 Found 350 hits with Last Name = 'zawadzke' and Initial = 'le'
Found 350 hits with Last Name = 'zawadzke' and Initial = 'le' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50432208
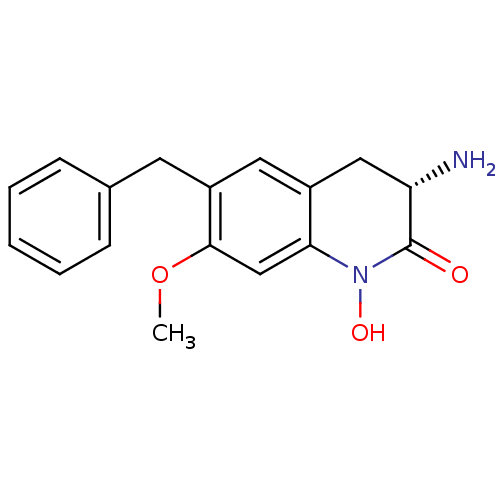
(CHEMBL2347110)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Cc1ccccc1 |r| Show InChI InChI=1S/C17H18N2O3/c1-22-16-10-15-12(9-14(18)17(20)19(15)21)8-13(16)7-11-5-3-2-4-6-11/h2-6,8,10,14,21H,7,9,18H2,1H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340
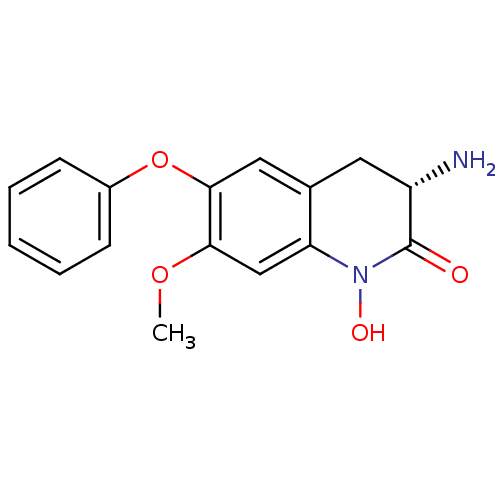
(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107730
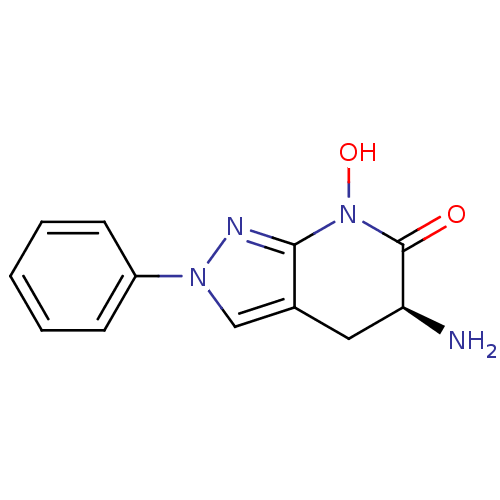
(CHEMBL2347108 | US8933095, 14)Show InChI InChI=1S/C12H12N4O2/c13-10-6-8-7-15(9-4-2-1-3-5-9)14-11(8)16(18)12(10)17/h1-5,7,10,18H,6,13H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341
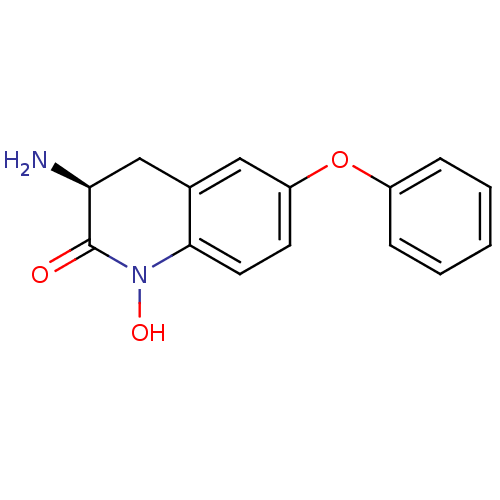
(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310
