 Found 1859 hits with Last Name = 'gobbi' and Initial = 'l'
Found 1859 hits with Last Name = 'gobbi' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603932

((+)-trans-Ethyl 2-ethyl-2-{[6-({-2-[(fluoromethoxy...)Show SMILES CCOC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2COCF)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603936

((+)-trans-2-Fluoroethyl 2-ethyl-2-{[6-{[-2-(hydrox...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CO)n1)C(=O)OCCF |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603935
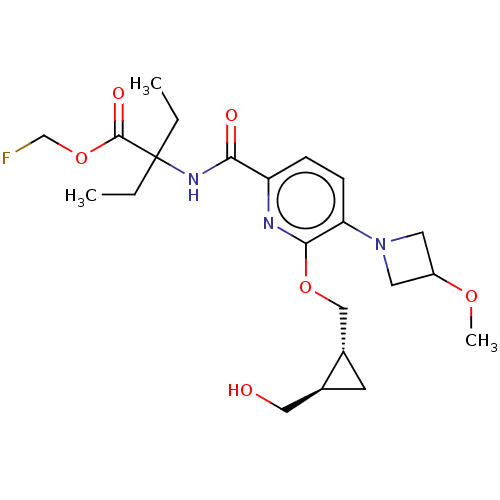
((−)-trans-Fluoromethyl 2-ethyl-2-{[6-{[-2-(h...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@@H]2C[C@H]2CO)n1)C(=O)OCF | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643722

((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1n[nH]c2=O |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603926
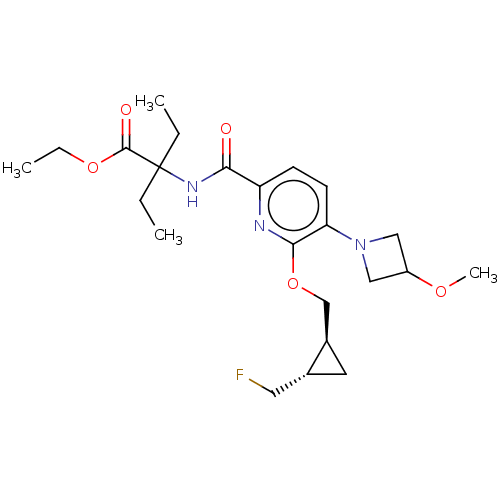
(Ethyl 2-ethyl-2-{[6-{[(1S,2S)-2-(fluoromethyl)cycl...)Show SMILES CCOC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CF)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50601872

(CHEMBL5174733)Show SMILES [11CH3]Oc1ccc2c(NC(=O)Nc3cccc(c3)C(F)(F)F)ccnc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643732
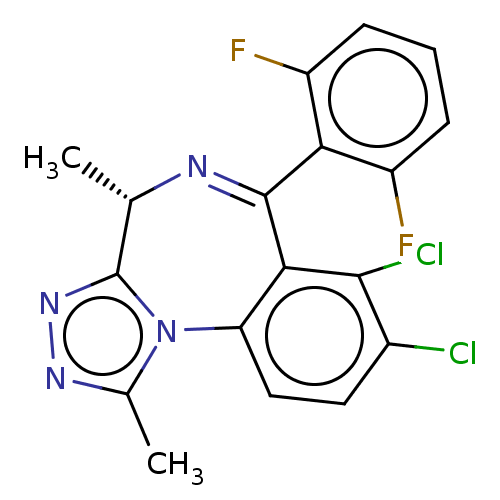
((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-1,4-dimet...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c(C)nnc12 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603927

(Ethyl 2-ethyl-2-{[6-{[(1R,2S)-2-(fluoromethyl)cycl...)Show SMILES CCOC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@@H]2C[C@@H]2CF)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603938
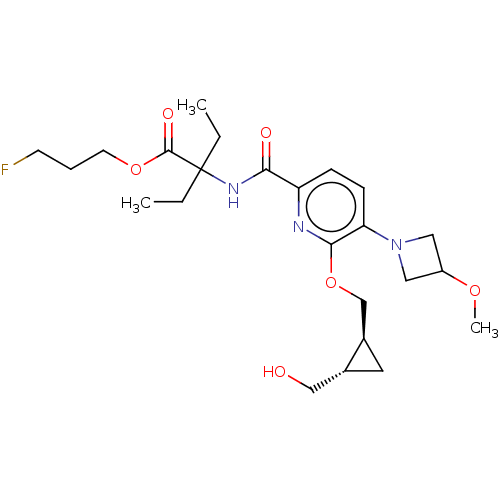
(3-Fluoropropyl 2-ethyl-2-{[6-{[(1S,2S)-2-(hydroxym...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CO)n1)C(=O)OCCCF |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072352
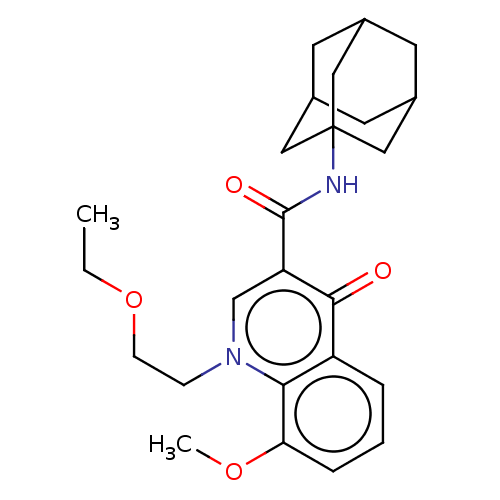
(CHEMBL3409318)Show SMILES CCOCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c(=O)c2cccc(OC)c12 |TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C25H32N2O4/c1-3-31-8-7-27-15-20(23(28)19-5-4-6-21(30-2)22(19)27)24(29)26-25-12-16-9-17(13-25)11-18(10-16)14-25/h4-6,15-18H,3,7-14H2,1-2H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643728

((4S)-8-bromo-7-chloro-6-(2,6-difluorophenyl)-1,4-d...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Br)ccc2-n2c(C)nnc12 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643750

