 Found 271 hits with Last Name = 'yuan' and Initial = 'l'
Found 271 hits with Last Name = 'yuan' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chitin synthase 1
(Candida albicans) | BDBM50559071

(CHEMBL4741395) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Candida albicans chitin synthase 1 expressed in Saccharomyces cerevisiae RRA400-1U cells using [3H]UDP-GlcNAc as substrate by scintilla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00748
BindingDB Entry DOI: 10.7270/Q2348Q30 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50602006

(CHEMBL5209436)Show SMILES ONC(=O)CN(c1cccc(c1)[N+]([O-])=O)S(=O)(=O)c1ccc(Br)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129043
BindingDB Entry DOI: 10.7270/Q23N27G1 |
More data for this
Ligand-Target Pair | |
Chitin synthase 1
(Candida albicans) | BDBM50559072

(CHEMBL4786444) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Candida albicans chitin synthase 1 expressed in Saccharomyces cerevisiae RRA400-1U cells using [3H]UDP-GlcNAc as substrate by scintilla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00748
BindingDB Entry DOI: 10.7270/Q2348Q30 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50602006

(CHEMBL5209436)Show SMILES ONC(=O)CN(c1cccc(c1)[N+]([O-])=O)S(=O)(=O)c1ccc(Br)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129043
BindingDB Entry DOI: 10.7270/Q23N27G1 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59188

(Dihydroisoxazole, 3e)Show SMILES BrC1=NOC(CNC(=O)[C@H](Cc2ccccc2)NC(=O)Oc2ccc3ccccc3c2)C1 |r,t:1| Show InChI InChI=1S/C24H22BrN3O4/c25-22-14-20(32-28-22)15-26-23(29)21(12-16-6-2-1-3-7-16)27-24(30)31-19-11-10-17-8-4-5-9-18(17)13-19/h1-11,13,20-21H,12,14-15H2,(H,26,29)(H,27,30)/t20?,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59187
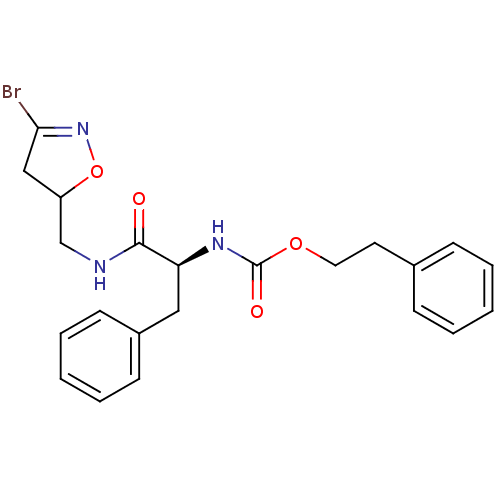
(Dihydroisoxazole, 3d)Show SMILES BrC1=NOC(CNC(=O)[C@H](Cc2ccccc2)NC(=O)OCCc2ccccc2)C1 |r,t:1| Show InChI InChI=1S/C22H24BrN3O4/c23-20-14-18(30-26-20)15-24-21(27)19(13-17-9-5-2-6-10-17)25-22(28)29-12-11-16-7-3-1-4-8-16/h1-10,18-19H,11-15H2,(H,24,27)(H,25,28)/t18?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59186

(Dihydroisoxazole, 3c)Show SMILES BrC1=NOC(CNC(=O)[C@H](Cc2ccccc2)NC(=O)Oc2cccnc2)C1 |r,t:1| Show InChI InChI=1S/C19H19BrN4O4/c20-17-10-15(28-24-17)12-22-18(25)16(9-13-5-2-1-3-6-13)23-19(26)27-14-7-4-8-21-11-14/h1-8,11,15-16H,9-10,12H2,(H,22,25)(H,23,26)/t15?,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59190