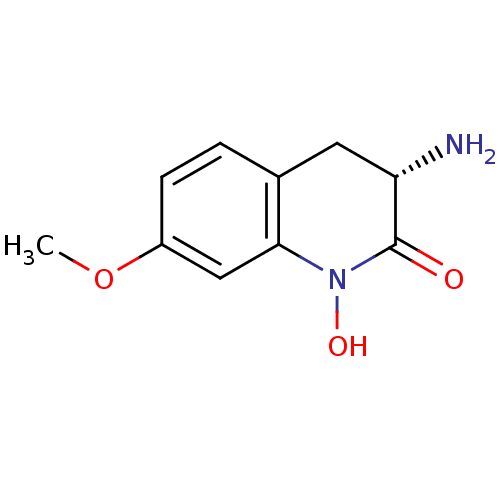
(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107747
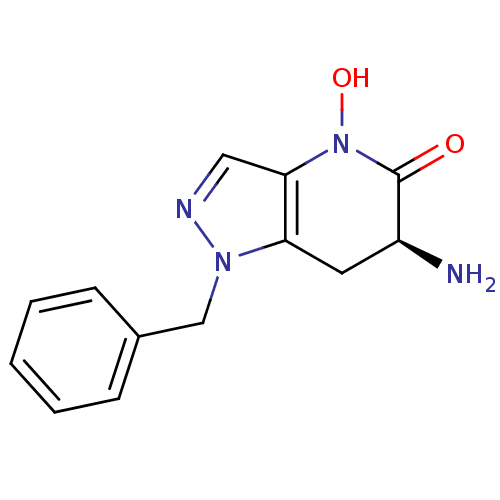
(CHEMBL2347115 | US8933095, 4)Show InChI InChI=1S/C13H14N4O2/c14-10-6-11-12(17(19)13(10)18)7-15-16(11)8-9-4-2-1-3-5-9/h1-5,7,10,19H,6,8,14H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292
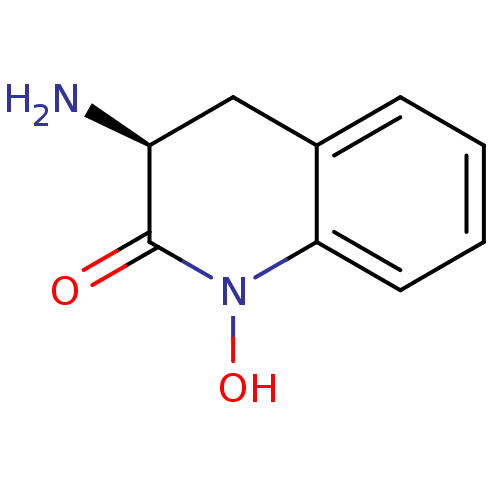
(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107738
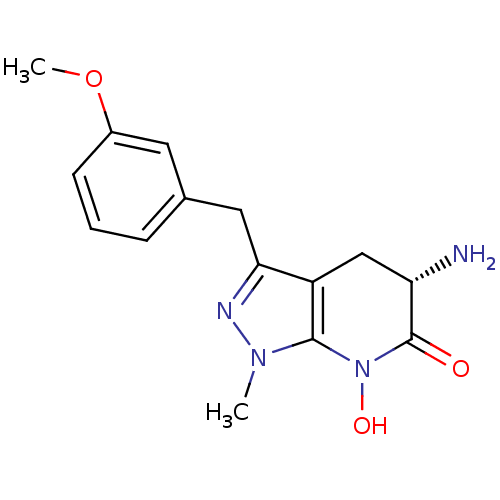
(CHEMBL2347113 | US8933095, 22)Show SMILES COc1cccc(Cc2nn(C)c3N(O)C(=O)[C@@H](N)Cc23)c1 |r| Show InChI InChI=1S/C15H18N4O3/c1-18-14-11(8-12(16)15(20)19(14)21)13(17-18)7-9-4-3-5-10(6-9)22-2/h3-6,12,21H,7-8,16H2,1-2H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107746
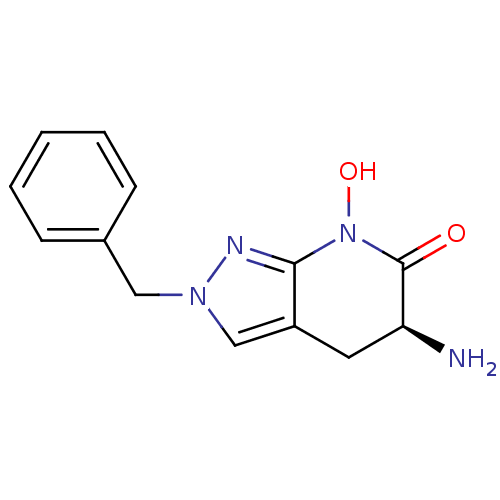
(CHEMBL2347107 | US8933095, 1)Show InChI InChI=1S/C13H14N4O2/c14-11-6-10-8-16(7-9-4-2-1-3-5-9)15-12(10)17(19)13(11)18/h1-5,8,11,19H,6-7,14H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107720