((4S)-7-chloro-6-(2,6-difluorophenyl)-8-iodo-1,4-di...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(I)ccc2-n2c(C)nnc12 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM419188
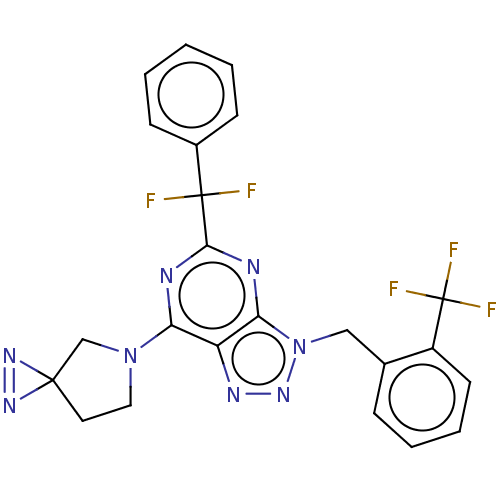
(5-[Difluoro(phenyl)methyl]-7-(1,2,5-triazaspiro[2....)Show SMILES FC(F)(F)c1ccccc1Cn1nnc2c(nc(nc12)C(F)(F)c1ccccc1)N1CCC2(C1)N=N2 |c:39| Show InChI InChI=1S/C23H17F5N8/c24-22(25,15-7-2-1-3-8-15)20-29-18(35-11-10-21(13-35)32-33-21)17-19(30-20)36(34-31-17)12-14-6-4-5-9-16(14)23(26,27)28/h1-9H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The affinity of the compounds of the invention for cannabinoid receptors was determined using recommended amounts of membrane preparations (PerkinElm... |
US Patent US10457684 (2019)
BindingDB Entry DOI: 10.7270/Q2HM5BT8 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643717

((4S)-8-bromo-7-chloro-6-(2,6-difluorophenyl)-4-met...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Br)ccc2-n2cnnc12 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603938

(3-Fluoropropyl 2-ethyl-2-{[6-{[(1S,2S)-2-(hydroxym...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CO)n1)C(=O)OCCCF |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643720
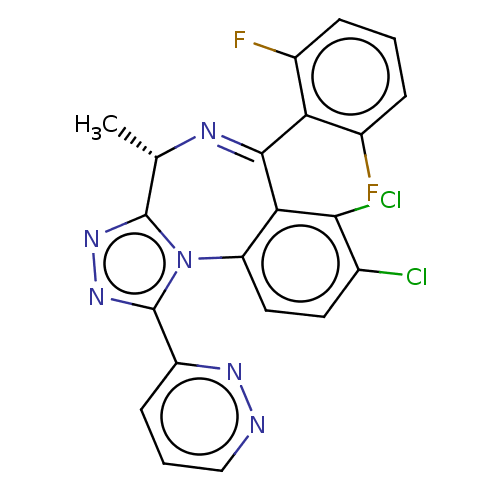
((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1nnc2-c1cccnn1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM419309

(2-[[5-tert-Butyl-7-(3,3-difluoropyrrolidin-1-yl)tr...)Show SMILES CC(C)(C)c1nc(N2CCC(F)(F)C2)c2nnn(Cc3c(cccc3S(F)(=O)=O)C#C)c2n1 Show InChI InChI=1S/C21H21F3N6O2S/c1-5-13-7-6-8-15(33(24,31)32)14(13)11-30-18-16(27-28-30)17(25-19(26-18)20(2,3)4)29-10-9-21(22,23)12-29/h1,6-8H,9-12H2,2-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
he affinity of the compounds of the invention for cannabinoid receptors was determined using recommended amounts of membrane preparations (PerkinElme... |
US Patent US10457685 (2019)
BindingDB Entry DOI: 10.7270/Q2CV4M4D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643749

(US20240002393, Example 101 | [(4R)-8-bromo-7-chlor...)Show SMILES Cc1nnc2[C@H](CO)N=C(c3c(F)cccc3F)c3c(Cl)c(Br)ccc3-n12 |r,t:8| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643719

((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1nnc2-c1cnn(C)c1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643718

((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1nnc2-c1ccncn1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643715
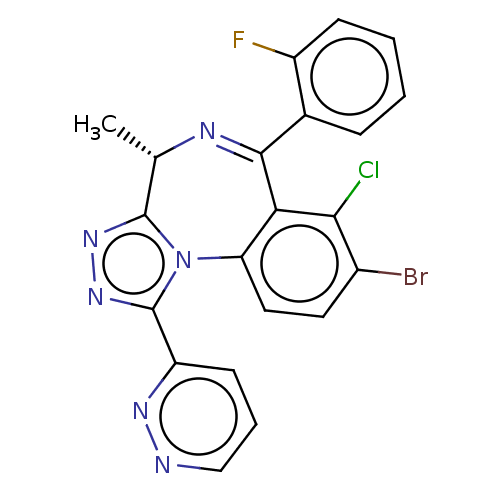
((4S)-8-bromo-7-chloro-6-(2-fluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2ccccc2F)c2c(Cl)c(Br)ccc2-n2c1nnc2-c1cccnn1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM106438
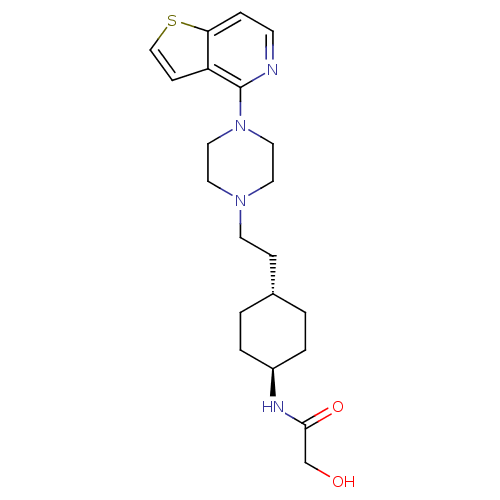
(US8586579, 27)Show SMILES OCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nccc3sccc23)CC1 |r,wU:5.4,wD:8.8,(9.63,1.85,;8.86,.52,;7.32,.52,;6.55,-.82,;6.55,1.85,;5.01,1.85,;4.23,.52,;2.69,.52,;1.92,1.85,;.38,1.85,;-.39,.52,;-1.93,.52,;-2.7,-.82,;-4.24,-.82,;-5.01,.52,;-4.24,1.85,;-2.7,1.85,;-6.55,.52,;-7.32,1.85,;-8.86,1.85,;-9.63,.52,;-8.86,-.82,;-9.33,-2.28,;-8.09,-3.19,;-6.84,-2.28,;-7.32,-.82,;2.69,3.19,;4.23,3.19,)| Show InChI InChI=1S/C21H30N4O2S/c26-15-20(27)23-17-3-1-16(2-4-17)6-9-24-10-12-25(13-11-24)21-18-7-14-28-19(18)5-8-22-21/h5,7-8,14,16-17,26H,1-4,6,9-13,15H2,(H,23,27)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... |
US Patent US8586579 (2013)
BindingDB Entry DOI: 10.7270/Q2QV3K55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM106438

