O)N(Cc3cc(CCCC#N)ccc3Cl)C3CC3)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C34H38Cl3N3O2/c1-23-17-31(36)33(32(37)18-23)42-16-4-6-24-7-9-26(10-8-24)19-27(21-39)34(41)40(29-12-13-29)22-28-20-25(5-2-3-15-38)11-14-30(28)35/h7-11,14,17-18,20,27,29H,2-6,12-13,16,19,21-22,39H2,1H3/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314655
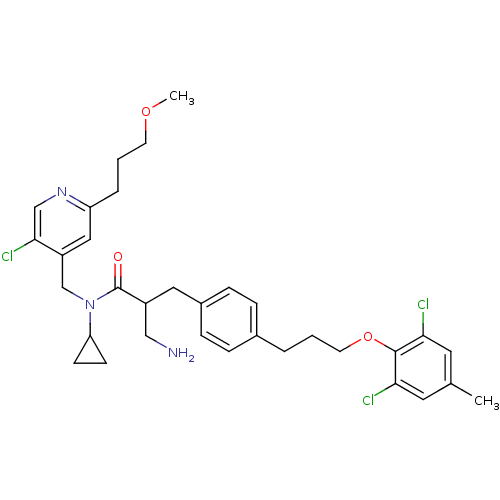
(3-amino-N-((5-chloro-2-(3-methoxypropyl)pyridin-4-...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)C(CN)Cc2ccc(CCCOc3c(Cl)cc(C)cc3Cl)cc2)c(Cl)cn1 Show InChI InChI=1S/C33H40Cl3N3O3/c1-22-15-29(34)32(30(35)16-22)42-14-3-5-23-7-9-24(10-8-23)17-25(19-37)33(40)39(28-11-12-28)21-26-18-27(6-4-13-41-2)38-20-31(26)36/h7-10,15-16,18,20,25,28H,3-6,11-14,17,19,21,37H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314648
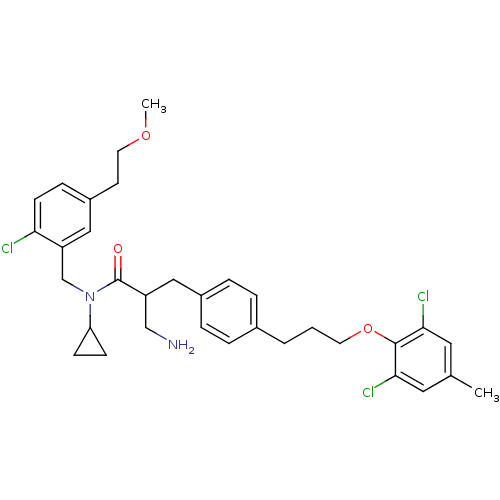
(3-amino-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)C(CN)Cc2ccc(CCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 Show InChI InChI=1S/C33H39Cl3N2O3/c1-22-16-30(35)32(31(36)17-22)41-14-3-4-23-5-7-24(8-6-23)18-26(20-37)33(39)38(28-10-11-28)21-27-19-25(13-15-40-2)9-12-29(27)34/h5-9,12,16-17,19,26,28H,3-4,10-11,13-15,18,20-21,37H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183808

