 Found 606 hits with Last Name = 'marshall' and Initial = 'l'
Found 606 hits with Last Name = 'marshall' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50204580
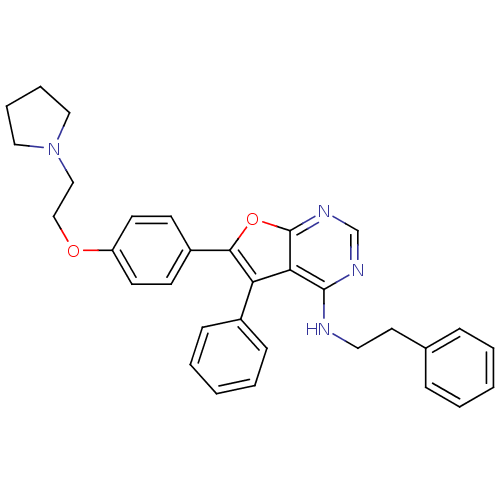
(CHEMBL247667 | N-phenethyl-5-phenyl-6-(4-(2-(pyrro...)Show SMILES C(Cc1ccccc1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C32H32N4O2/c1-3-9-24(10-4-1)17-18-33-31-29-28(25-11-5-2-6-12-25)30(38-32(29)35-23-34-31)26-13-15-27(16-14-26)37-22-21-36-19-7-8-20-36/h1-6,9-16,23H,7-8,17-22H2,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Ack1 |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591075
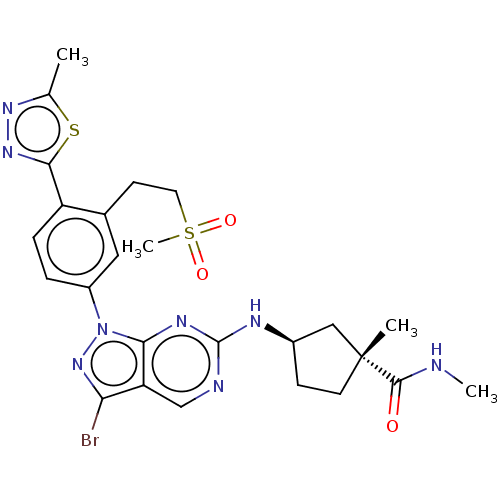
(CHEMBL5200118)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCS(C)(=O)=O)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591046
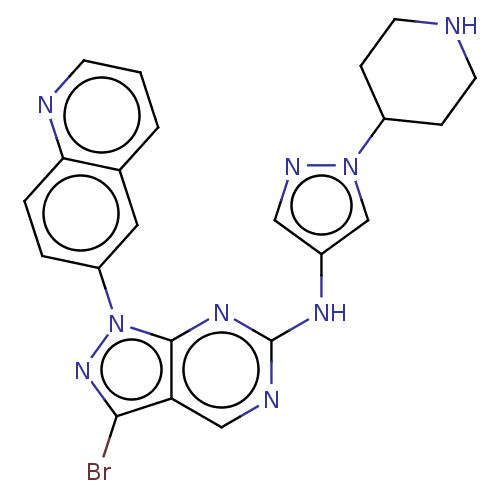
(CHEMBL5202342)Show SMILES Brc1nn(-c2ccc3ncccc3c2)c2nc(Nc3cnn(c3)C3CCNCC3)ncc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591054
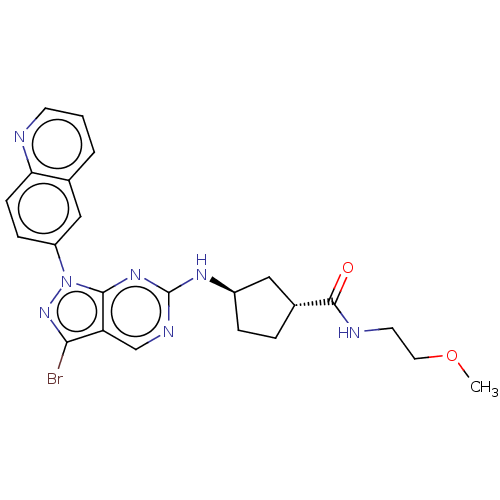
(CHEMBL5179922)Show SMILES COCCNC(=O)[C@@H]1CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591066
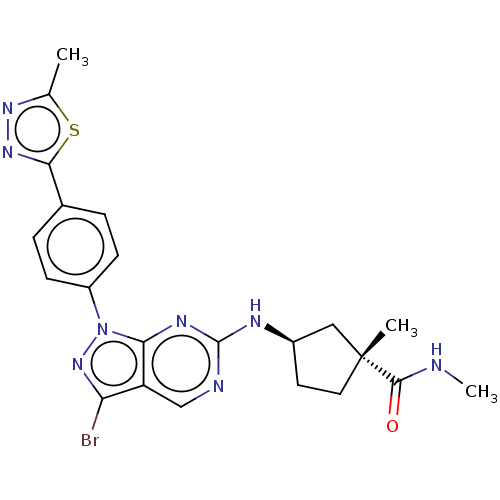
(CHEMBL5190023)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)s3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204580