(Dihydroisoxazole, 4)Show SMILES Oc1ccc2[nH]cc(C[C@H](NC(=O)OCC3=Cc4ccccc4S3(=O)=O)C(=O)NCC3CC(Br)=NO3)c2c1 |r,c:35,t:15| Show InChI InChI=1S/C25H23BrN4O7S/c26-23-10-17(37-30-23)12-28-24(32)21(8-15-11-27-20-6-5-16(31)9-19(15)20)29-25(33)36-13-18-7-14-3-1-2-4-22(14)38(18,34)35/h1-7,9,11,17,21,27,31H,8,10,12-13H2,(H,28,32)(H,29,33)/t17?,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59185
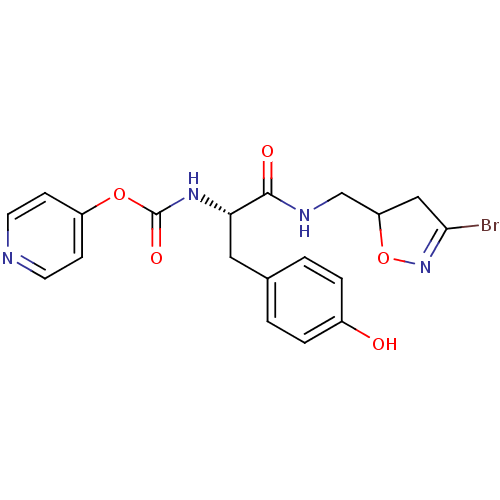
(Dihydroisoxazole, 3b)Show SMILES Oc1ccc(C[C@H](NC(=O)Oc2ccncc2)C(=O)NCC2CC(Br)=NO2)cc1 |r,c:25| Show InChI InChI=1S/C19H19BrN4O5/c20-17-10-15(29-24-17)11-22-18(26)16(9-12-1-3-13(25)4-2-12)23-19(27)28-14-5-7-21-8-6-14/h1-8,15-16,25H,9-11H2,(H,22,26)(H,23,27)/t15?,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59189
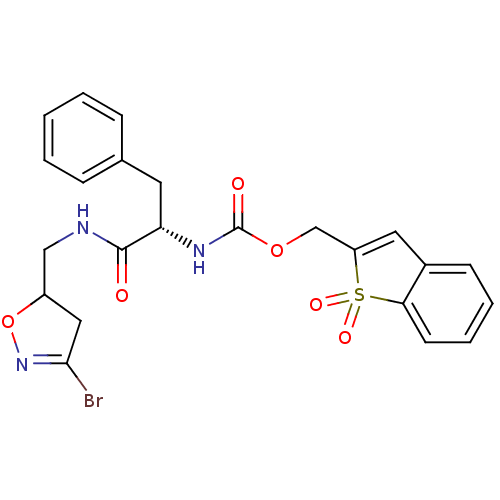
(Dihydroisoxazole, 3f)Show SMILES BrC1=NOC(CNC(=O)[C@H](Cc2ccccc2)NC(=O)OCC2=Cc3ccccc3S2(=O)=O)C1 |r,t:1,23| Show InChI InChI=1S/C23H22BrN3O6S/c24-21-12-17(33-27-21)13-25-22(28)19(10-15-6-2-1-3-7-15)26-23(29)32-14-18-11-16-8-4-5-9-20(16)34(18,30)31/h1-9,11,17,19H,10,12-14H2,(H,25,28)(H,26,29)/t17?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59177
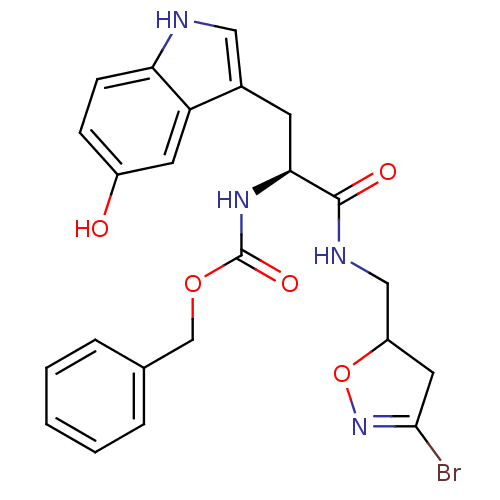
(Dihydroisoxazole, 1e | Dihydroisoxazole, 1f)Show SMILES Oc1ccc2[nH]cc(C[C@H](NC(=O)OCc3ccccc3)C(=O)NCC3CC(Br)=NO3)c2c1 |r,c:29| Show InChI InChI=1S/C23H23BrN4O5/c24-21-10-17(33-28-21)12-26-22(30)20(27-23(31)32-13-14-4-2-1-3-5-14)8-15-11-25-19-7-6-16(29)9-18(15)19/h1-7,9,11,17,20,25,29H,8,10,12-13H2,(H,26,30)(H,27,31)/t17?,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59179
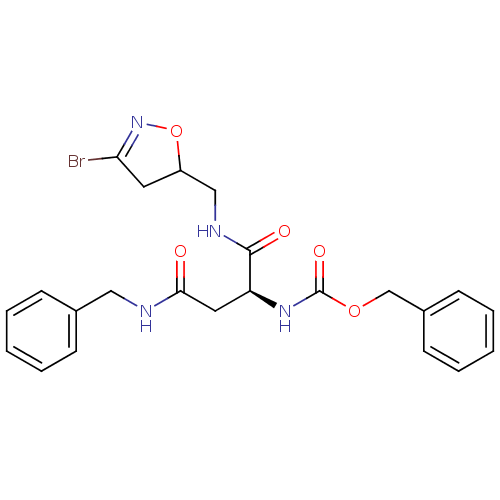
(Dihydroisoxazole, 1g)Show SMILES BrC1=NOC(CNC(=O)[C@H](CC(=O)NCc2ccccc2)NC(=O)OCc2ccccc2)C1 |r,t:1| Show InChI InChI=1S/C23H25BrN4O5/c24-20-11-18(33-28-20)14-26-22(30)19(12-21(29)25-13-16-7-3-1-4-8-16)27-23(31)32-15-17-9-5-2-6-10-17/h1-10,18-19H,11-15H2,(H,25,29)(H,26,30)(H,27,31)/t18?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59183
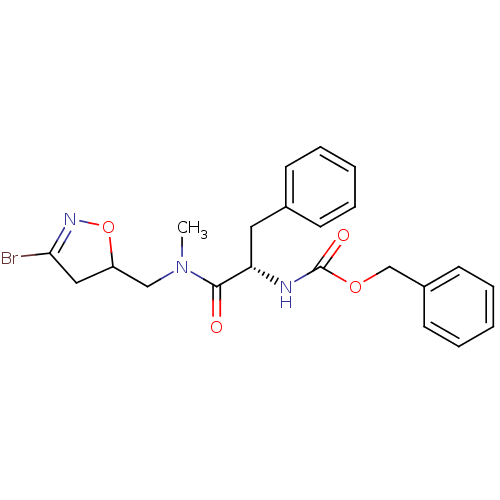
(Dihydroisoxazole, 2b)Show SMILES CN(CC1CC(Br)=NO1)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r,c:6| Show InChI InChI=1S/C22H24BrN3O4/c1-26(14-18-13-20(23)25-30-18)21(27)19(12-16-8-4-2-5-9-16)24-22(28)29-15-17-10-6-3-7-11-17/h2-11,18-19H,12-15H2,1H3,(H,24,28)/t18?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59177