(US8586579, 27)Show SMILES OCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nccc3sccc23)CC1 |r,wU:5.4,wD:8.8,(9.63,1.85,;8.86,.52,;7.32,.52,;6.55,-.82,;6.55,1.85,;5.01,1.85,;4.23,.52,;2.69,.52,;1.92,1.85,;.38,1.85,;-.39,.52,;-1.93,.52,;-2.7,-.82,;-4.24,-.82,;-5.01,.52,;-4.24,1.85,;-2.7,1.85,;-6.55,.52,;-7.32,1.85,;-8.86,1.85,;-9.63,.52,;-8.86,-.82,;-9.33,-2.28,;-8.09,-3.19,;-6.84,-2.28,;-7.32,-.82,;2.69,3.19,;4.23,3.19,)| Show InChI InChI=1S/C21H30N4O2S/c26-15-20(27)23-17-3-1-16(2-4-17)6-9-24-10-12-25(13-11-24)21-18-7-14-28-19(18)5-8-22-21/h5,7-8,14,16-17,26H,1-4,6,9-13,15H2,(H,23,27)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... |
US Patent US8586579 (2013)
BindingDB Entry DOI: 10.7270/Q2QV3K55 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643736
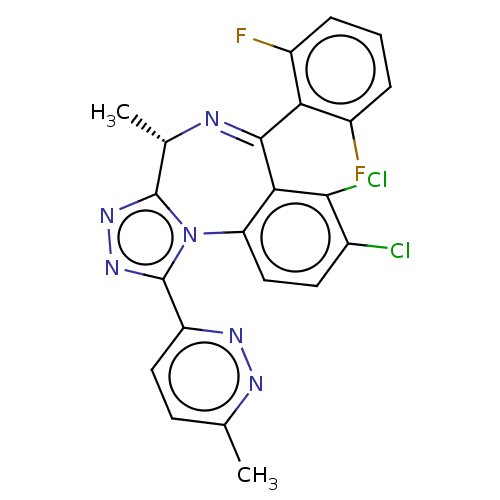
((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1nnc2-c1ccc(C)nn1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180718
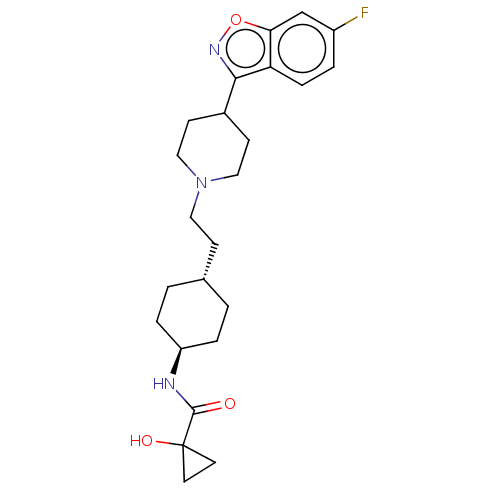
(US8829029, 18)Show SMILES OC1(CC1)C(=O)N[C@H]1CC[C@H](CCN2CCC(CC2)c2noc3cc(F)ccc23)CC1 |r,wU:7.7,wD:10.11,(7.88,5.3,;6.39,4.9,;5.99,3.41,;7.48,3.81,;5.99,6.39,;7.08,7.48,;4.5,6.79,;3.41,5.7,;3.81,4.21,;2.72,3.12,;1.23,3.52,;.15,2.43,;-1.34,2.83,;-2.43,1.74,;-2.03,.25,;-3.12,-.84,;-4.61,-.44,;-5.01,1.05,;-3.92,2.14,;-5.7,-1.53,;-7.18,-1.13,;-7.88,-3.7,;-6.39,-4.1,;-5.99,-5.59,;-4.5,-5.99,;-4.1,-7.48,;-3.41,-4.9,;-3.81,-3.41,;-5.3,-3.01,;.84,5.01,;1.93,6.1,)| Show InChI InChI=1S/C24H32FN3O3/c25-18-3-6-20-21(15-18)31-27-22(20)17-8-13-28(14-9-17)12-7-16-1-4-19(5-2-16)26-23(29)24(30)10-11-24/h3,6,15-17,19,30H,1-2,4-5,7-14H2,(H,26,29)/t16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643743
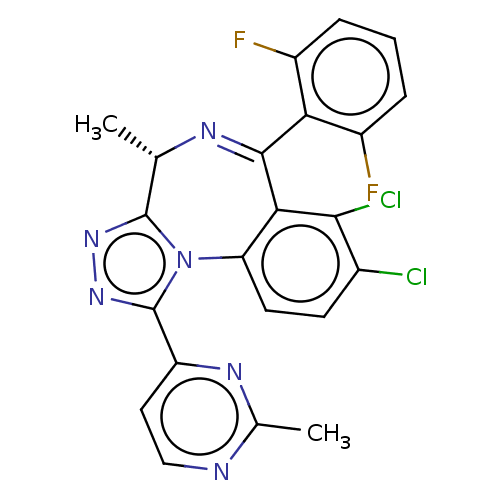
((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1nnc2-c1ccnc(C)n1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM106429
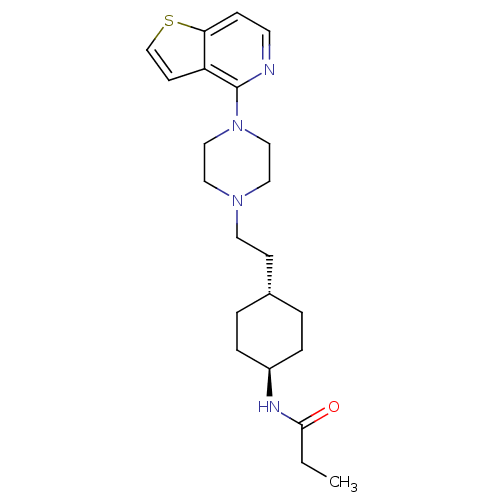
(US8586579, 18)Show SMILES CCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nccc3sccc23)CC1 |r,wU:5.4,wD:8.8,(9.63,1.85,;8.85,.52,;7.31,.52,;6.54,-.82,;6.54,1.85,;5,1.85,;4.23,.52,;2.69,.52,;1.93,1.85,;.38,1.85,;-.38,.52,;-1.93,.52,;-2.69,-.82,;-4.23,-.82,;-5,.52,;-4.23,1.85,;-2.69,1.85,;-6.54,.52,;-7.31,1.85,;-8.85,1.85,;-9.63,.52,;-8.85,-.82,;-9.33,-2.28,;-8.08,-3.19,;-6.84,-2.28,;-7.31,-.82,;2.69,3.19,;4.23,3.19,)| Show InChI InChI=1S/C22H32N4OS/c1-2-21(27)24-18-5-3-17(4-6-18)8-11-25-12-14-26(15-13-25)22-19-9-16-28-20(19)7-10-23-22/h7,9-10,16-18H,2-6,8,11-15H2,1H3,(H,24,27)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... |
US Patent US8586579 (2013)
BindingDB Entry DOI: 10.7270/Q2QV3K55 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180713
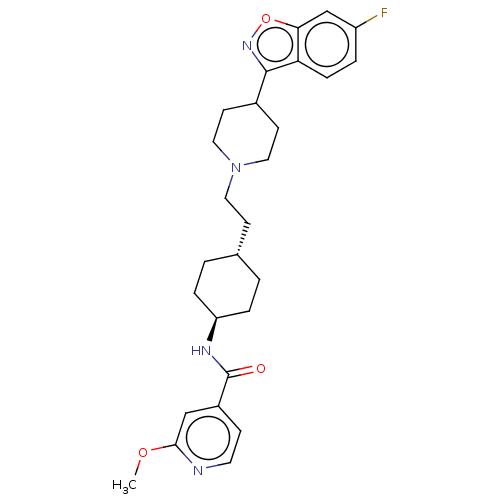
(US8829029, 13)Show SMILES COc1cc(ccn1)C(=O)N[C@H]1CC[C@H](CCN2CCC(CC2)c2noc3cc(F)ccc23)CC1 |r,wU:11.11,wD:14.15,(9.02,1.13,;8.62,2.62,;7.13,3.01,;6.73,4.5,;5.25,4.9,;4.16,3.81,;4.56,2.32,;6.04,1.93,;4.85,6.39,;5.94,7.48,;3.36,6.79,;2.27,5.7,;2.67,4.21,;1.58,3.12,;.09,3.52,;-1,2.43,;-2.48,2.83,;-3.57,1.74,;-3.17,.25,;-4.26,-.84,;-5.75,-.44,;-6.15,1.05,;-5.06,2.14,;-6.84,-1.53,;-8.33,-1.13,;-9.02,-3.7,;-7.53,-4.1,;-7.13,-5.59,;-5.64,-5.99,;-5.25,-7.48,;-4.56,-4.9,;-4.95,-3.41,;-6.44,-3.01,;-.31,5.01,;.78,6.1,)| Show InChI InChI=1S/C27H33FN4O3/c1-34-25-16-20(8-12-29-25)27(33)30-22-5-2-18(3-6-22)9-13-32-14-10-19(11-15-32)26-23-7-4-21(28)17-24(23)35-31-26/h4,7-8,12,16-19,22H,2-3,5-6,9-11,13-15H2,1H3,(H,30,33)/t18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180752
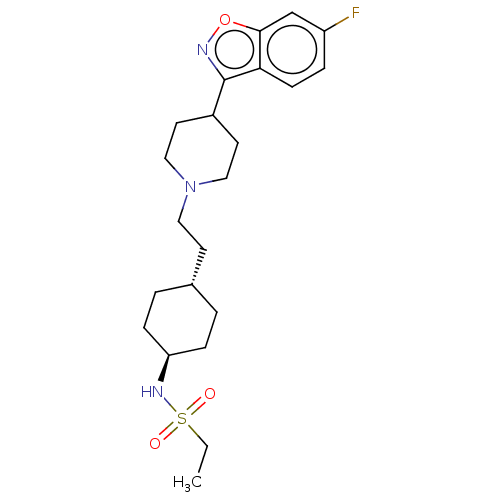
(US8829029, 47)Show SMILES CCS(=O)(=O)N[C@H]1CC[C@H](CCN2CCC(CC2)c2noc3cc(F)ccc23)CC1 |r,wU:6.5,wD:9.9,(8.67,2.41,;8.67,3.95,;7.34,4.72,;8.11,6.06,;6.57,3.39,;6,5.49,;4.67,4.72,;4.67,3.18,;3.33,2.41,;2,3.18,;.67,2.41,;-.67,3.18,;-2,2.41,;-2,.87,;-3.33,.1,;-4.67,.87,;-4.67,2.41,;-3.33,3.18,;-6,.1,;-7.34,.87,;-8.67,-1.44,;-7.34,-2.21,;-7.34,-3.75,;-6,-4.52,;-6,-6.06,;-4.67,-3.75,;-4.67,-2.21,;-6,-1.44,;2,4.72,;3.33,5.49,)| Show InChI InChI=1S/C22H32FN3O3S/c1-2-30(27,28)25-19-6-3-16(4-7-19)9-12-26-13-10-17(11-14-26)22-20-8-5-18(23)15-21(20)29-24-22/h5,8,15-17,19,25H,2-4,6-7,9-14H2,1H3/t16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM106435
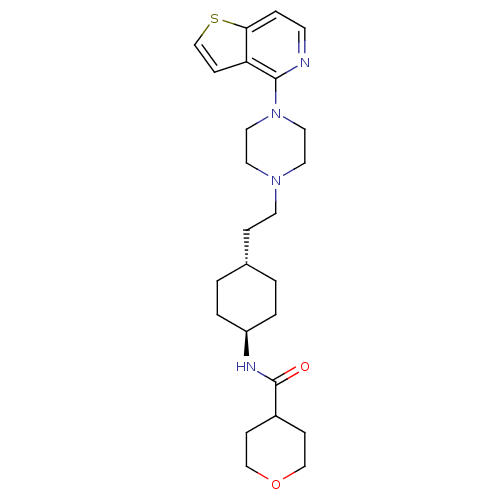
(US8586579, 24)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nccc3sccc23)CC1)C1CCOCC1 |r,wU:3.2,wD:6.6,(5.39,-.82,;6.16,.52,;5.39,1.85,;3.85,1.85,;3.08,.52,;1.54,.52,;.77,1.85,;-.77,1.85,;-1.54,.52,;-3.08,.52,;-3.85,-.82,;-5.39,-.82,;-6.16,.52,;-5.39,1.85,;-3.85,1.85,;-7.7,.52,;-8.47,1.85,;-10.01,1.85,;-10.78,.52,;-10.01,-.82,;-10.49,-2.28,;-9.24,-3.19,;-7.99,-2.28,;-8.47,-.82,;1.54,3.19,;3.08,3.19,;7.7,.52,;8.47,-.82,;10.01,-.82,;10.78,.52,;10.01,1.85,;8.47,1.85,)| Show InChI InChI=1S/C25H36N4O2S/c30-25(20-7-16-31-17-8-20)27-21-3-1-19(2-4-21)6-11-28-12-14-29(15-13-28)24-22-9-18-32-23(22)5-10-26-24/h5,9-10,18-21H,1-4,6-8,11-17H2,(H,27,30)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... |
US Patent US8586579 (2013)
BindingDB Entry DOI: 10.7270/Q2QV3K55 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603952