(CHEMBL383225 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)c3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H29N3O3S/c1-34(2,23-35)28-20-27-13-8-18-36-32(27)31(21-28)26-12-7-9-24(19-26)22-37(33(38)25-10-5-4-6-11-25)29-14-16-30(17-15-29)41(3,39)40/h4-21H,22H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314647
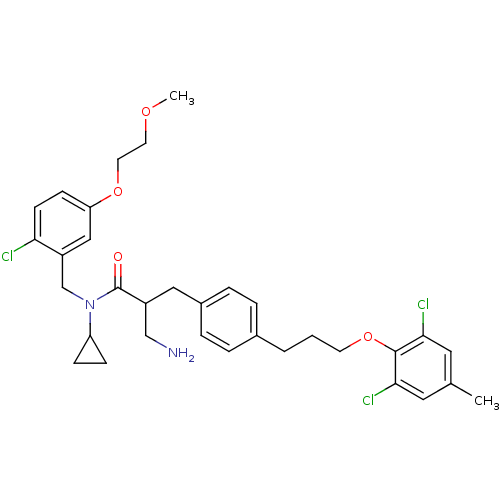
(3-amino-N-(2-chloro-5-(2-methoxyethoxy)benzyl)-N-c...)Show SMILES COCCOc1ccc(Cl)c(CN(C2CC2)C(=O)C(CN)Cc2ccc(CCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 Show InChI InChI=1S/C33H39Cl3N2O4/c1-22-16-30(35)32(31(36)17-22)42-13-3-4-23-5-7-24(8-6-23)18-25(20-37)33(39)38(27-9-10-27)21-26-19-28(11-12-29(26)34)41-15-14-40-2/h5-8,11-12,16-17,19,25,27H,3-4,9-10,13-15,18,20-21,37H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314651
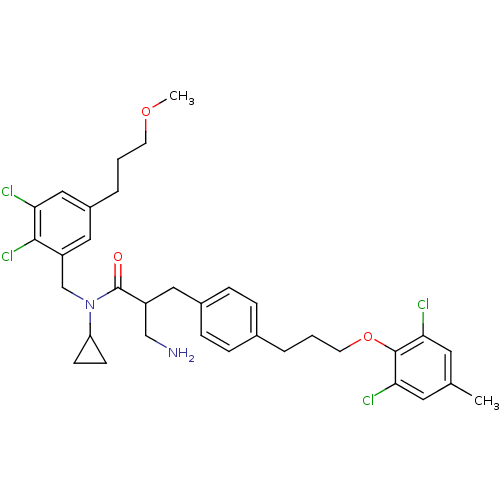
(3-amino-N-cyclopropyl-2-(4-(3-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(Cl)c(Cl)c(CN(C2CC2)C(=O)C(CN)Cc2ccc(CCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 Show InChI InChI=1S/C34H40Cl4N2O3/c1-22-15-30(36)33(31(37)16-22)43-14-4-5-23-7-9-24(10-8-23)17-26(20-39)34(41)40(28-11-12-28)21-27-18-25(6-3-13-42-2)19-29(35)32(27)38/h7-10,15-16,18-19,26,28H,3-6,11-14,17,20-21,39H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314645

((+/-)-3-amino-N-(2-chloro-5-(3-methoxypropyl)benzy...)Show SMILES COCCCc1ccc(Cl)c(CN(C2CC2)C(=O)C(CN)Cc2ccc(CCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 Show InChI InChI=1S/C34H41Cl3N2O3/c1-23-17-31(36)33(32(37)18-23)42-16-4-5-24-7-9-26(10-8-24)19-27(21-38)34(40)39(29-12-13-29)22-28-20-25(6-3-15-41-2)11-14-30(28)35/h7-11,14,17-18,20,27,29H,3-6,12-13,15-16,19,21-22,38H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314652
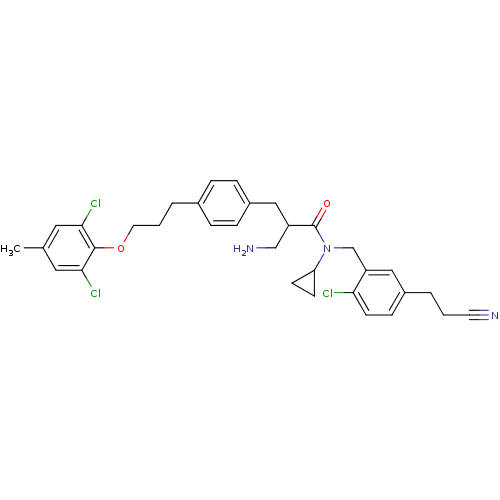
(3-amino-N-(2-chloro-5-(2-cyanoethyl)benzyl)-N-cycl...)Show SMILES Cc1cc(Cl)c(OCCCc2ccc(CC(CN)C(=O)N(Cc3cc(CCC#N)ccc3Cl)C3CC3)cc2)c(Cl)c1 Show InChI InChI=1S/C33H36Cl3N3O2/c1-22-16-30(35)32(31(36)17-22)41-15-3-5-23-6-8-25(9-7-23)18-26(20-38)33(40)39(28-11-12-28)21-27-19-24(4-2-14-37)10-13-29(27)34/h6-10,13,16-17,19,26,28H,2-5,11-12,15,18,20-21,38H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183803