(CHEMBL247667 | N-phenethyl-5-phenyl-6-(4-(2-(pyrro...)Show SMILES C(Cc1ccccc1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C32H32N4O2/c1-3-9-24(10-4-1)17-18-33-31-29-28(25-11-5-2-6-12-25)30(38-32(29)35-23-34-31)26-13-15-27(16-14-26)37-22-21-36-19-7-8-20-36/h1-6,9-16,23H,7-8,17-22H2,(H,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 23-8 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FT6 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591045

(CHEMBL5209076)Show SMILES Clc1nn(-c2ccc3ncccc3c2)c2nc(Nc3cnn(c3)C3CCNCC3)ncc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545768
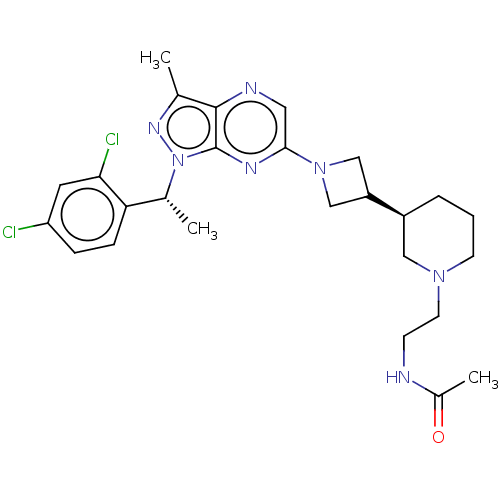
(CHEMBL4641127)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCNC(C)=O)C1 |r| Show InChI InChI=1S/C26H33Cl2N7O/c1-16-25-26(35(32-16)17(2)22-7-6-21(27)11-23(22)28)31-24(12-30-25)34-14-20(15-34)19-5-4-9-33(13-19)10-8-29-18(3)36/h6-7,11-12,17,19-20H,4-5,8-10,13-15H2,1-3H3,(H,29,36)/t17-,19+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204587
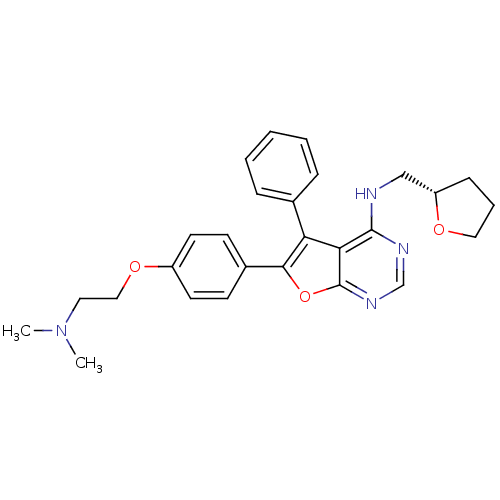
((S)-6-(4-(2-(dimethylamino)ethoxy)phenyl)-5-phenyl...)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C27H30N4O3/c1-31(2)14-16-33-21-12-10-20(11-13-21)25-23(19-7-4-3-5-8-19)24-26(29-18-30-27(24)34-25)28-17-22-9-6-15-32-22/h3-5,7-8,10-13,18,22H,6,9,14-17H2,1-2H3,(H,28,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204590

((S)-6-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-5...)Show SMILES CN1CCN(Cc2ccc(cc2)-c2oc3ncnc(NC[C@@H]4CCCO4)c3c2-c2ccccc2)CC1 Show InChI InChI=1S/C29H33N5O2/c1-33-13-15-34(16-14-33)19-21-9-11-23(12-10-21)27-25(22-6-3-2-4-7-22)26-28(31-20-32-29(26)36-27)30-18-24-8-5-17-35-24/h2-4,6-7,9-12,20,24H,5,8,13-19H2,1H3,(H,30,31,32)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Rattus norvegicus) | BDBM50366958
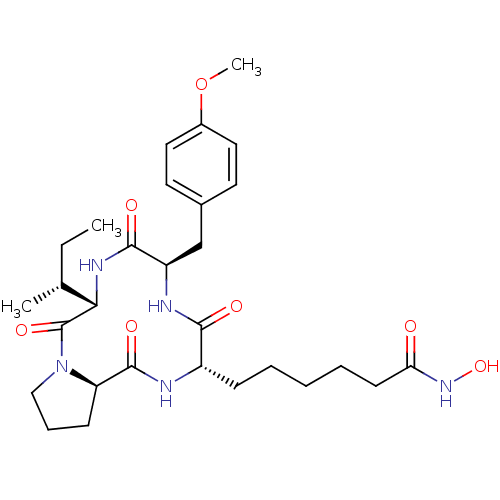
(CHEMBL1790587)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@@H](Cc2ccc(OC)cc2)NC(=O)[C@H](CCCCCC(=O)NO)NC(=O)[C@H]2CCCN2C1=O Show InChI InChI=1S/C29H43N5O7/c1-4-18(2)25-29(39)34-16-8-10-23(34)28(38)30-21(9-6-5-7-11-24(35)33-40)26(36)31-22(27(37)32-25)17-19-12-14-20(41-3)15-13-19/h12-15,18,21-23,25,40H,4-11,16-17H2,1-3H3,(H,30,38)(H,31,36)(H,32,37)(H,33,35)/t18-,21+,22-,23-,25+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver Histone deacetylase with substrate 5b |
J Med Chem 47: 5235-43 (2004)
Article DOI: 10.1021/jm0497592
BindingDB Entry DOI: 10.7270/Q2086839 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50204590