(3-Fluoropropyl 2-ethyl-2-{[5-(3-fluoroazetidin-1-y...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(F)C2)c(OC[C@H]2C[C@@H]2CO)n1)C(=O)OCCCF |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180723
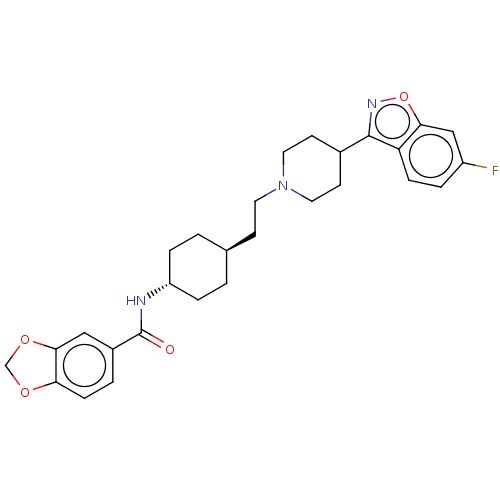
(US8829029, 23)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CC[C@H]2CC[C@@H](CC2)NC(=O)c2ccc3OCOc3c2)CC1 |r,wU:19.24,wD:16.17,(-4.99,-7.48,;-5.39,-5.99,;-4.3,-4.9,;-4.7,-3.41,;-6.18,-3.01,;-6.58,-1.53,;-8.07,-1.13,;-8.76,-3.7,;-7.27,-4.1,;-6.87,-5.59,;-5.49,-.44,;-4.01,-.84,;-2.92,.25,;-3.32,1.74,;-2.23,2.83,;-.74,2.43,;.35,3.52,;1.84,3.12,;2.93,4.21,;2.53,5.7,;1.04,6.1,;-.05,5.01,;3.62,6.79,;5.1,6.39,;6.19,7.48,;5.5,4.9,;4.41,3.81,;4.81,2.32,;6.3,1.93,;7,.55,;8.52,.79,;8.76,2.32,;7.39,3.01,;6.99,4.5,;-4.8,2.14,;-5.89,1.05,)| Show InChI InChI=1S/C28H32FN3O4/c29-21-4-7-23-25(16-21)36-31-27(23)19-10-13-32(14-11-19)12-9-18-1-5-22(6-2-18)30-28(33)20-3-8-24-26(15-20)35-17-34-24/h3-4,7-8,15-16,18-19,22H,1-2,5-6,9-14,17H2,(H,30,33)/t18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180709
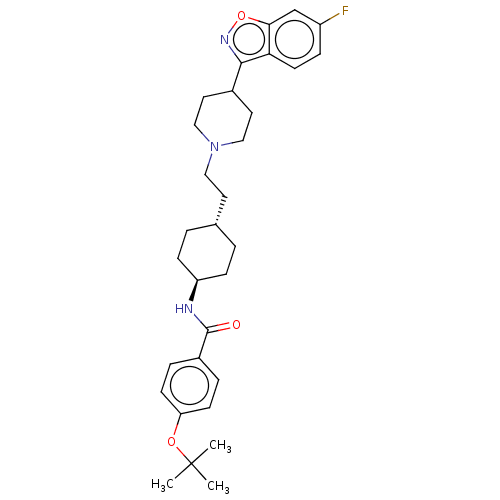
(US8829029, 9)Show SMILES CC(C)(C)Oc1ccc(cc1)C(=O)N[C@H]1CC[C@H](CCN2CCC(CC2)c2noc3cc(F)ccc23)CC1 |r,wU:14.14,wD:17.18,(5.64,-1.05,;7.18,-1.05,;6.1,-2.14,;8.67,-1.45,;6.79,.44,;6.39,1.93,;4.9,2.32,;4.5,3.81,;5.59,4.9,;7.08,4.5,;7.48,3.01,;5.19,6.39,;6.28,7.48,;3.7,6.79,;2.62,5.7,;3.01,4.21,;1.93,3.12,;.44,3.52,;-.65,2.43,;-2.14,2.83,;-3.23,1.74,;-2.83,.25,;-3.92,-.84,;-5.41,-.44,;-5.8,1.05,;-4.72,2.14,;-6.49,-1.53,;-7.98,-1.13,;-8.67,-3.7,;-7.18,-4.1,;-6.79,-5.59,;-5.3,-5.99,;-4.9,-7.48,;-4.21,-4.9,;-4.61,-3.41,;-6.1,-3.01,;.04,5.01,;1.13,6.1,)| Show InChI InChI=1S/C31H40FN3O3/c1-31(2,3)37-26-11-6-23(7-12-26)30(36)33-25-9-4-21(5-10-25)14-17-35-18-15-22(16-19-35)29-27-13-8-24(32)20-28(27)38-34-29/h6-8,11-13,20-22,25H,4-5,9-10,14-19H2,1-3H3,(H,33,36)/t21-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180698
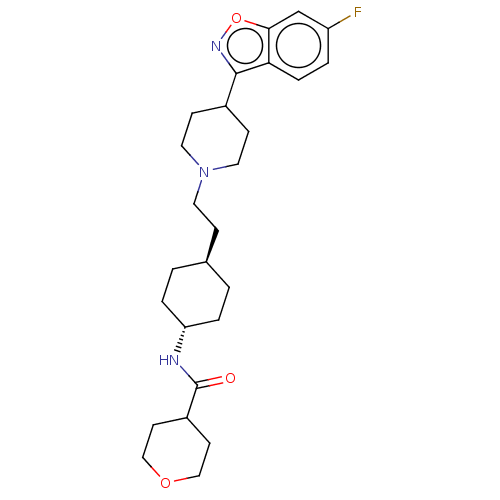
(US8829029, 2)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CC[C@H]2CC[C@@H](CC2)NC(=O)C2CCOCC2)CC1 |r,wU:19.24,wD:16.17,(-6,-5.78,;-6,-4.24,;-4.67,-3.47,;-4.67,-1.93,;-6,-1.16,;-6,.38,;-7.34,1.15,;-8.67,-1.16,;-7.34,-1.93,;-7.34,-3.47,;-4.67,1.15,;-3.33,.38,;-2,1.15,;-2,2.69,;-.67,3.46,;.67,2.69,;2,3.46,;3.33,2.69,;4.67,3.46,;4.67,5,;3.33,5.78,;2,5,;6,5.77,;7.34,5,;8.67,5.77,;7.34,3.46,;6,2.69,;6,1.15,;7.34,.38,;8.67,1.15,;8.67,2.69,;-3.33,3.46,;-4.67,2.69,)| Show InChI InChI=1S/C26H36FN3O3/c27-21-3-6-23-24(17-21)33-29-25(23)19-8-13-30(14-9-19)12-7-18-1-4-22(5-2-18)28-26(31)20-10-15-32-16-11-20/h3,6,17-20,22H,1-2,4-5,7-16H2,(H,28,31)/t18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603947
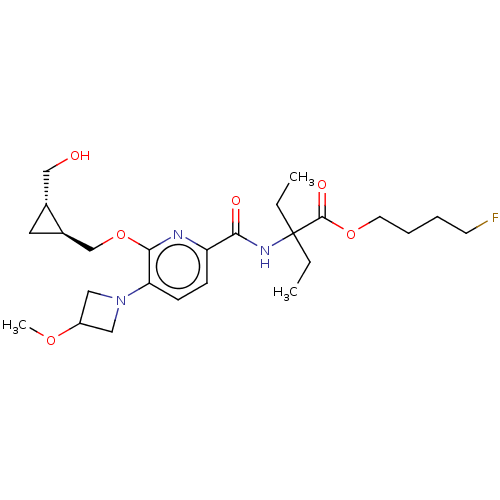
(4-Fluorobutyl 2-ethyl-2-[[6-[[(1S,2S)-2-(hydroxyme...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CO)n1)C(=O)OCCCCF |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603924
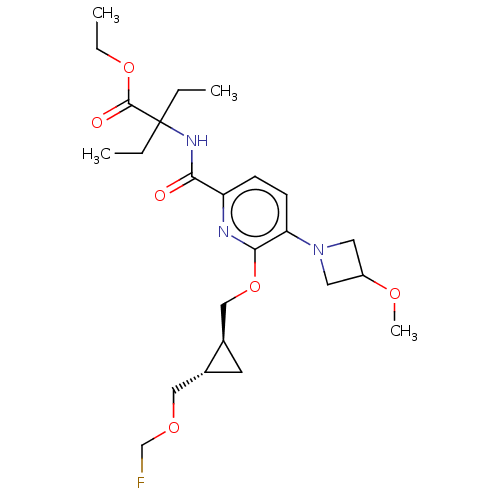
(Ethyl 2-ethyl-2-{[6-({(1S,2S)-2-[(fluoromethoxy)me...)Show SMILES CCOC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2COCF)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643740

