 Found 1380 hits with Last Name = 'rauser' and Initial = 'l'
Found 1380 hits with Last Name = 'rauser' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818
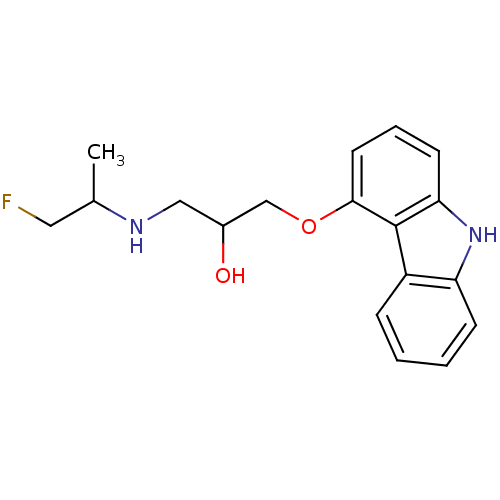
(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818

(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818

(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50099066
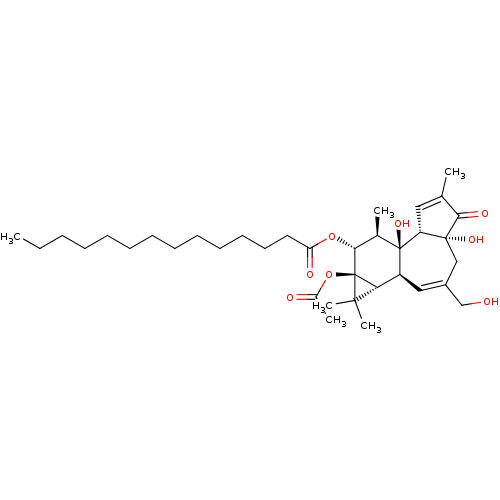
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818

(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50330860
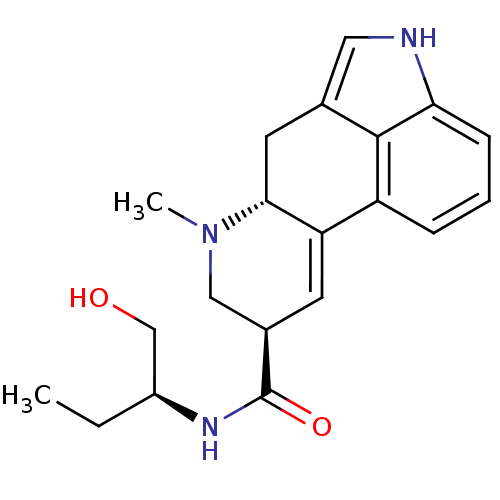
(CHEMBL1201356 | METHYLERGONOVINE | Methylergometri...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |r,c:23| Show InChI InChI=1S/C20H25N3O2/c1-3-14(11-24)22-20(25)13-7-16-15-5-4-6-17-19(15)12(9-21-17)8-18(16)23(2)10-13/h4-7,9,13-14,18,21,24H,3,8,10-11H2,1-2H3,(H,22,25)/t13-,14+,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50027065
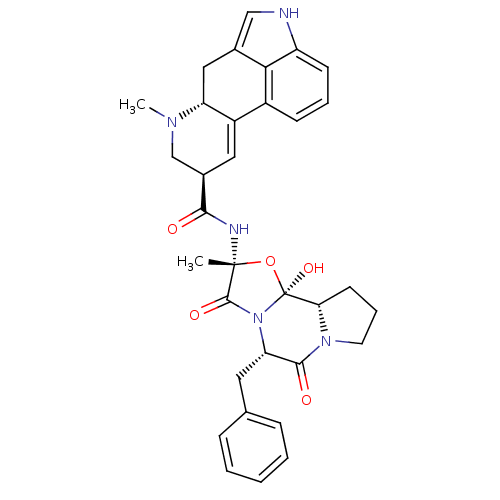
((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human 5-hydroxytryptamine 1D receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C delta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50031942
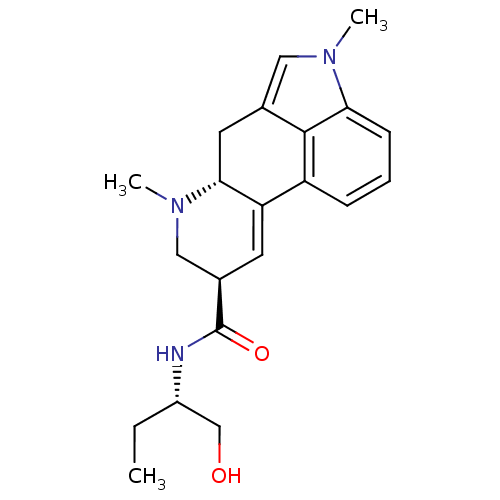
((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C alpha |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50027065