(Dihydroisoxazole, 1e | Dihydroisoxazole, 1f)Show SMILES Oc1ccc2[nH]cc(C[C@H](NC(=O)OCc3ccccc3)C(=O)NCC3CC(Br)=NO3)c2c1 |r,c:29| Show InChI InChI=1S/C23H23BrN4O5/c24-21-10-17(33-28-21)12-26-22(30)20(27-23(31)32-13-14-4-2-1-3-5-14)8-15-11-25-19-7-6-16(29)9-18(15)19/h1-7,9,11,17,20,25,29H,8,10,12-13H2,(H,26,30)(H,27,31)/t17?,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59180
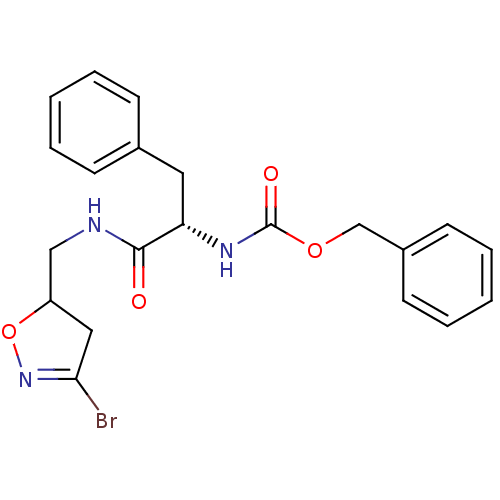
(Dihydroisoxazole, 1h)Show SMILES BrC1=NOC(CNC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C1 |r,t:1| Show InChI InChI=1S/C21H22BrN3O4/c22-19-12-17(29-25-19)13-23-20(26)18(11-15-7-3-1-4-8-15)24-21(27)28-14-16-9-5-2-6-10-16/h1-10,17-18H,11-14H2,(H,23,26)(H,24,27)/t17?,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59176
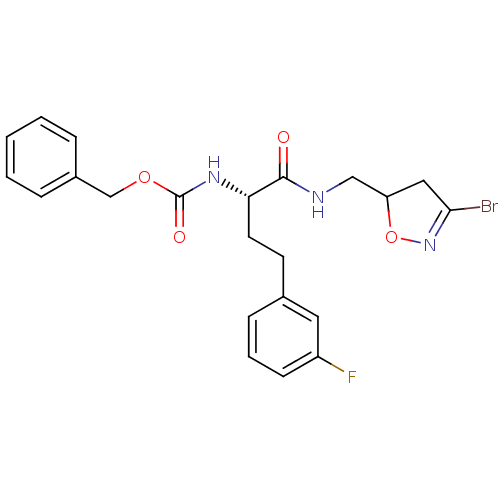
(Dihydroisoxazole, 1d)Show SMILES Fc1cccc(CC[C@H](NC(=O)OCc2ccccc2)C(=O)NCC2CC(Br)=NO2)c1 |r,c:28| Show InChI InChI=1S/C22H23BrFN3O4/c23-20-12-18(31-27-20)13-25-21(28)19(10-9-15-7-4-8-17(24)11-15)26-22(29)30-14-16-5-2-1-3-6-16/h1-8,11,18-19H,9-10,12-14H2,(H,25,28)(H,26,29)/t18?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59174
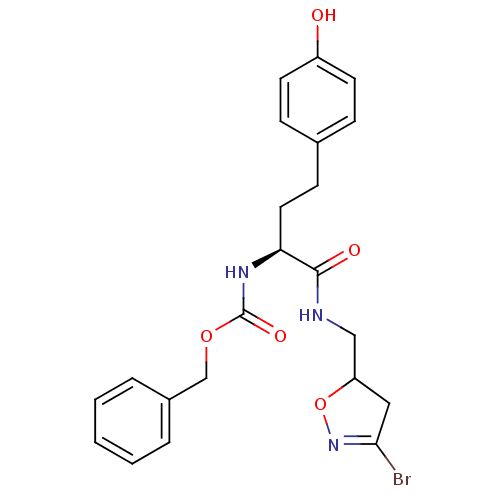
(Dihydroisoxazole, 1b)Show SMILES Oc1ccc(CC[C@H](NC(=O)OCc2ccccc2)C(=O)NCC2CC(Br)=NO2)cc1 |r,c:27| Show InChI InChI=1S/C22H24BrN3O5/c23-20-12-18(31-26-20)13-24-21(28)19(11-8-15-6-9-17(27)10-7-15)25-22(29)30-14-16-4-2-1-3-5-16/h1-7,9-10,18-19,27H,8,11-14H2,(H,24,28)(H,25,29)/t18?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59175
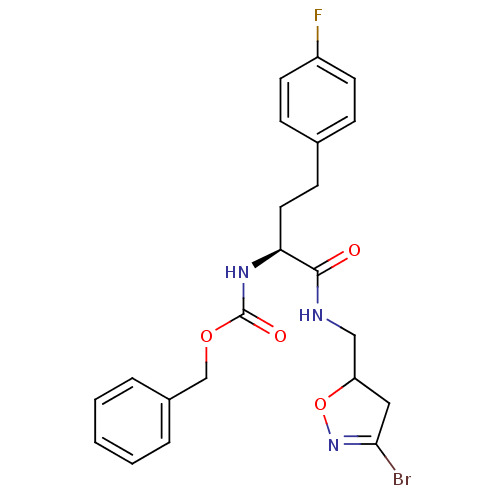
(Dihydroisoxazole, 1c)Show SMILES Fc1ccc(CC[C@H](NC(=O)OCc2ccccc2)C(=O)NCC2CC(Br)=NO2)cc1 |r,c:27| Show InChI InChI=1S/C22H23BrFN3O4/c23-20-12-18(31-27-20)13-25-21(28)19(11-8-15-6-9-17(24)10-7-15)26-22(29)30-14-16-4-2-1-3-5-16/h1-7,9-10,18-19H,8,11-14H2,(H,25,28)(H,26,29)/t18?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59173
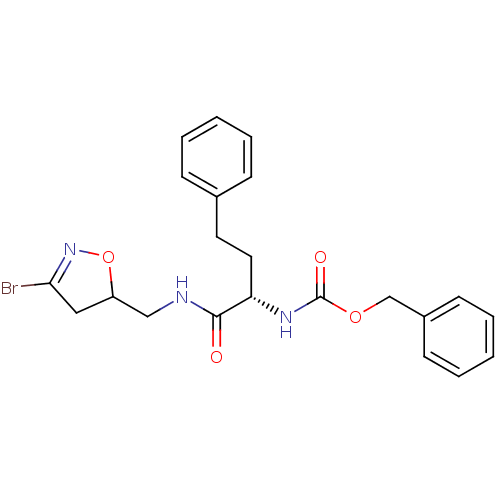
(Dihydroisoxazole, 1a)Show SMILES BrC1=NOC(CNC(=O)[C@H](CCc2ccccc2)NC(=O)OCc2ccccc2)C1 |r,t:1| Show InChI InChI=1S/C22H24BrN3O4/c23-20-13-18(30-26-20)14-24-21(27)19(12-11-16-7-3-1-4-8-16)25-22(28)29-15-17-9-5-2-6-10-17/h1-10,18-19H,11-15H2,(H,24,27)(H,25,28)/t18?,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59181
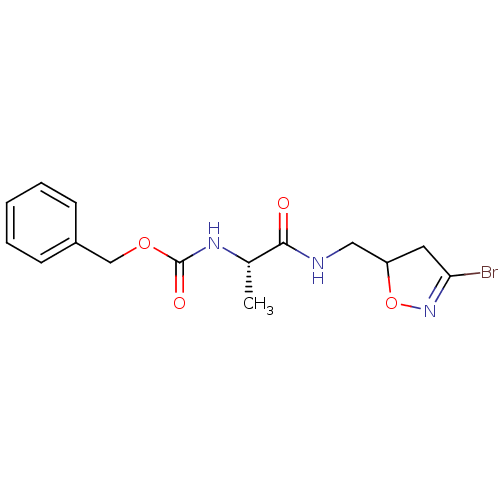
(Dihydroisoxazole, 1i)Show SMILES C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC1CC(Br)=NO1 |r,c:21| Show InChI InChI=1S/C15H18BrN3O4/c1-10(14(20)17-8-12-7-13(16)19-23-12)18-15(21)22-9-11-5-3-2-4-6-11/h2-6,10,12H,7-9H2,1H3,(H,17,20)(H,18,21)/t10-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59182
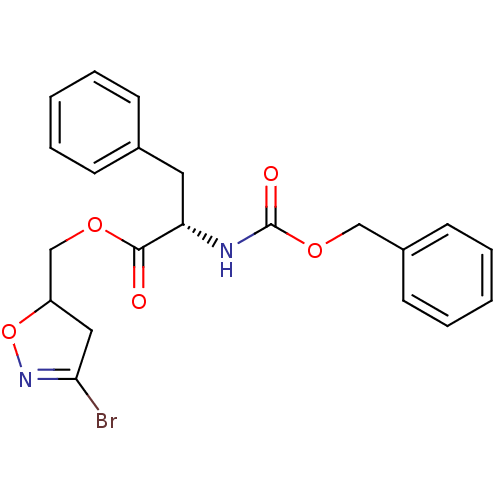
(Dihydroisoxazole, 2a)Show SMILES BrC1=NOC(COC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C1 |r,t:1| Show InChI InChI=1S/C21H21BrN2O5/c22-19-12-17(29-24-19)14-27-20(25)18(11-15-7-3-1-4-8-15)23-21(26)28-13-16-9-5-2-6-10-16/h1-10,17-18H,11-14H2,(H,23,26)/t17?,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM59184