((4S)-7-chloro-6-(2,6-difluorophenyl)-1,4-dimethyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(ccc2-n2c(C)nnc12)C(F)(F)F |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50090950
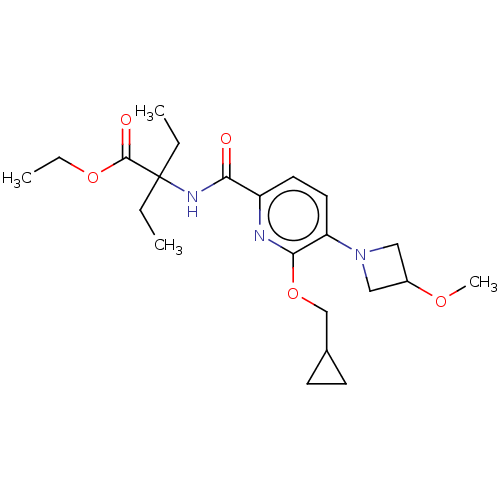
(CHEMBL3582008)Show SMILES CCOC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OCC2CC2)n1 Show InChI InChI=1S/C22H33N3O5/c1-5-22(6-2,21(27)29-7-3)24-19(26)17-10-11-18(25-12-16(13-25)28-4)20(23-17)30-14-15-8-9-15/h10-11,15-16H,5-9,12-14H2,1-4H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in CHO cell membranes |
J Med Chem 58: 4266-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00283
BindingDB Entry DOI: 10.7270/Q2542QBG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180699