(CHEMBL206968 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES Cc1cc(no1)C(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H28N4O4S/c1-21-15-29(35-40-21)31(37)36(26-10-12-27(13-11-26)41(4,38)39)19-22-7-5-8-23(16-22)28-18-25(32(2,3)20-33)17-24-9-6-14-34-30(24)28/h5-18H,19H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183808

(CHEMBL383225 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)c3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H29N3O3S/c1-34(2,23-35)28-20-27-13-8-18-36-32(27)31(21-28)26-12-7-9-24(19-26)22-37(33(38)25-10-5-4-6-11-25)29-14-16-30(17-15-29)41(3,39)40/h4-21H,22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183798
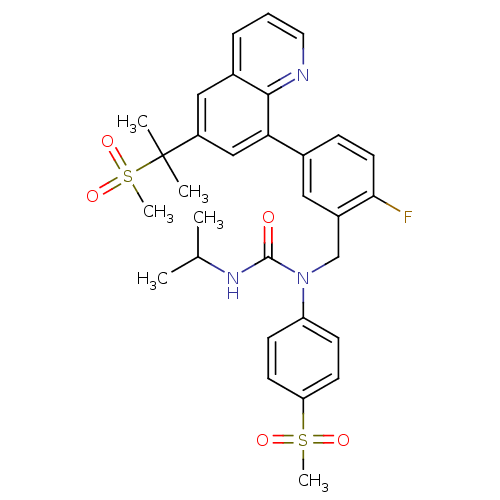
(1-((2-fluoro-5-(6-(2-(methylsulfonyl)propan-2-yl)q...)Show SMILES CC(C)NC(=O)N(Cc1cc(ccc1F)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H34FN3O5S2/c1-20(2)34-30(36)35(25-10-12-26(13-11-25)41(5,37)38)19-23-16-21(9-14-28(23)32)27-18-24(31(3,4)42(6,39)40)17-22-8-7-15-33-29(22)27/h7-18,20H,19H2,1-6H3,(H,34,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183791
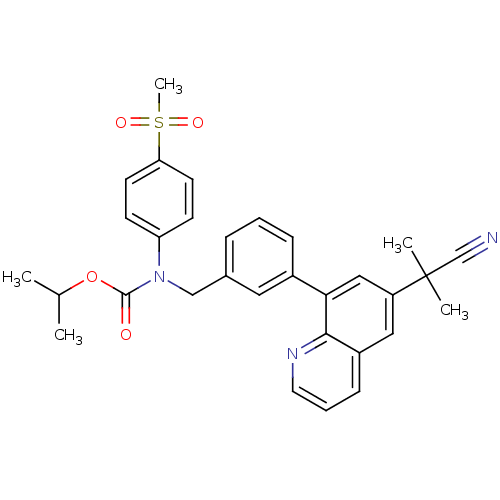
(CHEMBL209295 | isopropyl (3-(6-(2-cyanopropan-2-yl...)Show SMILES CC(C)OC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H31N3O4S/c1-21(2)38-30(35)34(26-11-13-27(14-12-26)39(5,36)37)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183791

(CHEMBL209295 | isopropyl (3-(6-(2-cyanopropan-2-yl...)Show SMILES CC(C)OC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H31N3O4S/c1-21(2)38-30(35)34(26-11-13-27(14-12-26)39(5,36)37)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183791