((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50001915
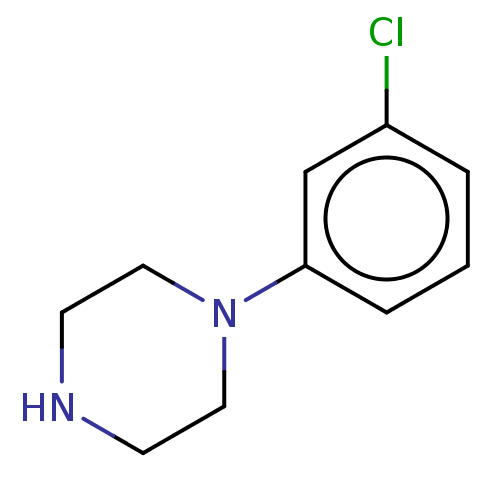
(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30130
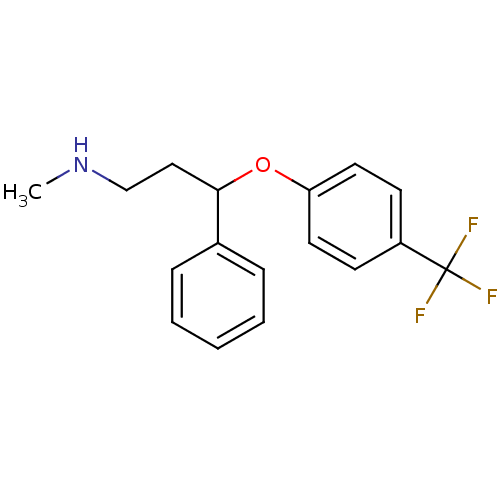
(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human serotonin transporter |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM10755
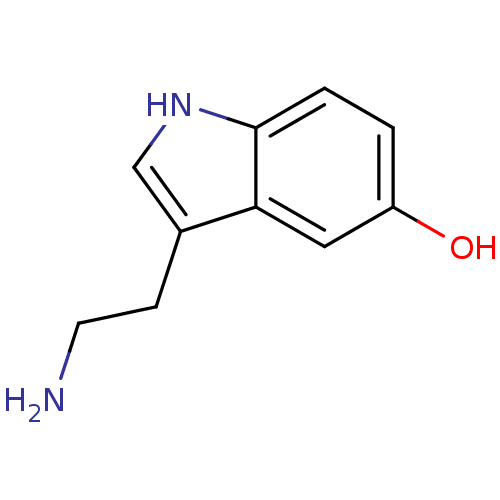
(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C gamma |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human NET (norepinephrine) transporter |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50027065

((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human 5-hydroxytryptamine 7 receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards rat 5-hydroxytryptamine 2A receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50031942