(Dihydroisoxazole, 3a)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC1CC(Br)=NO1 |r,c:20| Show InChI InChI=1S/C15H18BrN3O3/c1-10(20)18-13(7-11-5-3-2-4-6-11)15(21)17-9-12-8-14(16)19-22-12/h2-6,12-13H,7-9H2,1H3,(H,17,21)(H,18,20)/t12?,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Stanford University
| Assay Description
Recombinant of human TG2 was expressed in E.coli and purified to >90% homogeneity. |
Chem Biol 12: 469-75 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.007
BindingDB Entry DOI: 10.7270/Q2XK8D0V |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of fluormone VDR red from human full-length VDR after 4 hrs by fluorescence polarization assay |
Eur J Med Chem 157: 1174-1191 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.085
BindingDB Entry DOI: 10.7270/Q2WH2SQJ |
More data for this
Ligand-Target Pair | |
Endochitinase B1
(Aspergillus fumigatus) | BDBM50559069

(CHEMBL4756641)Show SMILES CNC(=O)NC(=N)NCCC[C@H]1CN(C)[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(=O)N[C@H](CC(=O)N[C@H](C)C(=O)N1)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Aspergillus fumigatus chitinase B1 expressed in Escherichia coli using 4MU-GlcNAc2 as substrate by fluorescence method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00748
BindingDB Entry DOI: 10.7270/Q2348Q30 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50605474