(CHEMBL209295 | isopropyl (3-(6-(2-cyanopropan-2-yl...)Show SMILES CC(C)OC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H31N3O4S/c1-21(2)38-30(35)34(26-11-13-27(14-12-26)39(5,36)37)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314656
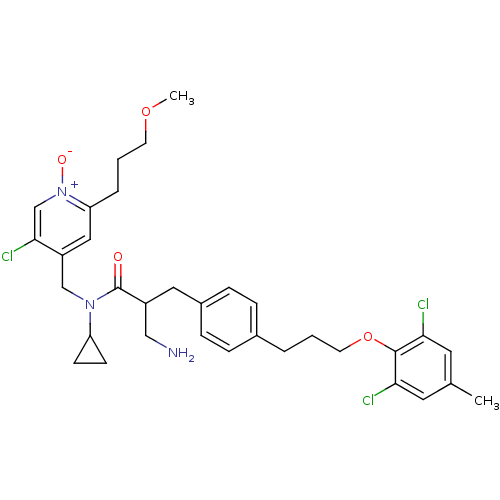
(4-((3-amino-N-cyclopropyl-2-(4-(3-(2,6-dichloro-4-...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)C(CN)Cc2ccc(CCCOc3c(Cl)cc(C)cc3Cl)cc2)c(Cl)c[n+]1[O-] Show InChI InChI=1S/C33H40Cl3N3O4/c1-22-15-29(34)32(30(35)16-22)43-14-3-5-23-7-9-24(10-8-23)17-25(19-37)33(40)38(27-11-12-27)20-26-18-28(6-4-13-42-2)39(41)21-31(26)36/h7-10,15-16,18,21,25,27H,3-6,11-14,17,19-20,37H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347344
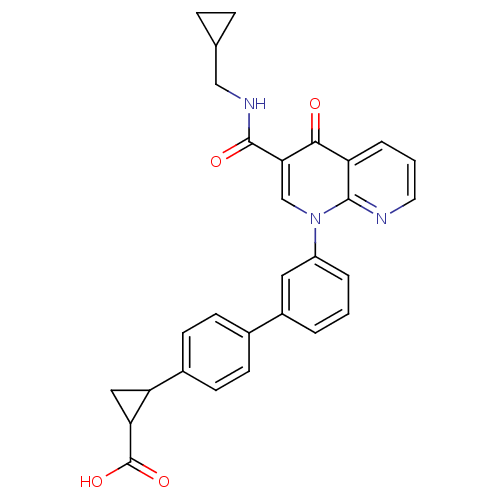
(CHEMBL1801156)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C29H25N3O4/c33-26-22-5-2-12-30-27(22)32(16-25(26)28(34)31-15-17-6-7-17)21-4-1-3-20(13-21)18-8-10-19(11-9-18)23-14-24(23)29(35)36/h1-5,8-13,16-17,23-24H,6-7,14-15H2,(H,31,34)(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50347344

(CHEMBL1801156)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C29H25N3O4/c33-26-22-5-2-12-30-27(22)32(16-25(26)28(34)31-15-17-6-7-17)21-4-1-3-20(13-21)18-8-10-19(11-9-18)23-14-24(23)29(35)36/h1-5,8-13,16-17,23-24H,6-7,14-15H2,(H,31,34)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4B |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347344