((S)-6-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-5...)Show SMILES CN1CCN(Cc2ccc(cc2)-c2oc3ncnc(NC[C@@H]4CCCO4)c3c2-c2ccccc2)CC1 Show InChI InChI=1S/C29H33N5O2/c1-33-13-15-34(16-14-33)19-21-9-11-23(12-10-21)27-25(22-6-3-2-4-7-22)26-28(31-20-32-29(26)36-27)30-18-24-8-5-17-35-24/h2-4,6-7,9-12,20,24H,5,8,13-19H2,1H3,(H,30,31,32)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Ack1 |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591070
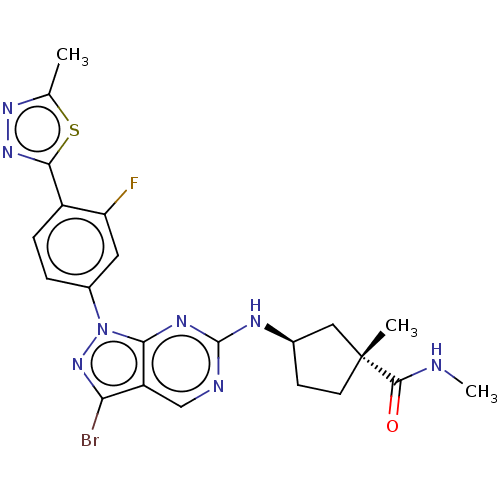
(CHEMBL5208600)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(F)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591079
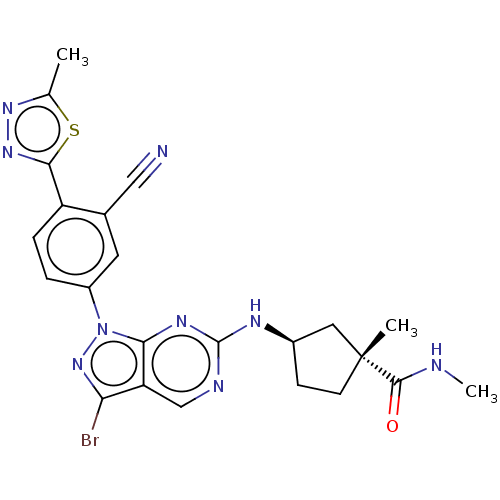
(CHEMBL5196751)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(c3)C#N)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204586

(6-(3-methoxy-4-(2-(piperidin-1-yl)ethoxy)phenyl)-5...)Show SMILES COc1cc(ccc1OCCN1CCCCC1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C31H36N4O4/c1-36-26-19-23(12-13-25(26)38-18-16-35-14-6-3-7-15-35)29-27(22-9-4-2-5-10-22)28-30(33-21-34-31(28)39-29)32-20-24-11-8-17-37-24/h2,4-5,9-10,12-13,19,21,24H,3,6-8,11,14-18,20H2,1H3,(H,32,33,34)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069610
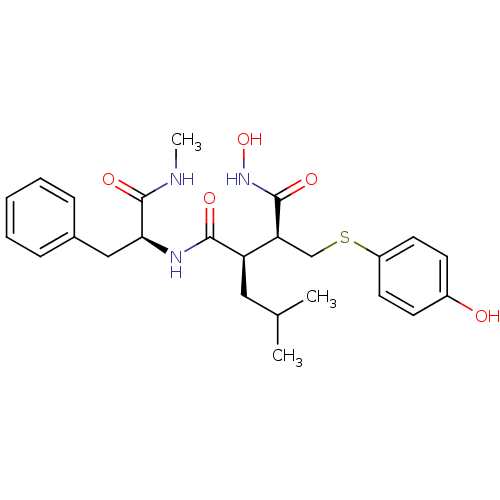
((2R,3S)-N*1*-Hydroxy-2-(4-hydroxy-phenylsulfanylme...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1ccc(O)cc1)C(=O)NO Show InChI InChI=1S/C25H33N3O5S/c1-16(2)13-20(21(24(31)28-33)15-34-19-11-9-18(29)10-12-19)23(30)27-22(25(32)26-3)14-17-7-5-4-6-8-17/h4-12,16,20-22,29,33H,13-15H2,1-3H3,(H,26,32)(H,27,30)(H,28,31)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545779