((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human 5-hydroxytryptamine 6 receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85530
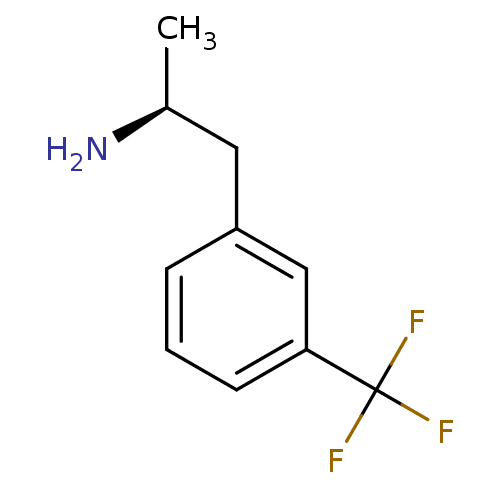
(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50027065

((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50330860

(CHEMBL1201356 | METHYLERGONOVINE | Methylergometri...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |r,c:23| Show InChI InChI=1S/C20H25N3O2/c1-3-14(11-24)22-20(25)13-7-16-15-5-4-6-17-19(15)12(9-21-17)8-18(16)23(2)10-13/h4-7,9,13-14,18,21,24H,3,8,10-11H2,1-2H3,(H,22,25)/t13-,14+,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50330860

(CHEMBL1201356 | METHYLERGONOVINE | Methylergometri...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |r,c:23| Show InChI InChI=1S/C20H25N3O2/c1-3-14(11-24)22-20(25)13-7-16-15-5-4-6-17-19(15)12(9-21-17)8-18(16)23(2)10-13/h4-7,9,13-14,18,21,24H,3,8,10-11H2,1-2H3,(H,22,25)/t13-,14+,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50031942

((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM34142
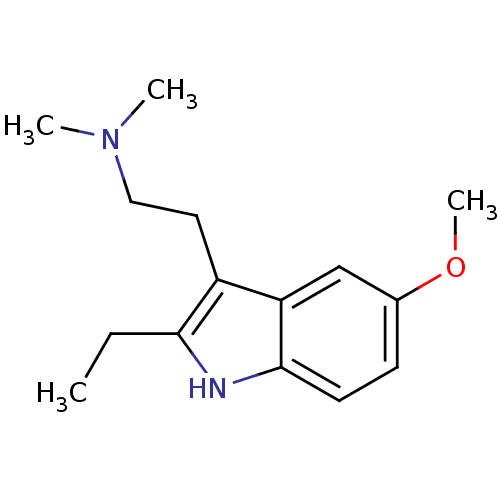
(2-ethyl-5-methoxy-N,N-dimethyltryptamine | CHEMBL2...)Show InChI InChI=1S/C15H22N2O/c1-5-14-12(8-9-17(2)3)13-10-11(18-4)6-7-15(13)16-14/h6-7,10,16H,5,8-9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human 5-hydroxytryptamine 6 receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50073444
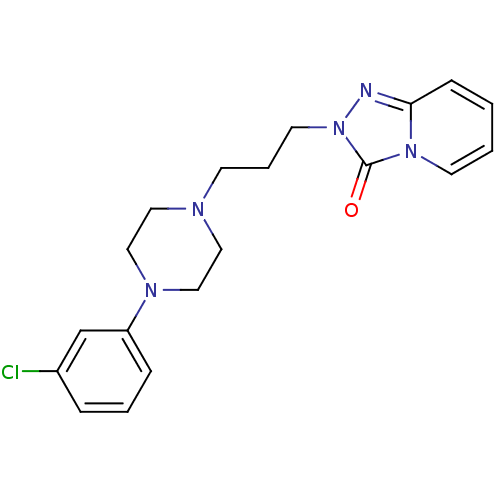
(2-{3-[4-(3-chlorophenyl)piperazin-1-yl]propyl}[1,2...)Show InChI InChI=1S/C19H22ClN5O/c20-16-5-3-6-17(15-16)23-13-11-22(12-14-23)8-4-10-25-19(26)24-9-2-1-7-18(24)21-25/h1-3,5-7,9,15H,4,8,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50085970
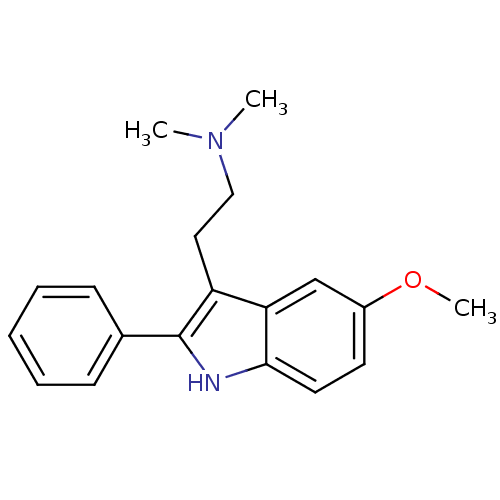
(CHEMBL7318 | [2-(5-Methoxy-2-phenyl-1H-indol-3-yl)...)Show InChI InChI=1S/C19H22N2O/c1-21(2)12-11-16-17-13-15(22-3)9-10-18(17)20-19(16)14-7-5-4-6-8-14/h4-10,13,20H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human 5-hydroxytryptamine 6 receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM50027065