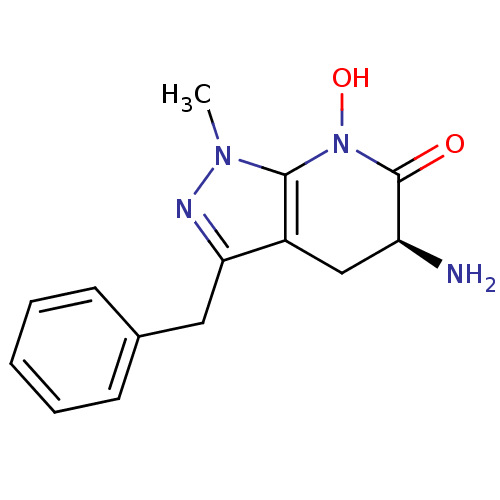
(CHEMBL2347112 | US8598200, 2)Show SMILES Cn1nc(Cc2ccccc2)c2C[C@H](N)C(=O)N(O)c12 |r| Show InChI InChI=1S/C14H16N4O2/c1-17-13-10(8-11(15)14(19)18(13)20)12(16-17)7-9-5-3-2-4-6-9/h2-6,11,20H,7-8,15H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50432200
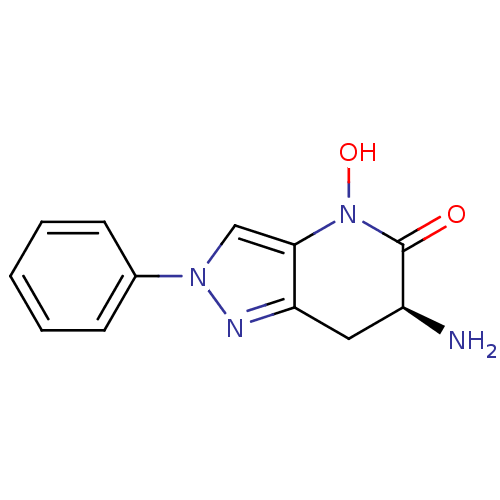
(CHEMBL2347109 | US8933095, 16)Show InChI InChI=1S/C12H12N4O2/c13-9-6-10-11(16(18)12(9)17)7-15(14-10)8-4-2-1-3-5-8/h1-5,7,9,18H,6,13H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107722
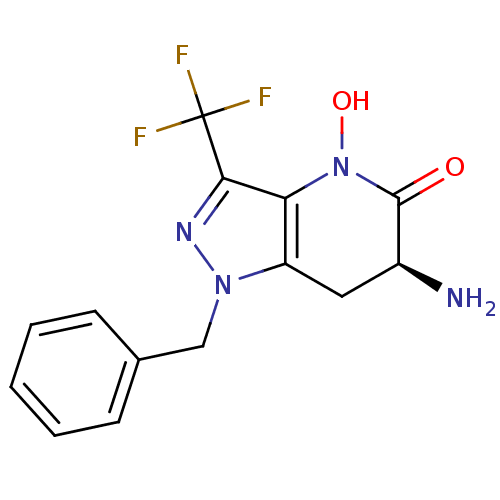
(CHEMBL2347114 | US8933095, 5)Show SMILES N[C@H]1Cc2c(N(O)C1=O)c(nn2Cc1ccccc1)C(F)(F)F |r| Show InChI InChI=1S/C14H13F3N4O2/c15-14(16,17)12-11-10(6-9(18)13(22)21(11)23)20(19-12)7-8-4-2-1-3-5-8/h1-5,9,23H,6-7,18H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503407