(CHEMBL4642563)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C(N)=O)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCO)C1 |r| Show InChI InChI=1S/C24H29Cl2N7O2/c1-14(18-5-4-17(25)9-19(18)26)33-24-22(21(30-33)23(27)35)28-10-20(29-24)32-12-16(13-32)15-3-2-6-31(11-15)7-8-34/h4-5,9-10,14-16,34H,2-3,6-8,11-13H2,1H3,(H2,27,35)/t14-,15+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591075

(CHEMBL5200118)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCS(C)(=O)=O)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204582
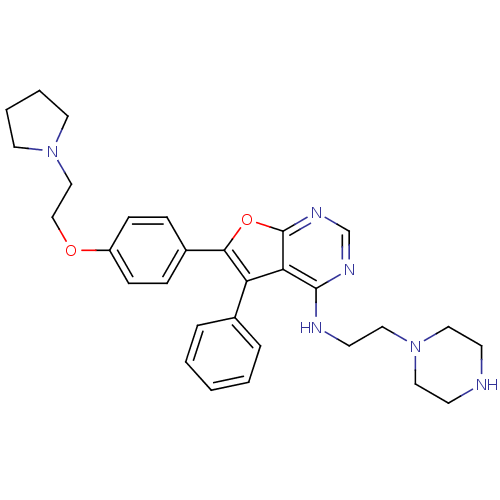
(5-phenyl-N-(2-(piperazin-1-yl)ethyl)-6-(4-(2-(pyrr...)Show SMILES C(CN1CCNCC1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C30H36N6O2/c1-2-6-23(7-3-1)26-27-29(32-14-19-36-17-12-31-13-18-36)33-22-34-30(27)38-28(26)24-8-10-25(11-9-24)37-21-20-35-15-4-5-16-35/h1-3,6-11,22,31H,4-5,12-21H2,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545782
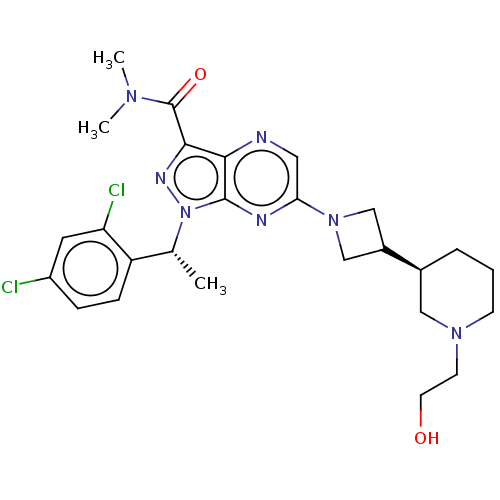
(CHEMBL4637695)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C(=O)N(C)C)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCO)C1 |r| Show InChI InChI=1S/C26H33Cl2N7O2/c1-16(20-7-6-19(27)11-21(20)28)35-25-23(24(31-35)26(37)32(2)3)29-12-22(30-25)34-14-18(15-34)17-5-4-8-33(13-17)9-10-36/h6-7,11-12,16-18,36H,4-5,8-10,13-15H2,1-3H3/t16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591081
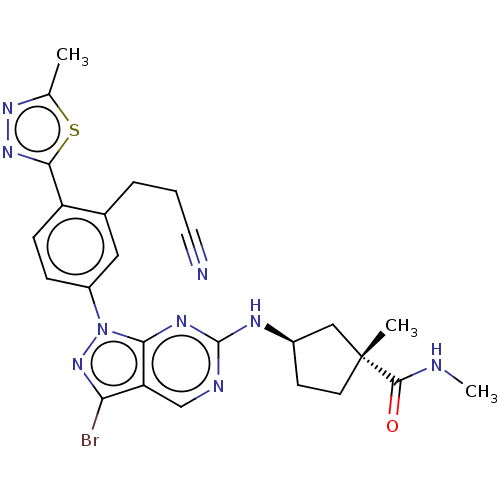
(CHEMBL5193210)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCC#N)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591044
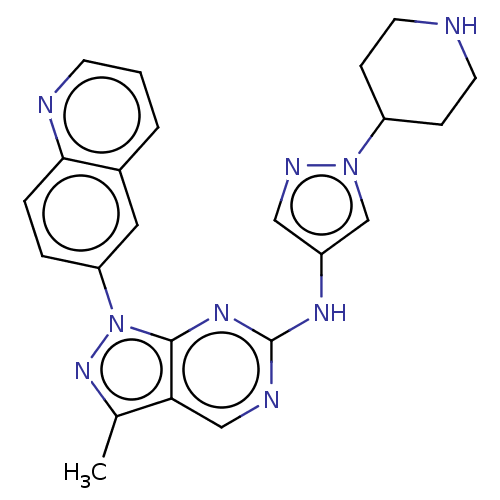
(CHEMBL5202445)Show SMILES Cc1nn(-c2ccc3ncccc3c2)c2nc(Nc3cnn(c3)C3CCNCC3)ncc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204585