((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human 5-hydroxytryptamine 5A receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards rat r5-hydroxytryptamine 2C receptor |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50057509
((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C alpha |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50020712
(10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 33.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Centre for Addiction and Mental Health
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 427-33 (2001)
Article DOI: 10.1124/mol.59.3.427
BindingDB Entry DOI: 10.7270/Q22Z1438 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM85818

(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50333880
(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50217942
(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50057509

((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50057509

((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C gamma |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50014406
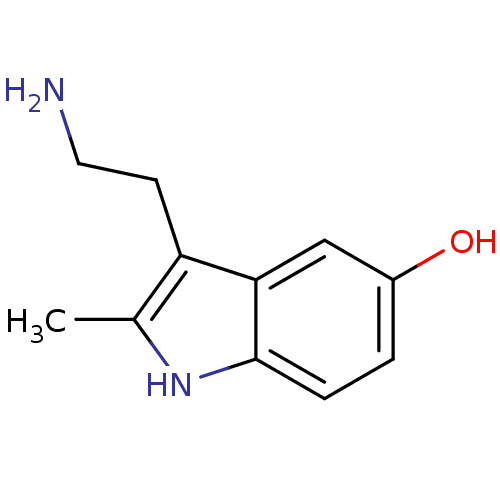
(2-Me 5-HT | 2-Methyl-5-hydroxytryptamine | 2-methy...)Show InChI InChI=1S/C11H14N2O/c1-7-9(4-5-12)10-6-8(14)2-3-11(10)13-7/h2-3,6,13-14H,4-5,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 6 receptor expressed in HEK 293 human embryonic kidney cells, [3H]-lysergic acid diethylamide as radio... |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 50.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Centre for Addiction and Mental Health
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 427-33 (2001)
Article DOI: 10.1124/mol.59.3.427
BindingDB Entry DOI: 10.7270/Q22Z1438 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM34142

(2-ethyl-5-methoxy-N,N-dimethyltryptamine | CHEMBL2...)Show InChI InChI=1S/C15H22N2O/c1-5-14-12(8-9-17(2)3)13-10-11(18-4)6-7-15(13)16-14/h6-7,10,16H,5,8-9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 6 receptor expressed in HEK 293 human embryonic kidney cells, [3H]-lysergic acid diethylamide as radio... |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50085970

(CHEMBL7318 | [2-(5-Methoxy-2-phenyl-1H-indol-3-yl)...)Show InChI InChI=1S/C19H22N2O/c1-21(2)12-11-16-17-13-15(22-3)9-10-18(17)20-19(16)14-7-5-4-6-8-14/h4-10,13,20H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 6 receptor expressed in HEK 293 human embryonic kidney cells, [3H]-lysergic acid diethylamide as radio... |
J Med Chem 43: 1011-8 (2000)
BindingDB Entry DOI: 10.7270/Q2CF9PBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50001915

(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by PDSP Ki Database
| |
Circulation 102: 2836-41 (2000)
Article DOI: 10.1161/01.cir.102.23.2836
BindingDB Entry DOI: 10.7270/Q2H70DD1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data