(CHEMBL4440829)Show SMILES CCOc1ncc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C23H26N4O4/c1-3-5-8-27-15-19(17-6-7-24-20(17)23(27)29)16-13-18(21(25-14-16)31-4-2)22(28)26-9-11-30-12-10-26/h3,6-7,13-15,24H,1,4-5,8-12H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503399

(CHEMBL4441257)Show SMILES Clc1ccc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H22ClN3O3/c1-2-3-8-26-14-18(16-6-7-24-20(16)22(26)28)15-4-5-19(23)17(13-15)21(27)25-9-11-29-12-10-25/h2,4-7,13-14,24H,1,3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321463

(6-(but-3-en-1-yl)-4-(3-(difluoromethoxy)-5-(morpho...)Show SMILES FC(F)Oc1cc(cc(c1)-c1cn(CCC=C)c(=O)c2[nH]ccc12)C(=O)N1CCOCC1 Show InChI InChI=1S/C23H23F2N3O4/c1-2-3-6-28-14-19(18-4-5-26-20(18)22(28)30)15-11-16(13-17(12-15)32-23(24)25)21(29)27-7-9-31-10-8-27/h2,4-5,11-14,23,26H,1,3,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321467
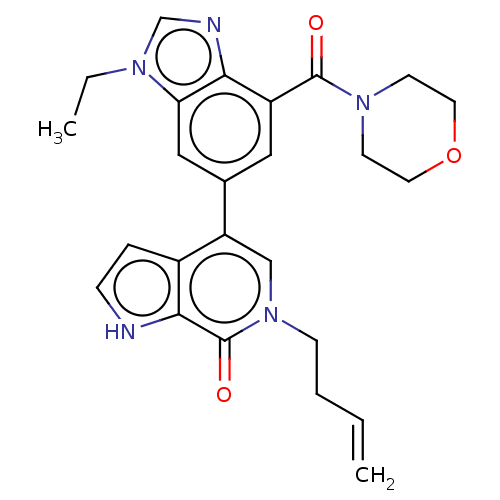
(6-but-3-enyl-4-[3-ethyl-7-(morpholine-4-carbonyl)b...)Show SMILES CCn1cnc2c(cc(cc12)-c1cn(CCC=C)c(=O)c2[nH]ccc12)C(=O)N1CCOCC1 Show InChI InChI=1S/C25H27N5O3/c1-3-5-8-30-15-20(18-6-7-26-23(18)25(30)32)17-13-19(24(31)29-9-11-33-12-10-29)22-21(14-17)28(4-2)16-27-22/h3,6-7,13-16,26H,1,4-5,8-12H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503413

(CHEMBL4566599)Show SMILES COc1ncc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H24N4O4/c1-3-4-7-26-14-18(16-5-6-23-19(16)22(26)28)15-12-17(20(29-2)24-13-15)21(27)25-8-10-30-11-9-25/h3,5-6,12-14,23H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503406
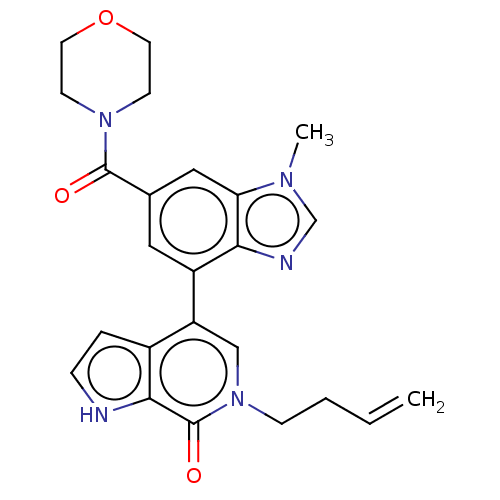
(CHEMBL4463538)Show SMILES Cn1cnc2c(cc(cc12)C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C24H25N5O3/c1-3-4-7-29-14-19(17-5-6-25-22(17)24(29)31)18-12-16(13-20-21(18)26-15-27(20)2)23(30)28-8-10-32-11-9-28/h3,5-6,12-15,25H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321462