(CHEMBL1801156)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C29H25N3O4/c33-26-22-5-2-12-30-27(22)32(16-25(26)28(34)31-15-17-6-7-17)21-4-1-3-20(13-21)18-8-10-19(11-9-18)23-14-24(23)29(35)36/h1-5,8-13,16-17,23-24H,6-7,14-15H2,(H,31,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183797
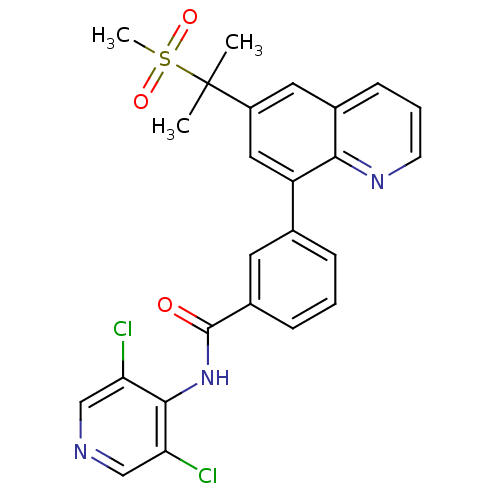
(CHEMBL207996 | N-(3,5-dichloropyridin-4-yl)-3-(6-(...)Show SMILES CC(C)(c1cc(-c2cccc(c2)C(=O)Nc2c(Cl)cncc2Cl)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C25H21Cl2N3O3S/c1-25(2,34(3,32)33)18-11-16-8-5-9-29-22(16)19(12-18)15-6-4-7-17(10-15)24(31)30-23-20(26)13-28-14-21(23)27/h4-14H,1-3H3,(H,28,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50314644
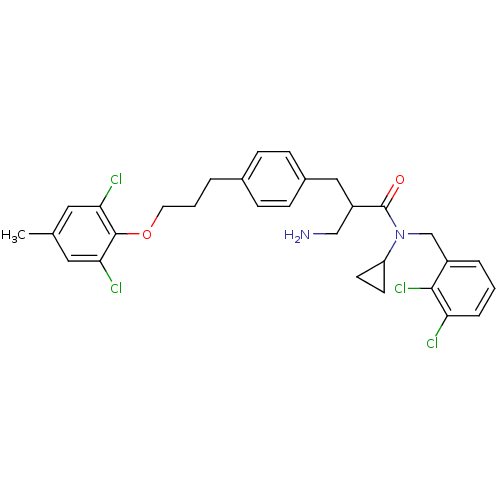
(3-amino-N-cyclopropyl-2-(4-(3-(2,6-dichloro-4-meth...)Show SMILES Cc1cc(Cl)c(OCCCc2ccc(CC(CN)C(=O)N(Cc3cccc(Cl)c3Cl)C3CC3)cc2)c(Cl)c1 Show InChI InChI=1S/C30H32Cl4N2O2/c1-19-14-26(32)29(27(33)15-19)38-13-3-4-20-7-9-21(10-8-20)16-23(17-35)30(37)36(24-11-12-24)18-22-5-2-6-25(31)28(22)34/h2,5-10,14-15,23-24H,3-4,11-13,16-18,35H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer at pH 7.4 |
Bioorg Med Chem Lett 20: 2204-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.036
BindingDB Entry DOI: 10.7270/Q2W37WGD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183804
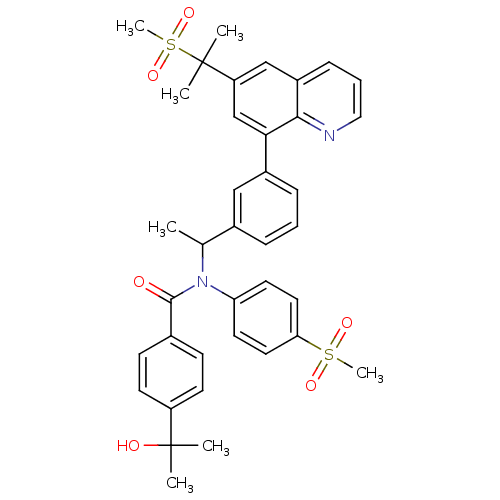
(4-(2-hydroxypropan-2-yl)-N-(4-(methylsulfonyl)phen...)Show SMILES CC(N(C(=O)c1ccc(cc1)C(C)(C)O)c1ccc(cc1)S(C)(=O)=O)c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C38H40N2O6S2/c1-25(40(32-17-19-33(20-18-32)47(6,43)44)36(41)26-13-15-30(16-14-26)37(2,3)42)27-10-8-11-28(22-27)34-24-31(38(4,5)48(7,45)46)23-29-12-9-21-39-35(29)34/h8-25,42H,1-7H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50347349
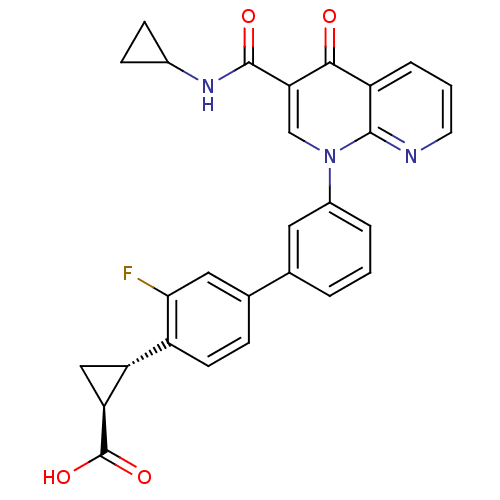
(CHEMBL1801161)Show SMILES OC(=O)[C@H]1C[C@@H]1c1ccc(cc1F)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 |r| Show InChI InChI=1S/C28H22FN3O4/c29-24-12-16(6-9-19(24)21-13-22(21)28(35)36)15-3-1-4-18(11-15)32-14-23(27(34)31-17-7-8-17)25(33)20-5-2-10-30-26(20)32/h1-6,9-12,14,17,21-22H,7-8,13H2,(H,31,34)(H,35,36)/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4B |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347349