(CHEMBL5189857)Show SMILES Oc1ccc(NC(=O)c2ccc(cc2)-c2ccccc2)cc1NS(=O)(=O)c1ccc(F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01948
BindingDB Entry DOI: 10.7270/Q2CZ3C8Z |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50469127
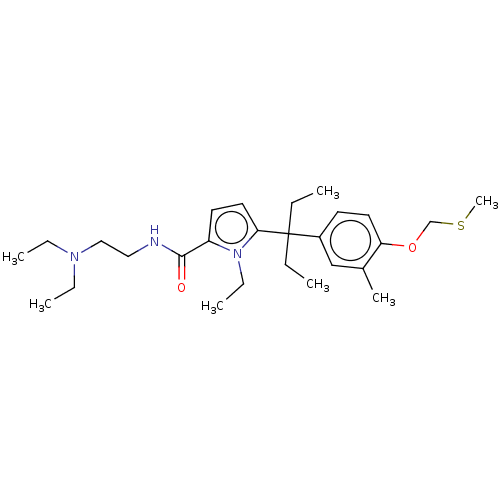
(CHEMBL4288947)Show SMILES CCN(CC)CCNC(=O)c1ccc(n1CC)C(CC)(CC)c1ccc(OCSC)c(C)c1 Show InChI InChI=1S/C27H43N3O2S/c1-8-27(9-2,22-13-15-24(21(6)19-22)32-20-33-7)25-16-14-23(30(25)12-5)26(31)28-17-18-29(10-3)11-4/h13-16,19H,8-12,17-18,20H2,1-7H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of fluormone VDR red from human full-length VDR after 4 hrs by fluorescence polarization assay |
Eur J Med Chem 157: 1174-1191 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.085
BindingDB Entry DOI: 10.7270/Q2WH2SQJ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50126186
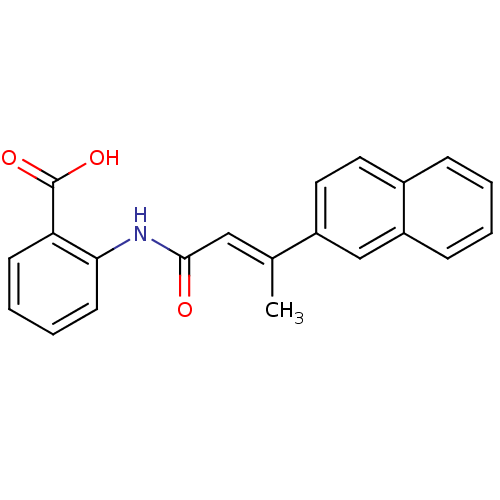
(2-(3-Naphthalen-2-yl-but-2-enoylamino)-benzoic aci...)Show InChI InChI=1S/C21H17NO3/c1-14(16-11-10-15-6-2-3-7-17(15)13-16)12-20(23)22-19-9-5-4-8-18(19)21(24)25/h2-13H,1H3,(H,22,23)(H,24,25)/b14-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase |
Eur J Med Chem 112: 231-51 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.009
BindingDB Entry DOI: 10.7270/Q2SB47N4 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50441900
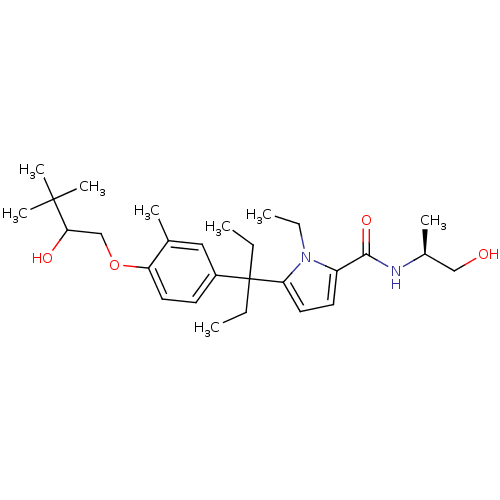
(CHEMBL2434990)Show SMILES CCn1c(ccc1C(CC)(CC)c1ccc(OCC(O)C(C)(C)C)c(C)c1)C(=O)N[C@@H](C)CO |r| Show InChI InChI=1S/C28H44N2O4/c1-9-28(10-2,24-15-13-22(30(24)11-3)26(33)29-20(5)17-31)21-12-14-23(19(4)16-21)34-18-25(32)27(6,7)8/h12-16,20,25,31-32H,9-11,17-18H2,1-8H3,(H,29,33)/t20-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of fluormone VDR red from human full-length VDR after 4 hrs by fluorescence polarization assay |
Eur J Med Chem 157: 1174-1191 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.085
BindingDB Entry DOI: 10.7270/Q2WH2SQJ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50469128
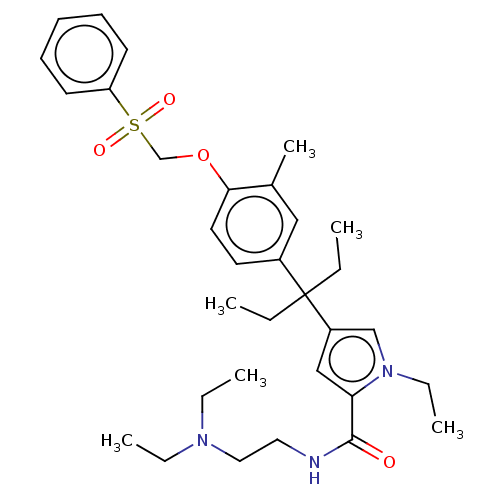
(CHEMBL4290851)Show SMILES CCN(CC)CCNC(=O)c1cc(cn1CC)C(CC)(CC)c1ccc(OCS(=O)(=O)c2ccccc2)c(C)c1 Show InChI InChI=1S/C32H45N3O4S/c1-7-32(8-2,27-22-29(35(11-5)23-27)31(36)33-19-20-34(9-3)10-4)26-17-18-30(25(6)21-26)39-24-40(37,38)28-15-13-12-14-16-28/h12-18,21-23H,7-11,19-20,24H2,1-6H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of fluormone VDR red from human full-length VDR after 4 hrs by fluorescence polarization assay |
Eur J Med Chem 157: 1174-1191 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.085
BindingDB Entry DOI: 10.7270/Q2WH2SQJ |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50602006