(6-but-3-enyl-4-[4-fluoro-3-(morpholine-4-carbonyl)...)Show SMILES Fc1ccc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H22FN3O3/c1-2-3-8-26-14-18(16-6-7-24-20(16)22(26)28)15-4-5-19(23)17(13-15)21(27)25-9-11-29-12-10-25/h2,4-7,13-14,24H,1,3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503404

(CHEMBL4455513)Show SMILES Cn1cnc2cc(cc(C(=O)N3CCOCC3)c12)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C24H25N5O3/c1-3-4-7-29-14-19(17-5-6-25-21(17)24(29)31)16-12-18(22-20(13-16)26-15-27(22)2)23(30)28-8-10-32-11-9-28/h3,5-6,12-15,25H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321458

(6-but-3-enyl-4-[3,4-difluoro-5-(morpholine-4-carbo...)Show SMILES Fc1cc(cc(C(=O)N2CCOCC2)c1F)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H21F2N3O3/c1-2-3-6-27-13-17(15-4-5-25-20(15)22(27)29)14-11-16(19(24)18(23)12-14)21(28)26-7-9-30-10-8-26/h2,4-5,11-13,25H,1,3,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503408

(CHEMBL4459660)Show SMILES Cc1ncc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H24N4O3/c1-3-4-7-26-14-19(17-5-6-23-20(17)22(26)28)16-12-18(15(2)24-13-16)21(27)25-8-10-29-11-9-25/h3,5-6,12-14,23H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321460

(3-(6-but-3-enyl-7-oxo-1H-pyrrolo[2,3-c]pyridin-4-y...)Show SMILES C=CCCn1cc(-c2cc(cc(c2)C(=O)N2CCOCC2)C#N)c2cc[nH]c2c1=O Show InChI InChI=1S/C23H22N4O3/c1-2-3-6-27-15-20(19-4-5-25-21(19)23(27)29)17-11-16(14-24)12-18(13-17)22(28)26-7-9-30-10-8-26/h2,4-5,11-13,15,25H,1,3,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503398

(CHEMBL4445482)Show SMILES CCn1cnc2cc(cc(C(=O)N3CCOCC3)c12)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C25H27N5O3/c1-3-5-8-30-15-20(18-6-7-26-22(18)25(30)32)17-13-19(24(31)29-9-11-33-12-10-29)23-21(14-17)27-16-28(23)4-2/h3,6-7,13-16,26H,1,4-5,8-12H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321464

(6-but-3-enyl-4-[7-(morpholine-4-carbonyl)-3H-benzi...)Show SMILES C=CCCn1cc(-c2cc(C(=O)N3CCOCC3)c3nc[nH]c3c2)c2cc[nH]c2c1=O Show InChI InChI=1S/C23H23N5O3/c1-2-3-6-28-13-18(16-4-5-24-21(16)23(28)30)15-11-17(20-19(12-15)25-14-26-20)22(29)27-7-9-31-10-8-27/h2,4-5,11-14,24H,1,3,6-10H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321465
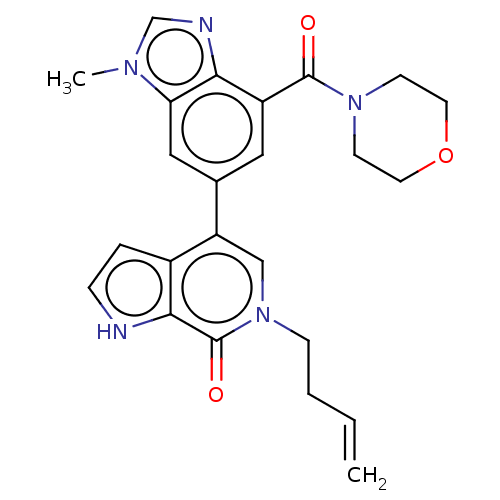
(6-but-3-enyl-4-[3-methyl-7-(morpholine-4-carbonyl)...)Show SMILES Cn1cnc2c(cc(cc12)-c1cn(CCC=C)c(=O)c2[nH]ccc12)C(=O)N1CCOCC1 Show InChI InChI=1S/C24H25N5O3/c1-3-4-7-29-14-19(17-5-6-25-22(17)24(29)31)16-12-18(21-20(13-16)27(2)15-26-21)23(30)28-8-10-32-11-9-28/h3,5-6,12-15,25H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503412