((S)-5-phenyl-6-(4-(2-(pyrrolidin-1-yl)ethoxy)pheny...)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C29H32N4O3/c1-2-7-21(8-3-1)25-26-28(30-19-24-9-6-17-34-24)31-20-32-29(26)36-27(25)22-10-12-23(13-11-22)35-18-16-33-14-4-5-15-33/h1-3,7-8,10-13,20,24H,4-6,9,14-19H2,(H,30,31,32)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204583

((S)-(4-ethylpiperazin-1-yl)(4-(5-phenyl-4-((tetrah...)Show SMILES CCN1CCN(CC1)C(=O)c1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C30H33N5O3/c1-2-34-14-16-35(17-15-34)30(36)23-12-10-22(11-13-23)27-25(21-7-4-3-5-8-21)26-28(32-20-33-29(26)38-27)31-19-24-9-6-18-37-24/h3-5,7-8,10-13,20,24H,2,6,9,14-19H2,1H3,(H,31,32,33)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204576
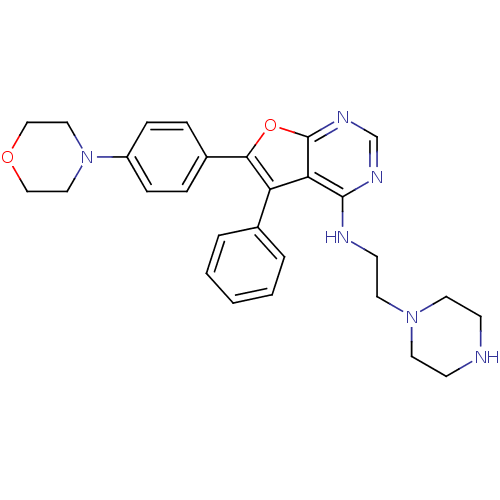
(6-(4-morpholinophenyl)-5-phenyl-N-(2-(piperazin-1-...)Show SMILES C(CN1CCNCC1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C28H32N6O2/c1-2-4-21(5-3-1)24-25-27(30-12-15-33-13-10-29-11-14-33)31-20-32-28(25)36-26(24)22-6-8-23(9-7-22)34-16-18-35-19-17-34/h1-9,20,29H,10-19H2,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50204587

((S)-6-(4-(2-(dimethylamino)ethoxy)phenyl)-5-phenyl...)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C27H30N4O3/c1-31(2)14-16-33-21-12-10-20(11-13-21)25-23(19-7-4-3-5-8-19)24-26(29-18-30-27(24)34-25)28-17-22-9-6-15-32-22/h3-5,7-8,10-13,18,22H,6,9,14-17H2,1-2H3,(H,28,29,30)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Ack1 |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Rattus norvegicus) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat liver histone deacetylase (HDAC) with substrate 3a |
J Med Chem 47: 5235-43 (2004)
Article DOI: 10.1021/jm0497592
BindingDB Entry DOI: 10.7270/Q2086839 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069616
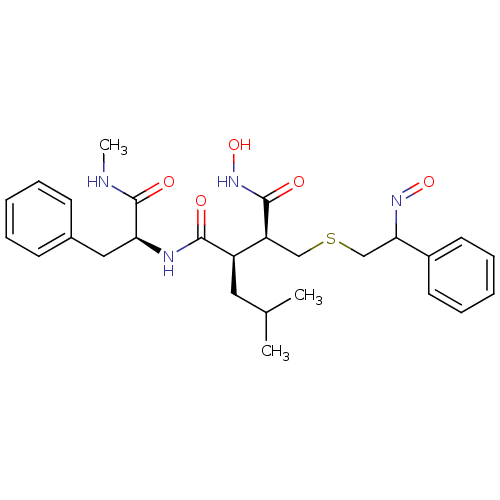
((2R,3S)-N*1*-Hydroxy-2-{2-[(E)-hydroxyimino]-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSCC(N=O)c1ccccc1)C(=O)NO Show InChI InChI=1S/C27H36N4O5S/c1-18(2)14-21(25(32)29-23(27(34)28-3)15-19-10-6-4-7-11-19)22(26(33)31-36)16-37-17-24(30-35)20-12-8-5-9-13-20/h4-13,18,21-24,36H,14-17H2,1-3H3,(H,28,34)(H,29,32)(H,31,33)/t21-,22+,23+,24?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Rattus norvegicus) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat liver histone deacetylase (HDAC) with substrate 3b |
J Med Chem 47: 5235-43 (2004)
Article DOI: 10.1021/jm0497592
BindingDB Entry DOI: 10.7270/Q2086839 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50204583