(US8829029, 3)Show SMILES COCCC(=O)N[C@H]1CC[C@H](CCN2CCC(CC2)c2noc3cc(F)ccc23)CC1 |r,wU:7.6,wD:10.10,(9.34,.38,;8,1.15,;8,2.69,;6.67,3.46,;6.67,5,;8,5.77,;5.33,5.77,;4,5,;4,3.46,;2.67,2.69,;1.33,3.46,;,2.69,;-1.33,3.46,;-2.67,2.69,;-2.67,1.15,;-4,.38,;-5.33,1.15,;-5.33,2.69,;-4,3.46,;-6.67,.38,;-8,1.15,;-9.34,-1.16,;-8,-1.93,;-8,-3.47,;-6.67,-4.24,;-6.67,-5.78,;-5.33,-3.47,;-5.33,-1.93,;-6.67,-1.16,;1.33,5,;2.67,5.78,)| Show InChI InChI=1S/C24H34FN3O3/c1-30-15-11-23(29)26-20-5-2-17(3-6-20)8-12-28-13-9-18(10-14-28)24-21-7-4-19(25)16-22(21)31-27-24/h4,7,16-18,20H,2-3,5-6,8-15H2,1H3,(H,26,29)/t17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM106426
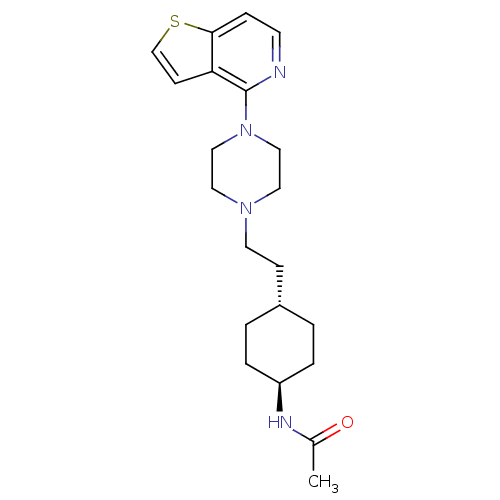
(US8586579, 15)Show SMILES CC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nccc3sccc23)CC1 |r,wU:4.3,wD:7.7,(9.24,.52,;7.7,.52,;6.93,-.82,;6.93,1.85,;5.39,1.85,;4.62,.52,;3.08,.52,;2.31,1.85,;.77,1.85,;,.52,;-1.54,.52,;-2.31,-.82,;-3.85,-.82,;-4.62,.52,;-3.85,1.85,;-2.31,1.85,;-6.16,.52,;-6.93,1.85,;-8.47,1.85,;-9.24,.52,;-8.47,-.82,;-8.95,-2.28,;-7.7,-3.19,;-6.45,-2.28,;-6.93,-.82,;3.08,3.19,;4.62,3.19,)| Show InChI InChI=1S/C21H30N4OS/c1-16(26)23-18-4-2-17(3-5-18)7-10-24-11-13-25(14-12-24)21-19-8-15-27-20(19)6-9-22-21/h6,8-9,15,17-18H,2-5,7,10-14H2,1H3,(H,23,26)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... |
US Patent US8586579 (2013)
BindingDB Entry DOI: 10.7270/Q2QV3K55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM106430
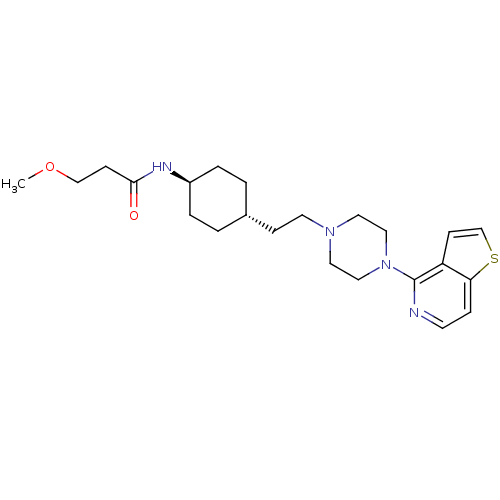
(US8586579, 19)Show SMILES COCCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nccc3sccc23)CC1 |r,wU:7.6,wD:10.10,(10.78,-2.15,;10.01,-.82,;8.47,-.82,;7.7,.52,;6.16,.52,;5.39,-.82,;5.39,1.85,;3.85,1.85,;3.08,.52,;1.54,.52,;.77,1.85,;-.77,1.85,;-1.54,.52,;-3.08,.52,;-3.85,-.82,;-5.39,-.82,;-6.16,.52,;-5.39,1.85,;-3.85,1.85,;-7.7,.52,;-8.47,1.85,;-10.01,1.85,;-10.78,.52,;-10.01,-.82,;-10.49,-2.28,;-9.24,-3.19,;-7.99,-2.28,;-8.47,-.82,;1.54,3.19,;3.08,3.19,)| Show InChI InChI=1S/C23H34N4O2S/c1-29-16-8-22(28)25-19-4-2-18(3-5-19)7-11-26-12-14-27(15-13-26)23-20-9-17-30-21(20)6-10-24-23/h6,9-10,17-19H,2-5,7-8,11-16H2,1H3,(H,25,28)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... |
US Patent US8586579 (2013)
BindingDB Entry DOI: 10.7270/Q2QV3K55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM180755