(CHEMBL5209436)Show SMILES ONC(=O)CN(c1cccc(c1)[N+]([O-])=O)S(=O)(=O)c1ccc(Br)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129043
BindingDB Entry DOI: 10.7270/Q23N27G1 |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274307
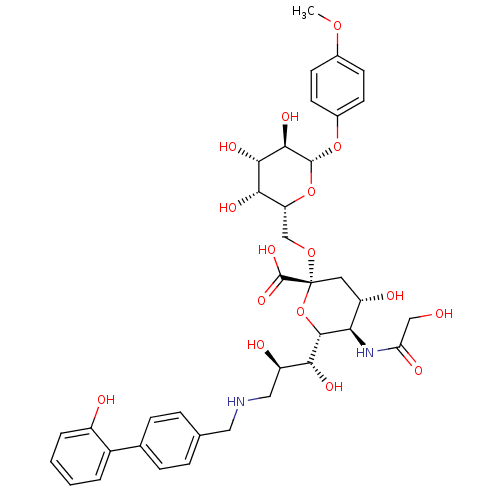
(CHEMBL511111 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNCc3ccc(cc3)-c3ccccc3O)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C37H46N2O16/c1-51-21-10-12-22(13-11-21)53-35-33(48)32(47)31(46)27(54-35)18-52-37(36(49)50)14-25(42)29(39-28(44)17-40)34(55-37)30(45)26(43)16-38-15-19-6-8-20(9-7-19)23-4-2-3-5-24(23)41/h2-13,25-27,29-35,38,40-43,45-48H,14-18H2,1H3,(H,39,44)(H,49,50)/t25-,26+,27+,29+,30+,31-,32-,33+,34+,35+,37+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274303
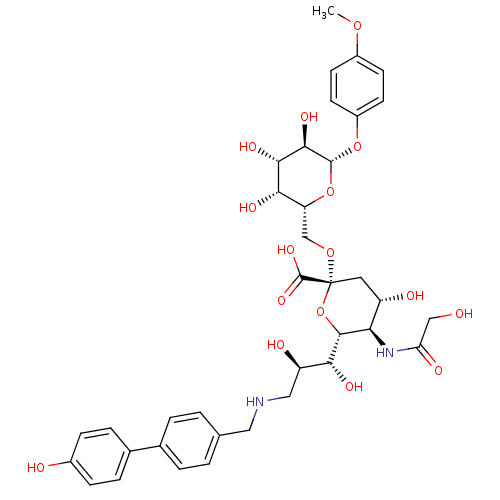
(CHEMBL502862 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNCc3ccc(cc3)-c3ccc(O)cc3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C37H46N2O16/c1-51-23-10-12-24(13-11-23)53-35-33(48)32(47)31(46)27(54-35)18-52-37(36(49)50)14-25(42)29(39-28(44)17-40)34(55-37)30(45)26(43)16-38-15-19-2-4-20(5-3-19)21-6-8-22(41)9-7-21/h2-13,25-27,29-35,38,40-43,45-48H,14-18H2,1H3,(H,39,44)(H,49,50)/t25-,26+,27+,29+,30+,31-,32-,33+,34+,35+,37+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274292

(CHEMBL509768 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNC(=O)c3ccc(cc3)-c3ccc(O)cc3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C37H44N2O17/c1-52-22-10-12-23(13-11-22)54-35-32(48)31(47)30(46)26(55-35)17-53-37(36(50)51)14-24(42)28(39-27(44)16-40)33(56-37)29(45)25(43)15-38-34(49)20-4-2-18(3-5-20)19-6-8-21(41)9-7-19/h2-13,24-26,28-33,35,40-43,45-48H,14-17H2,1H3,(H,38,49)(H,39,44)(H,50,51)/t24-,25+,26+,28+,29+,30-,31-,32+,33+,35+,37+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50446421

(CHEMBL3109740)Show SMILES C\C(=C/C(=O)Nc1ccc(cc1C(O)=O)N1CCOCC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C25H24N2O4/c1-17(19-7-6-18-4-2-3-5-20(18)15-19)14-24(28)26-23-9-8-21(16-22(23)25(29)30)27-10-12-31-13-11-27/h2-9,14-16H,10-13H2,1H3,(H,26,28)(H,29,30)/b17-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase |
Eur J Med Chem 112: 231-51 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.009
BindingDB Entry DOI: 10.7270/Q2SB47N4 |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274300
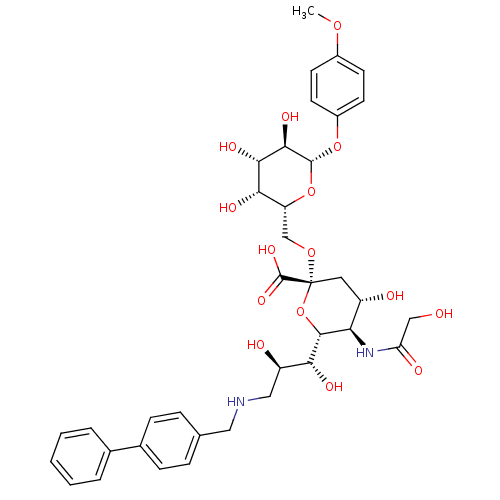
(CHEMBL509633 | p-Methoxyphenyl (9-(4-biphenyl)meth...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNCc3ccc(cc3)-c3ccccc3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C37H46N2O15/c1-50-23-11-13-24(14-12-23)52-35-33(47)32(46)31(45)27(53-35)19-51-37(36(48)49)15-25(41)29(39-28(43)18-40)34(54-37)30(44)26(42)17-38-16-20-7-9-22(10-8-20)21-5-3-2-4-6-21/h2-14,25-27,29-35,38,40-42,44-47H,15-19H2,1H3,(H,39,43)(H,48,49)/t25-,26+,27+,29+,30+,31-,32-,33+,34+,35+,37+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50469128

(CHEMBL4290851)Show SMILES CCN(CC)CCNC(=O)c1cc(cn1CC)C(CC)(CC)c1ccc(OCS(=O)(=O)c2ccccc2)c(C)c1 Show InChI InChI=1S/C32H45N3O4S/c1-7-32(8-2,27-22-29(35(11-5)23-27)31(36)33-19-20-34(9-3)10-4)26-17-18-30(25(6)21-26)39-24-40(37,38)28-15-13-12-14-16-28/h12-18,21-23H,7-11,19-20,24H2,1-6H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to VDR in scrambled siRNA-transfected human MCF7 cells assessed as cell growth inhibition by measuring reduction in BrdU incorporati... |
Eur J Med Chem 157: 1174-1191 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.085
BindingDB Entry DOI: 10.7270/Q2WH2SQJ |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50602003
(CHEMBL5207136)Show SMILES ONC(=O)CN(c1cccc(c1)[N+]([O-])=O)S(=O)(=O)c1cccc(F)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129043
BindingDB Entry DOI: 10.7270/Q23N27G1 |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274296