((S)-(4-ethylpiperazin-1-yl)(4-(5-phenyl-4-((tetrah...)Show SMILES CCN1CCN(CC1)C(=O)c1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C30H33N5O3/c1-2-34-14-16-35(17-15-34)30(36)23-12-10-22(11-13-23)27-25(21-7-4-3-5-8-21)26-28(32-20-33-29(26)38-27)31-19-24-9-6-18-37-24/h3-5,7-8,10-13,20,24H,2,6,9,14-19H2,1H3,(H,31,32,33)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Ack1 |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069604
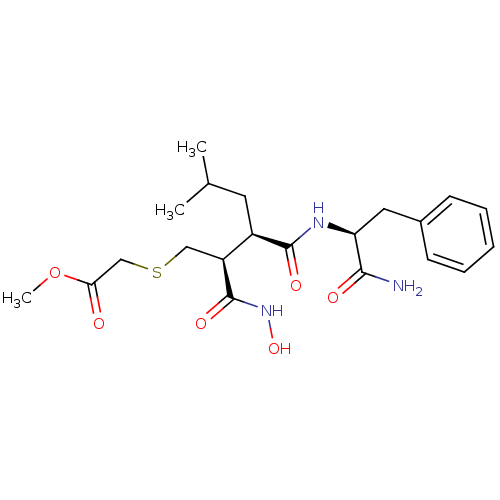
(CHEMBL118054 | [(2S,3R)-3-((S)-1-Carbamoyl-2-pheny...)Show SMILES COC(=O)CSC[C@@H]([C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)NO Show InChI InChI=1S/C21H31N3O6S/c1-13(2)9-15(16(21(28)24-29)11-31-12-18(25)30-3)20(27)23-17(19(22)26)10-14-7-5-4-6-8-14/h4-8,13,15-17,29H,9-12H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204588

(6-(4-(2-(dimethylamino)ethoxy)phenyl)-5-phenyl-N-(...)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCCN3CCNCC3)c2c1-c1ccccc1 Show InChI InChI=1S/C28H34N6O2/c1-33(2)18-19-35-23-10-8-22(9-11-23)26-24(21-6-4-3-5-7-21)25-27(31-20-32-28(25)36-26)30-14-17-34-15-12-29-13-16-34/h3-11,20,29H,12-19H2,1-2H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204591
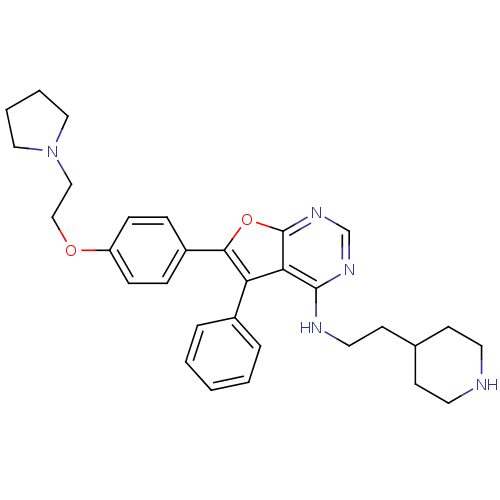
(5-phenyl-N-(2-(piperidin-4-yl)ethyl)-6-(4-(2-(pyrr...)Show SMILES C(CC1CCNCC1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C31H37N5O2/c1-2-6-24(7-3-1)27-28-30(33-17-14-23-12-15-32-16-13-23)34-22-35-31(28)38-29(27)25-8-10-26(11-9-25)37-21-20-36-18-4-5-19-36/h1-3,6-11,22-23,32H,4-5,12-21H2,(H,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591077
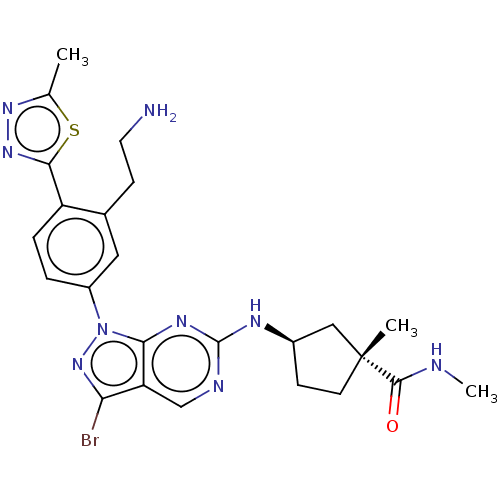
(CHEMBL5201930)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCN)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591065
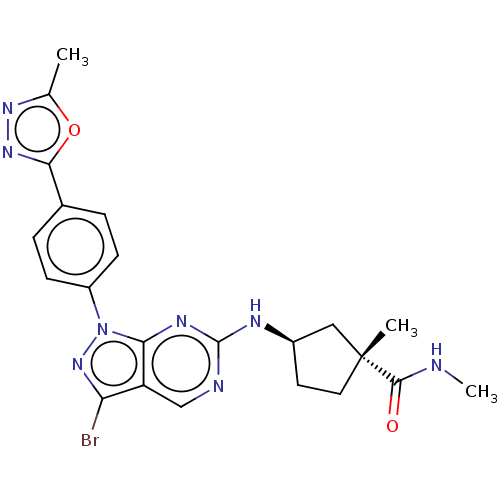
(CHEMBL5170361)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)o3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591072
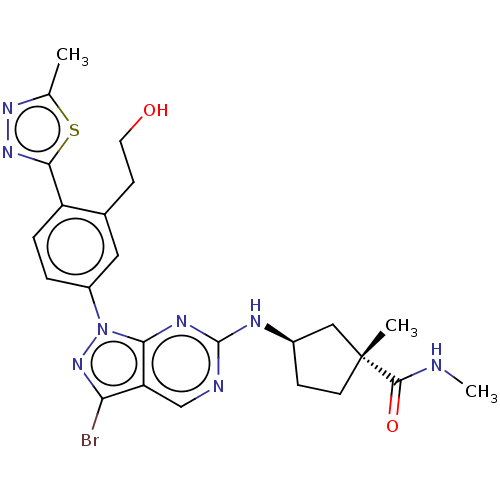
(CHEMBL5172690)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCO)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50204585