(CHEMBL4459431)Show SMILES C=CCCn1cc(-c2cncc(c2)C(=O)N2CCOCC2)c2cc[nH]c2c1=O Show InChI InChI=1S/C21H22N4O3/c1-2-3-6-25-14-18(17-4-5-23-19(17)21(25)27)15-11-16(13-22-12-15)20(26)24-7-9-28-10-8-24/h2,4-5,11-14,23H,1,3,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321461
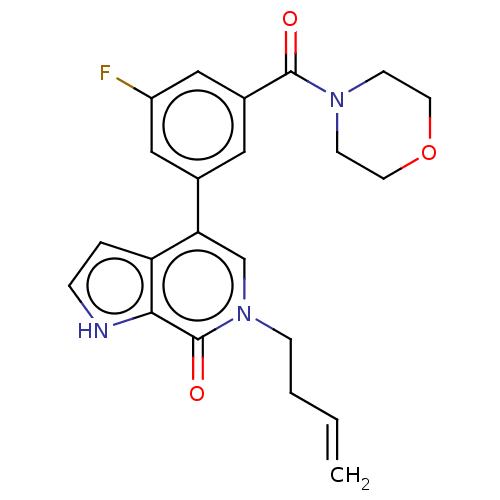
(6-but-3-enyl-4-[3-fluoro-5-(morpholine-4-carbonyl)...)Show SMILES Fc1cc(cc(c1)-c1cn(CCC=C)c(=O)c2[nH]ccc12)C(=O)N1CCOCC1 Show InChI InChI=1S/C22H22FN3O3/c1-2-3-6-26-14-19(18-4-5-24-20(18)22(26)28)15-11-16(13-17(23)12-15)21(27)25-7-9-29-10-8-25/h2,4-5,11-14,24H,1,3,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310

(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310

(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503403

(CHEMBL4463398)Show SMILES C=CCCn1cc(-c2cccc(c2)C(=O)N2CCOCC2)c2cc[nH]c2c1=O Show InChI InChI=1S/C22H23N3O3/c1-2-3-9-25-15-19(18-7-8-23-20(18)22(25)27)16-5-4-6-17(14-16)21(26)24-10-12-28-13-11-24/h2,4-8,14-15,23H,1,3,9-13H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341

(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107747

(CHEMBL2347115 | US8933095, 4)Show InChI InChI=1S/C13H14N4O2/c14-10-6-11-12(17(19)13(10)18)7-15-16(11)8-9-4-2-1-3-5-9/h1-5,7,10,19H,6,8,14H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107730

(CHEMBL2347108 | US8933095, 14)Show InChI InChI=1S/C12H12N4O2/c13-10-6-8-7-15(9-4-2-1-3-5-9)14-11(8)16(18)12(10)17/h1-5,7,10,18H,6,13H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503411