(CHEMBL501599 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNC(=O)Cc3ccc(cc3)-c3ccc(O)cc3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C38H46N2O17/c1-53-23-10-12-24(13-11-23)55-36-34(50)33(49)32(48)27(56-36)18-54-38(37(51)52)15-25(43)30(40-29(46)17-41)35(57-38)31(47)26(44)16-39-28(45)14-19-2-4-20(5-3-19)21-6-8-22(42)9-7-21/h2-13,25-27,30-36,41-44,47-50H,14-18H2,1H3,(H,39,45)(H,40,46)(H,51,52)/t25-,26+,27+,30+,31+,32-,33-,34+,35+,36+,38+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50469127

(CHEMBL4288947)Show SMILES CCN(CC)CCNC(=O)c1ccc(n1CC)C(CC)(CC)c1ccc(OCSC)c(C)c1 Show InChI InChI=1S/C27H43N3O2S/c1-8-27(9-2,22-13-15-24(21(6)19-22)32-20-33-7)25-16-14-23(30(25)12-5)26(31)28-17-18-29(10-3)11-4/h13-16,19H,8-12,17-18,20H2,1-7H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to VDR in scrambled siRNA-transfected human MCF7 cells assessed as cell growth inhibition by measuring reduction in BrdU incorporati... |
Eur J Med Chem 157: 1174-1191 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.085
BindingDB Entry DOI: 10.7270/Q2WH2SQJ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50160391
(CHEMBL3786231)Show SMILES CCCCN(CCCC)CC(=O)N1N=C(CC1c1ccccc1)c1cc2ccccc2oc1=O |c:13| Show InChI InChI=1S/C28H33N3O3/c1-3-5-16-30(17-6-4-2)20-27(32)31-25(21-12-8-7-9-13-21)19-24(29-31)23-18-22-14-10-11-15-26(22)34-28(23)33/h7-15,18,25H,3-6,16-17,19-20H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University
Curated by ChEMBL
| Assay Description
Inhibition of telomerase in human MGC-803 cells after 24 hrs by TRAP-PCR-ELISA assay |
Eur J Med Chem 112: 231-51 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.009
BindingDB Entry DOI: 10.7270/Q2SB47N4 |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274291
(CHEMBL502788 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNC(=O)Cc3ccc4ccccc4c3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C36H44N2O16/c1-50-21-8-10-22(11-9-21)52-34-32(47)31(46)30(45)25(53-34)17-51-36(35(48)49)14-23(40)28(38-27(43)16-39)33(54-36)29(44)24(41)15-37-26(42)13-18-6-7-19-4-2-3-5-20(19)12-18/h2-12,23-25,28-34,39-41,44-47H,13-17H2,1H3,(H,37,42)(H,38,43)(H,48,49)/t23-,24+,25+,28+,29+,30-,31-,32+,33+,34+,36+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274289

(CHEMBL503830 | p-Methoxyphenyl (9-(4-biphenylcarbo...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNC(=O)c3ccc(cc3)-c3ccccc3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C37H44N2O16/c1-51-22-11-13-23(14-12-22)53-35-32(47)31(46)30(45)26(54-35)18-52-37(36(49)50)15-24(41)28(39-27(43)17-40)33(55-37)29(44)25(42)16-38-34(48)21-9-7-20(8-10-21)19-5-3-2-4-6-19/h2-14,24-26,28-33,35,40-42,44-47H,15-18H2,1H3,(H,38,48)(H,39,43)(H,49,50)/t24-,25+,26+,28+,29+,30-,31-,32+,33+,35+,37+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50160320
(CHEMBL3786261)Show InChI InChI=1S/C14H15ClN2O3/c1-9-7-12(17(16-9)14(19)8-15)11-5-3-4-6-13(11)20-10(2)18/h3-6,12H,7-8H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University
Curated by ChEMBL
| Assay Description
Inhibition of telomerase in human MGC-803 cells after 24 hrs by TRAP-PCR-ELISA assay |
Eur J Med Chem 112: 231-51 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.009
BindingDB Entry DOI: 10.7270/Q2SB47N4 |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274306