(US8829029, 50)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CC[C@H]2CC[C@@H](CC2)NS(=O)(=O)c2cccnc2)CC1 |r,wU:19.24,wD:16.17,(-7.34,-6.06,;-7.34,-4.52,;-6,-3.75,;-6,-2.21,;-7.34,-1.44,;-7.34,.1,;-8.67,.87,;-10,-1.44,;-8.67,-2.21,;-8.67,-3.75,;-6,.87,;-4.67,.1,;-3.33,.87,;-3.33,2.41,;-2,3.18,;-.67,2.41,;.67,3.18,;2,2.41,;3.33,3.18,;3.33,4.72,;2,5.49,;.67,4.72,;4.67,5.49,;6,4.72,;6.77,6.06,;5.23,3.39,;7.34,3.95,;7.34,2.41,;8.67,1.64,;10,2.41,;10,3.95,;8.67,4.72,;-4.67,3.18,;-6,2.41,)| Show InChI InChI=1S/C25H31FN4O3S/c26-20-5-8-23-24(16-20)33-28-25(23)19-10-14-30(15-11-19)13-9-18-3-6-21(7-4-18)29-34(31,32)22-2-1-12-27-17-22/h1-2,5,8,12,16-19,21,29H,3-4,6-7,9-11,13-15H2/t18-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643755

((4S)-7-chloro-6-(2,6-difluorophenyl)-1-ethyl-4-met...)Show SMILES CCc1nnc2[C@H](C)N=C(c3c(F)cccc3F)c3c(Cl)c(ccc3-n12)C(F)(F)F |r,t:8| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643726
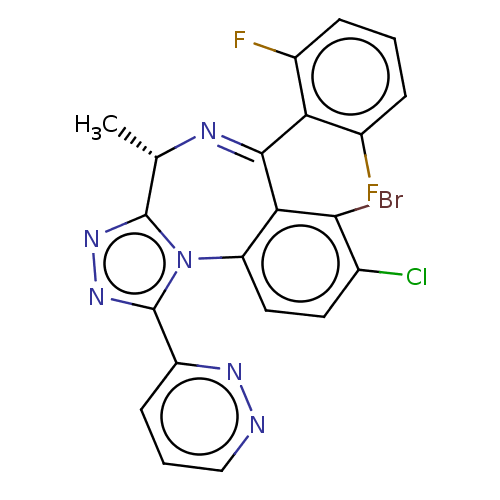
((4S)-7-bromo-8-chloro-6-(2,6-difluorophenyl)-4-met...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Br)c(Cl)ccc2-n2c1nnc2-c1cccnn1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643742
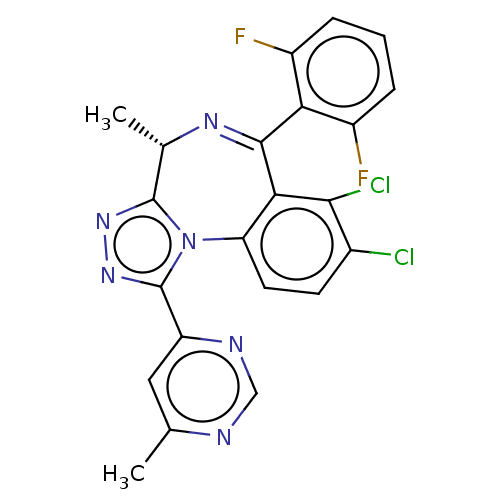
((4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-methyl-...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1nnc2-c1cc(C)ncn1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180707