(CHEMBL4448345)Show SMILES Nc1cc(cc(c1)-c1cn(CCC=C)c(=O)c2[nH]ccc12)C(=O)N1CCOCC1 Show InChI InChI=1S/C22H24N4O3/c1-2-3-6-26-14-19(18-4-5-24-20(18)22(26)28)15-11-16(13-17(23)12-15)21(27)25-7-9-29-10-8-25/h2,4-5,11-14,24H,1,3,6-10,23H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321468
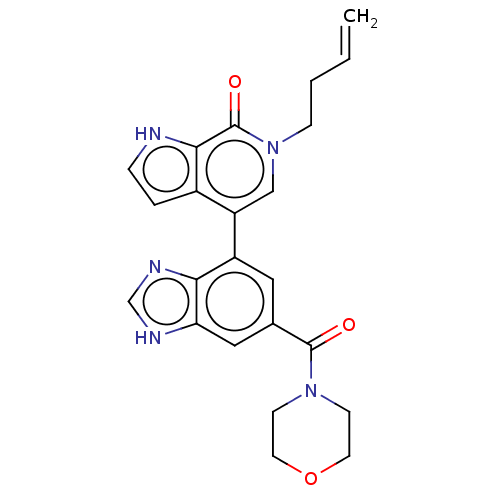
(6-but-3-enyl-4-[6-(morpholine-4-carbonyl)-1H-benzi...)Show SMILES C=CCCn1cc(-c2cc(cc3[nH]cnc23)C(=O)N2CCOCC2)c2cc[nH]c2c1=O Show InChI InChI=1S/C23H23N5O3/c1-2-3-6-28-13-18(16-4-5-24-21(16)23(28)30)17-11-15(12-19-20(17)26-14-25-19)22(29)27-7-9-31-10-8-27/h2,4-5,11-14,24H,1,3,6-10H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386308
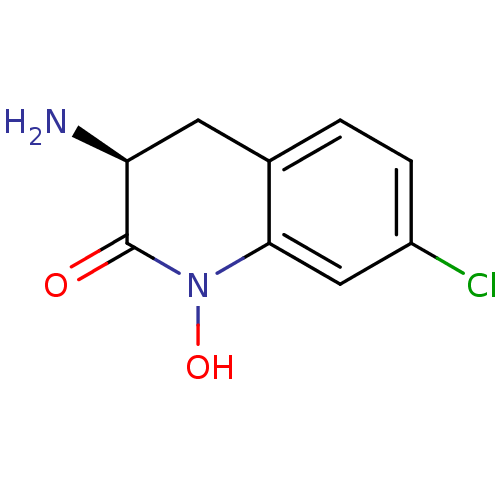
(CHEMBL2047861)Show InChI InChI=1S/C9H9ClN2O2/c10-6-2-1-5-3-7(11)9(13)12(14)8(5)4-6/h1-2,4,7,14H,3,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386294
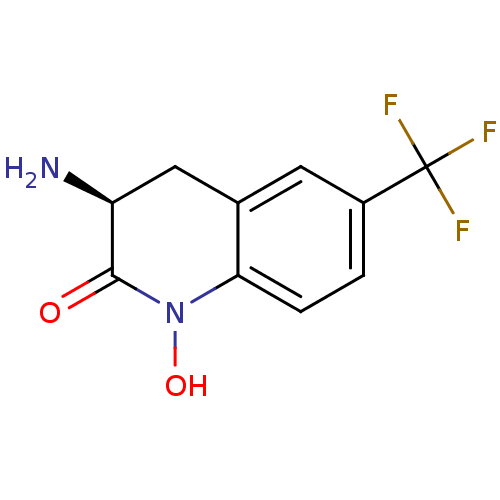
(CHEMBL2049095)Show InChI InChI=1S/C10H9F3N2O2/c11-10(12,13)6-1-2-8-5(3-6)4-7(14)9(16)15(8)17/h1-3,7,17H,4,14H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386312
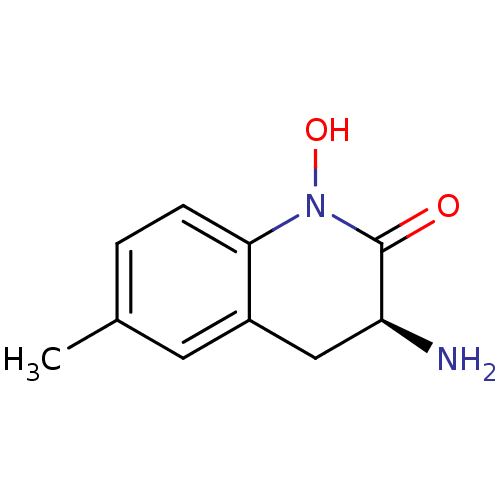
(CHEMBL2049094)Show InChI InChI=1S/C10H12N2O2/c1-6-2-3-9-7(4-6)5-8(11)10(13)12(9)14/h2-4,8,14H,5,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503409