(CHEMBL505326 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNCc3ccc(cc3)-c3cccc(O)c3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C37H46N2O16/c1-51-23-9-11-24(12-10-23)53-35-33(48)32(47)31(46)27(54-35)18-52-37(36(49)50)14-25(42)29(39-28(44)17-40)34(55-37)30(45)26(43)16-38-15-19-5-7-20(8-6-19)21-3-2-4-22(41)13-21/h2-13,25-27,29-35,38,40-43,45-48H,14-18H2,1H3,(H,39,44)(H,49,50)/t25-,26+,27+,29+,30+,31-,32-,33+,34+,35+,37+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50602005
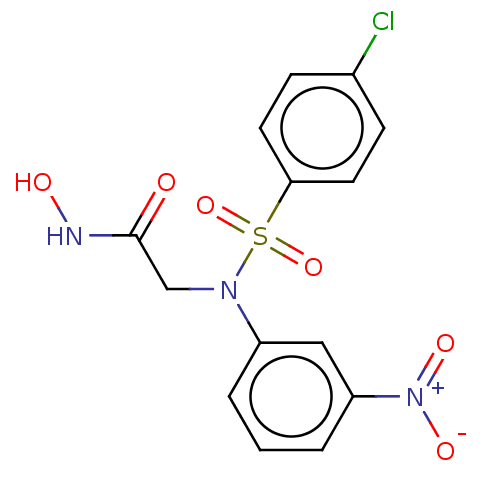
(CHEMBL5188176)Show SMILES ONC(=O)CN(c1cccc(c1)[N+]([O-])=O)S(=O)(=O)c1ccc(Cl)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129043
BindingDB Entry DOI: 10.7270/Q23N27G1 |
More data for this
Ligand-Target Pair | |
B-cell receptor CD22
(Homo sapiens (Human)) | BDBM50274308
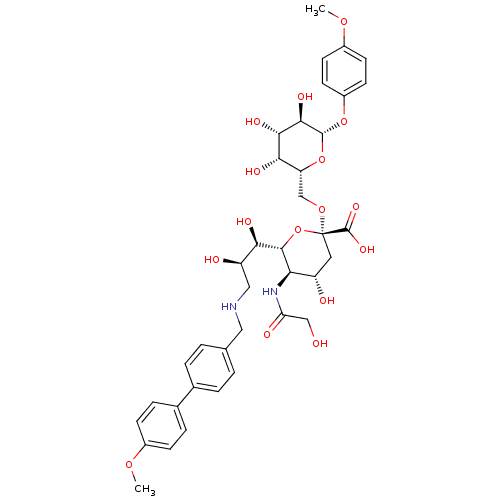
(CHEMBL504222 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...)Show SMILES COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNCc3ccc(cc3)-c3ccc(OC)cc3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C38H48N2O16/c1-51-23-9-7-22(8-10-23)21-5-3-20(4-6-21)16-39-17-27(43)31(45)35-30(40-29(44)18-41)26(42)15-38(56-35,37(49)50)53-19-28-32(46)33(47)34(48)36(55-28)54-25-13-11-24(52-2)12-14-25/h3-14,26-28,30-36,39,41-43,45-48H,15-19H2,1-2H3,(H,40,44)(H,49,50)/t26-,27+,28+,30+,31+,32-,33-,34+,35+,36+,38+/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry |
J Med Chem 51: 6665-81 (2008)
Article DOI: 10.1021/jm8000696
BindingDB Entry DOI: 10.7270/Q23X86GN |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50609626
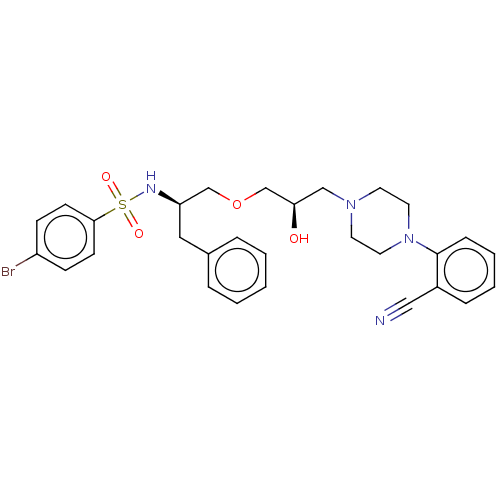
(CHEMBL5283504)Show SMILES O[C@@H](COC[C@@H](Cc1ccccc1)NS(=O)(=O)c1ccc(Br)cc1)CN1CCN(CC1)c1ccccc1C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM50184410
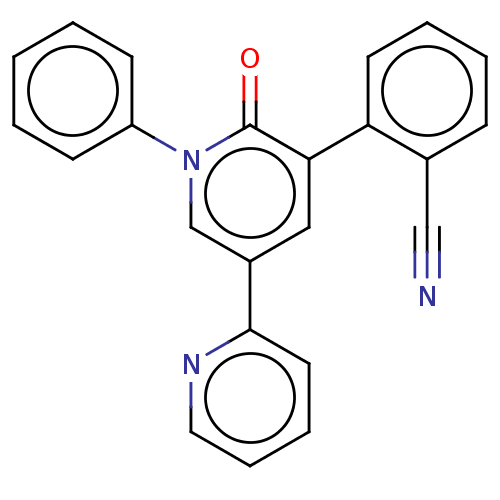
(CHEBI:71013 | E-2007 | E2007 | ER-155055-90 | Fyco...)Show SMILES O=c1c(cc(cn1-c1ccccc1)-c1ccccn1)-c1ccccc1C#N Show InChI InChI=1S/C23H15N3O/c24-15-17-8-4-5-11-20(17)21-14-18(22-12-6-7-13-25-22)16-26(23(21)27)19-9-2-1-3-10-19/h1-14,16H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50160330
(CHEMBL3785281)Show SMILES CN1CCN(CC(=O)N2N=C(C)CC2c2ccccc2O)CC1 |t:9| Show InChI InChI=1S/C17H24N4O2/c1-13-11-15(14-5-3-4-6-16(14)22)21(18-13)17(23)12-20-9-7-19(2)8-10-20/h3-6,15,22H,7-12H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University
Curated by ChEMBL
| Assay Description
Inhibition of telomerase in human MGC-803 cells after 24 hrs by TRAP-PCR-ELISA assay |
Eur J Med Chem 112: 231-51 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.009
BindingDB Entry DOI: 10.7270/Q2SB47N4 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50160326

(CHEMBL3787380)Show SMILES CC(=O)Oc1ccccc1C1CC(C)=NN1C(=O)CN1CCCCC1 |c:14| Show InChI InChI=1S/C19H25N3O3/c1-14-12-17(16-8-4-5-9-18(16)25-15(2)23)22(20-14)19(24)13-21-10-6-3-7-11-21/h4-5,8-9,17H,3,6-7,10-13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University
Curated by ChEMBL
| Assay Description
Inhibition of telomerase in human MGC-803 cells after 24 hrs by TRAP-PCR-ELISA assay |
Eur J Med Chem 112: 231-51 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.009
BindingDB Entry DOI: 10.7270/Q2SB47N4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data