(CHEMBL1801161)Show SMILES OC(=O)[C@H]1C[C@@H]1c1ccc(cc1F)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 |r| Show InChI InChI=1S/C28H22FN3O4/c29-24-12-16(6-9-19(24)21-13-22(21)28(35)36)15-3-1-4-18(11-15)32-14-23(27(34)31-17-7-8-17)25(33)20-5-2-10-30-26(20)32/h1-6,9-12,14,17,21-22H,7-8,13H2,(H,31,34)(H,35,36)/t21-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347349

(CHEMBL1801161)Show SMILES OC(=O)[C@H]1C[C@@H]1c1ccc(cc1F)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 |r| Show InChI InChI=1S/C28H22FN3O4/c29-24-12-16(6-9-19(24)21-13-22(21)28(35)36)15-3-1-4-18(11-15)32-14-23(27(34)31-17-7-8-17)25(33)20-5-2-10-30-26(20)32/h1-6,9-12,14,17,21-22H,7-8,13H2,(H,31,34)(H,35,36)/t21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183795

(2-(8-(3-(((4-fluorobenzyl)(4-(methylsulfonyl)pheny...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(Cc3ccc(F)cc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30FN3O2S/c1-34(2,23-36)28-19-27-8-5-17-37-33(27)32(20-28)26-7-4-6-25(18-26)22-38(21-24-9-11-29(35)12-10-24)30-13-15-31(16-14-30)41(3,39)40/h4-20H,21-22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183799
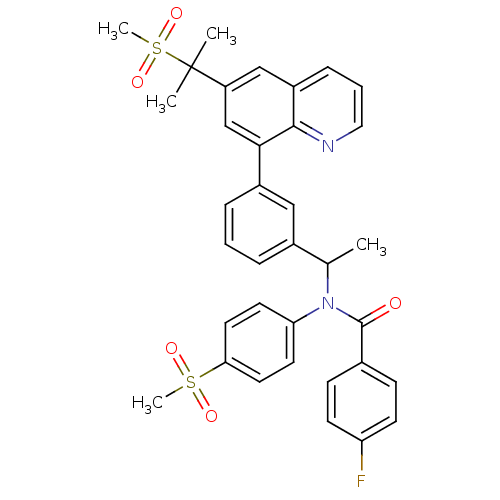
(4-fluoro-N-(4-(methylsulfonyl)phenyl)-N-(1-(3-(6-(...)Show SMILES CC(N(C(=O)c1ccc(F)cc1)c1ccc(cc1)S(C)(=O)=O)c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C35H33FN2O5S2/c1-23(38(34(39)24-11-13-29(36)14-12-24)30-15-17-31(18-16-30)44(4,40)41)25-8-6-9-26(20-25)32-22-28(35(2,3)45(5,42)43)21-27-10-7-19-37-33(27)32/h6-23H,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183804