((S)-5-phenyl-6-(4-(2-(pyrrolidin-1-yl)ethoxy)pheny...)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C29H32N4O3/c1-2-7-21(8-3-1)25-26-28(30-19-24-9-6-17-34-24)31-20-32-29(26)36-27(25)22-10-12-23(13-11-22)35-18-16-33-14-4-5-15-33/h1-3,7-8,10-13,20,24H,4-6,9,14-19H2,(H,30,31,32)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Ack1 |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591066

(CHEMBL5190023)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)s3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069601
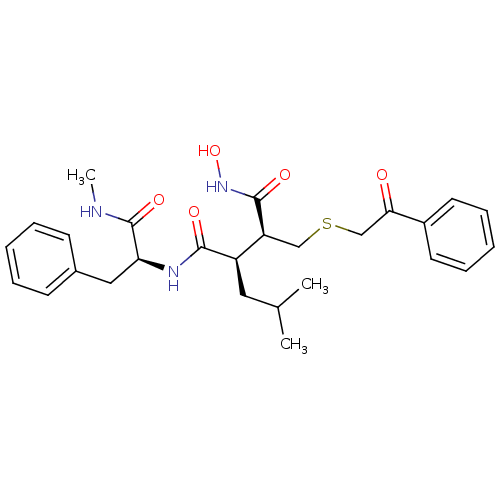
((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSCC(=O)c1ccccc1)C(=O)NO Show InChI InChI=1S/C27H35N3O5S/c1-18(2)14-21(25(32)29-23(27(34)28-3)15-19-10-6-4-7-11-19)22(26(33)30-35)16-36-17-24(31)20-12-8-5-9-13-20/h4-13,18,21-23,35H,14-17H2,1-3H3,(H,28,34)(H,29,32)(H,30,33)/t21-,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591065

(CHEMBL5170361)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)o3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50204576