(US8829029, 7)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CC[C@H]2CC[C@@H](CC2)NC(=O)Cc2ccc3OCOc3c2)CC1 |r,wU:19.24,wD:16.17,(-6.64,-7.48,;-7.03,-5.99,;-5.95,-4.9,;-6.34,-3.41,;-7.83,-3.01,;-8.23,-1.53,;-9.72,-1.13,;-10.41,-3.7,;-8.92,-4.1,;-8.52,-5.59,;-7.14,-.44,;-5.65,-.84,;-4.56,.25,;-4.96,1.74,;-3.87,2.83,;-2.39,2.43,;-1.3,3.52,;.19,3.12,;1.28,4.21,;.88,5.7,;-.61,6.1,;-1.7,5.01,;1.97,6.79,;3.46,6.39,;4.55,7.48,;3.86,4.9,;5.34,4.5,;5.74,3.01,;7.23,2.62,;8.32,3.7,;9.86,3.62,;10.41,5.06,;9.21,6.03,;7.92,5.19,;6.43,5.59,;-6.45,2.14,;-7.54,1.05,)| Show InChI InChI=1S/C29H34FN3O4/c30-22-4-7-24-26(17-22)37-32-29(24)21-10-13-33(14-11-21)12-9-19-1-5-23(6-2-19)31-28(34)16-20-3-8-25-27(15-20)36-18-35-25/h3-4,7-8,15,17,19,21,23H,1-2,5-6,9-14,16,18H2,(H,31,34)/t19-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-1
(Homo sapiens (Human)) | BDBM643737
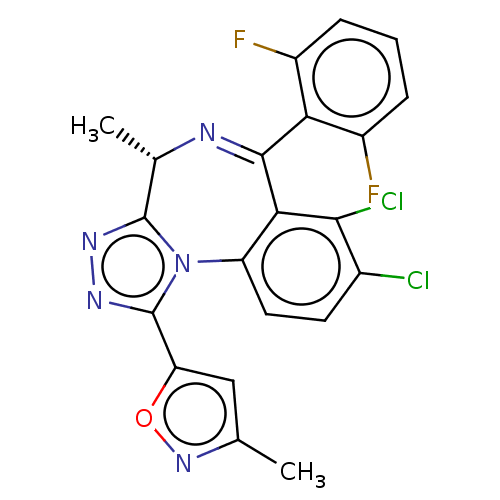
(5-[(4S)-7,8-dichloro-6-(2,6-difluorophenyl)-4-meth...)Show SMILES C[C@@H]1N=C(c2c(F)cccc2F)c2c(Cl)c(Cl)ccc2-n2c1nnc2-c1cc(C)no1 |r,t:2| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM106433
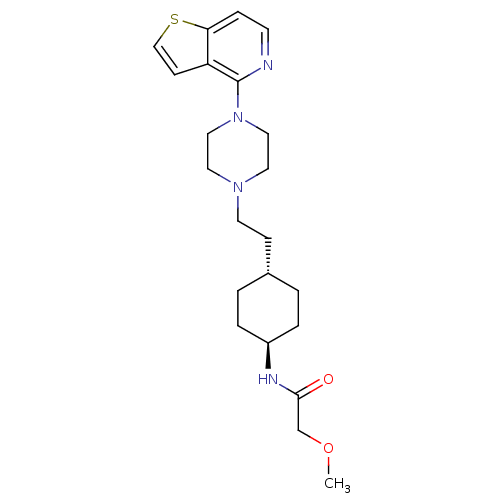
(US8586579, 22)Show SMILES COCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nccc3sccc23)CC1 |r,wU:6.5,wD:9.9,(10.4,-.82,;8.86,-.82,;8.09,.52,;6.55,.52,;5.78,-.82,;5.78,1.85,;4.24,1.85,;3.47,.52,;1.93,.52,;1.16,1.85,;-.38,1.85,;-1.15,.52,;-2.69,.52,;-3.46,-.82,;-5,-.82,;-5.77,.52,;-5,1.85,;-3.46,1.85,;-7.31,.52,;-8.08,1.85,;-9.62,1.85,;-10.39,.52,;-9.62,-.82,;-10.1,-2.28,;-8.85,-3.19,;-7.61,-2.28,;-8.08,-.82,;1.93,3.19,;3.47,3.19,)| Show InChI InChI=1S/C22H32N4O2S/c1-28-16-21(27)24-18-4-2-17(3-5-18)7-10-25-11-13-26(14-12-25)22-19-8-15-29-20(19)6-9-23-22/h6,8-9,15,17-18H,2-5,7,10-14,16H2,1H3,(H,24,27)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... |
US Patent US8586579 (2013)
BindingDB Entry DOI: 10.7270/Q2QV3K55 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180697
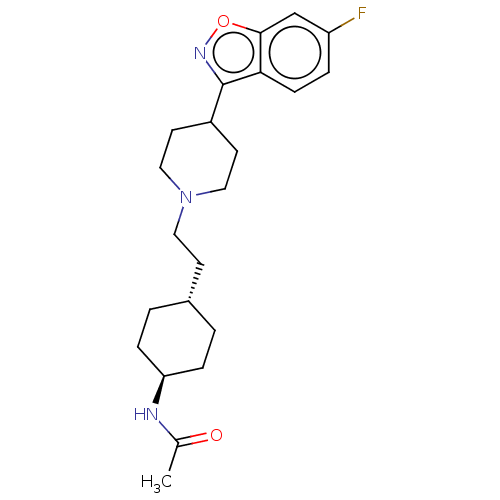
(US8829029, 1)Show SMILES CC(=O)N[C@H]1CC[C@H](CCN2CCC(CC2)c2noc3cc(F)ccc23)CC1 |r,wU:4.3,wD:7.7,(6,-5.78,;7.34,-5,;8.67,-5.78,;7.34,-3.47,;6,-2.69,;4.67,-3.47,;3.33,-2.69,;3.33,-1.15,;2,-.38,;2,1.15,;.67,1.93,;-.67,1.15,;-2,1.93,;-2,3.47,;-.67,4.24,;.67,3.47,;-3.33,4.23,;-3.33,5.78,;-6,5.78,;-6,4.23,;-7.34,3.47,;-7.34,1.93,;-8.67,1.15,;-6,1.15,;-4.67,1.93,;-4.67,3.47,;4.67,-.38,;6,-1.15,)| Show InChI InChI=1S/C22H30FN3O2/c1-15(27)24-19-5-2-16(3-6-19)8-11-26-12-9-17(10-13-26)22-20-7-4-18(23)14-21(20)28-25-22/h4,7,14,16-17,19H,2-3,5-6,8-13H2,1H3,(H,24,27)/t16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM180719

(US8829029, 19)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CC[C@H]2CC[C@@H](CC2)NC(=O)C2(CCC2)C(F)(F)F)CC1 |r,wU:19.24,wD:16.17,(-4.85,-7.48,;-5.25,-5.99,;-4.16,-4.9,;-4.56,-3.41,;-6.04,-3.01,;-6.44,-1.53,;-7.93,-1.13,;-8.62,-3.7,;-7.13,-4.1,;-6.73,-5.59,;-5.35,-.44,;-3.86,-.84,;-2.78,.25,;-3.17,1.74,;-2.09,2.83,;-.6,2.43,;.49,3.52,;1.98,3.12,;3.07,4.21,;2.67,5.7,;1.18,6.1,;.09,5.01,;3.76,6.79,;5.25,6.39,;6.33,7.48,;5.64,4.9,;4.87,3.57,;6.21,2.8,;6.98,4.13,;7.13,5.3,;8.62,5.7,;6.73,6.79,;7.53,3.81,;-4.66,2.14,;-5.75,1.05,)| Show InChI InChI=1S/C26H33F4N3O2/c27-19-4-7-21-22(16-19)35-32-23(21)18-9-14-33(15-10-18)13-8-17-2-5-20(6-3-17)31-24(34)25(11-1-12-25)26(28,29)30/h4,7,16-18,20H,1-3,5-6,8-15H2,(H,31,34)/t17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... |
US Patent US8829029 (2014)
BindingDB Entry DOI: 10.7270/Q22B8WSS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data