(4-(2-hydroxypropan-2-yl)-N-(4-(methylsulfonyl)phen...)Show SMILES CC(N(C(=O)c1ccc(cc1)C(C)(C)O)c1ccc(cc1)S(C)(=O)=O)c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C38H40N2O6S2/c1-25(40(32-17-19-33(20-18-32)47(6,43)44)36(41)26-13-15-30(16-14-26)37(2,3)42)27-10-8-11-28(22-27)34-24-31(38(4,5)48(7,45)46)23-29-12-9-21-39-35(29)34/h8-25,42H,1-7H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183801
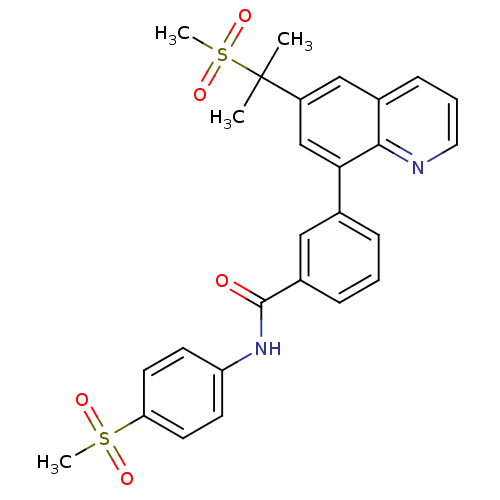
(CHEMBL208149 | N-(4-(methylsulfonyl)phenyl)-3-(6-(...)Show SMILES CC(C)(c1cc(-c2cccc(c2)C(=O)Nc2ccc(cc2)S(C)(=O)=O)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C27H26N2O5S2/c1-27(2,36(4,33)34)21-16-19-9-6-14-28-25(19)24(17-21)18-7-5-8-20(15-18)26(30)29-22-10-12-23(13-11-22)35(3,31)32/h5-17H,1-4H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50183799

(4-fluoro-N-(4-(methylsulfonyl)phenyl)-N-(1-(3-(6-(...)Show SMILES CC(N(C(=O)c1ccc(F)cc1)c1ccc(cc1)S(C)(=O)=O)c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C35H33FN2O5S2/c1-23(38(34(39)24-11-13-29(36)14-12-24)30-15-17-31(18-16-30)44(4,40)41)25-8-6-9-26(20-25)32-22-28(35(2,3)45(5,42)43)21-27-10-7-19-37-33(27)32/h6-23H,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4B |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347343
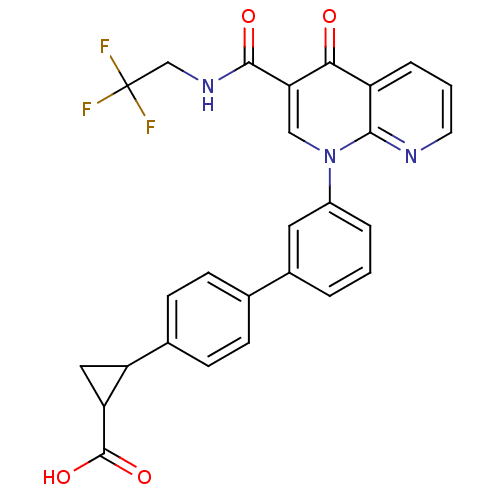
(CHEMBL1801155)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC(F)(F)F)c(=O)c2cccnc12 Show InChI InChI=1S/C27H20F3N3O4/c28-27(29,30)14-32-25(35)22-13-33(24-19(23(22)34)5-2-10-31-24)18-4-1-3-17(11-18)15-6-8-16(9-7-15)20-12-21(20)26(36)37/h1-11,13,20-21H,12,14H2,(H,32,35)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50347343

(CHEMBL1801155)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC(F)(F)F)c(=O)c2cccnc12 Show InChI InChI=1S/C27H20F3N3O4/c28-27(29,30)14-32-25(35)22-13-33(24-19(23(22)34)5-2-10-31-24)18-4-1-3-17(11-18)15-6-8-16(9-7-15)20-12-21(20)26(36)37/h1-11,13,20-21H,12,14H2,(H,32,35)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4B |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347343

(CHEMBL1801155)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC(F)(F)F)c(=O)c2cccnc12 Show InChI InChI=1S/C27H20F3N3O4/c28-27(29,30)14-32-25(35)22-13-33(24-19(23(22)34)5-2-10-31-24)18-4-1-3-17(11-18)15-6-8-16(9-7-15)20-12-21(20)26(36)37/h1-11,13,20-21H,12,14H2,(H,32,35)(H,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data