(6-(4-morpholinophenyl)-5-phenyl-N-(2-(piperazin-1-...)Show SMILES C(CN1CCNCC1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C28H32N6O2/c1-2-4-21(5-3-1)24-25-27(30-12-15-33-13-10-29-11-14-33)31-20-32-28(25)36-26(24)22-6-8-23(9-7-22)34-16-18-35-19-17-34/h1-9,20,29H,10-19H2,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Ack1 |
Bioorg Med Chem Lett 17: 2305-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.057
BindingDB Entry DOI: 10.7270/Q2697365 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591064
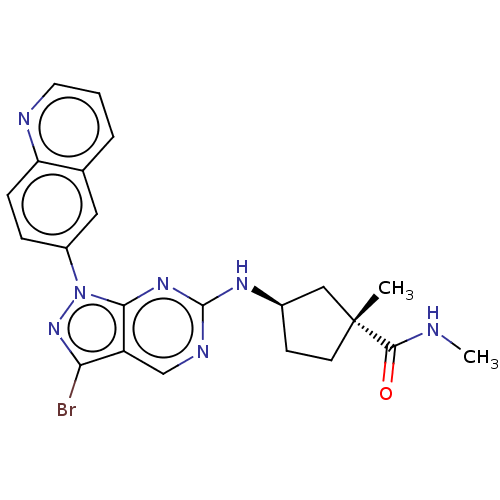
(CHEMBL5199369)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545769
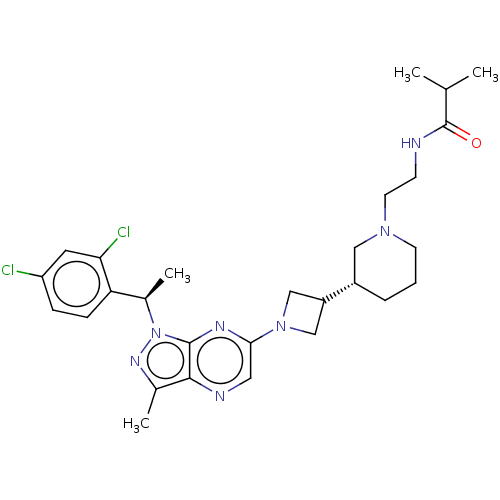
(CHEMBL4645325)Show SMILES CC(C)C(=O)NCCN1CCC[C@@H](C1)C1CN(C1)c1cnc2c(C)nn([C@H](C)c3ccc(Cl)cc3Cl)c2n1 |r| Show InChI InChI=1S/C28H37Cl2N7O/c1-17(2)28(38)31-9-11-35-10-5-6-20(14-35)21-15-36(16-21)25-13-32-26-18(3)34-37(27(26)33-25)19(4)23-8-7-22(29)12-24(23)30/h7-8,12-13,17,19-21H,5-6,9-11,14-16H2,1-4H3,(H,31,38)/t19-,20+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591054

(CHEMBL5179922)Show SMILES COCCNC(=O)[C@@H]1CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Rattus norvegicus) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat liver histone deacetylase (HDAC) with substrate 12a |
J Med Chem 47: 5235-43 (2004)
Article DOI: 10.1021/jm0497592
BindingDB Entry DOI: 10.7270/Q2086839 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069583

((R)-N*1*-((S)-1-Benzylcarbamoyl-2-phenyl-ethyl)-2-...)Show SMILES ONC(=O)C[C@@H](CC1CCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C26H33N3O4/c30-24(29-33)17-22(15-19-11-7-8-12-19)25(31)28-23(16-20-9-3-1-4-10-20)26(32)27-18-21-13-5-2-6-14-21/h1-6,9-10,13-14,19,22-23,33H,7-8,11-12,15-18H2,(H,27,32)(H,28,31)(H,29,30)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 23-8 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FT6 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082226
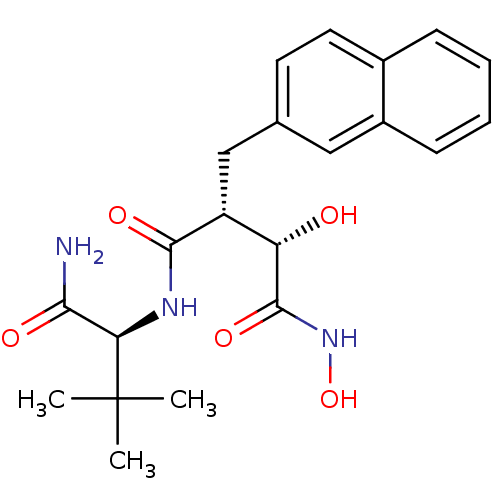
((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H27N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-10,15-17,25,29H,11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069620
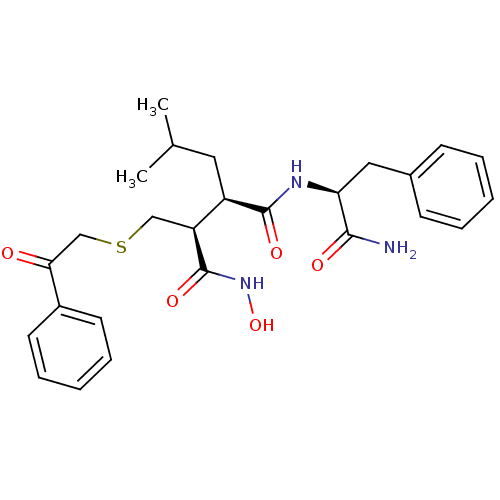
((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-N*4*...)Show SMILES CC(C)C[C@H]([C@H](CSCC(=O)c1ccccc1)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C26H33N3O5S/c1-17(2)13-20(25(32)28-22(24(27)31)14-18-9-5-3-6-10-18)21(26(33)29-34)15-35-16-23(30)19-11-7-4-8-12-19/h3-12,17,20-22,34H,13-16H2,1-2H3,(H2,27,31)(H,28,32)(H,29,33)/t20-,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data