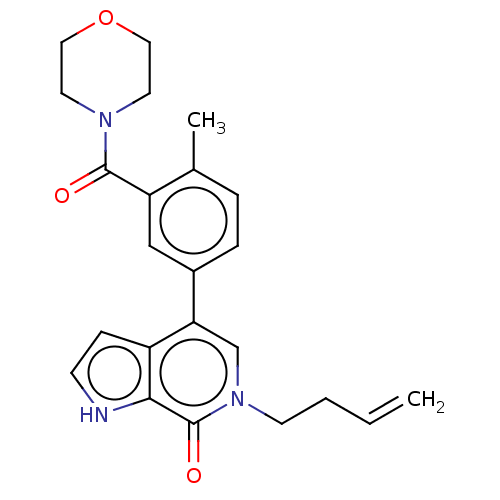
(CHEMBL4465624)Show SMILES Cc1ccc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C23H25N3O3/c1-3-4-9-26-15-20(18-7-8-24-21(18)23(26)28)17-6-5-16(2)19(14-17)22(27)25-10-12-29-13-11-25/h3,5-8,14-15,24H,1,4,9-13H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503400
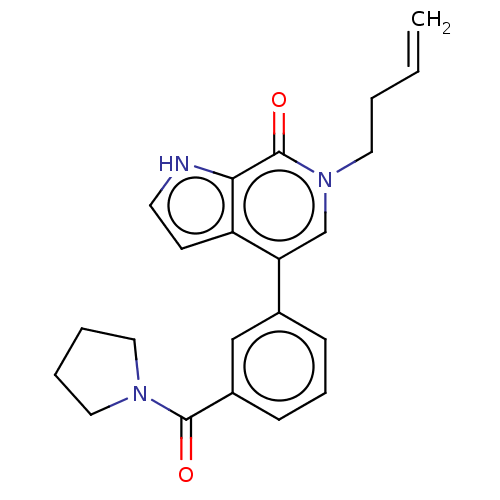
(CHEMBL4483624)Show SMILES C=CCCn1cc(-c2cccc(c2)C(=O)N2CCCC2)c2cc[nH]c2c1=O Show InChI InChI=1S/C22H23N3O2/c1-2-3-11-25-15-19(18-9-10-23-20(18)22(25)27)16-7-6-8-17(14-16)21(26)24-12-4-5-13-24/h2,6-10,14-15,23H,1,3-5,11-13H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386311
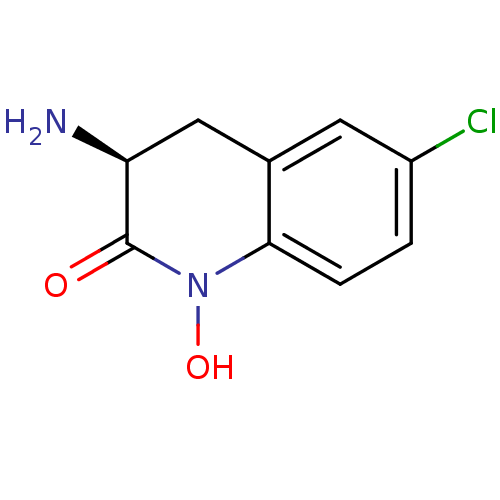
(CHEMBL2049093)Show InChI InChI=1S/C9H9ClN2O2/c10-6-1-2-8-5(3-6)4-7(11)9(13)12(8)14/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340

(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50432208

(CHEMBL2347110)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Cc1ccccc1 |r| Show InChI InChI=1S/C17H18N2O3/c1-22-16-10-15-12(9-14(18)17(20)19(15)21)8-13(16)7-11-5-3-2-4-6-11/h2-6,8,10,14,21H,7,9,18H2,1H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386309
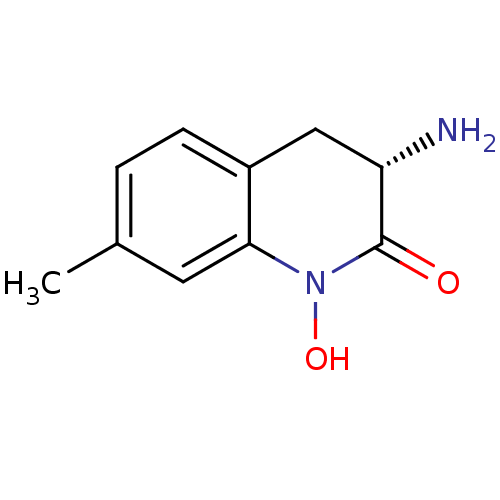
(CHEMBL2047862)Show InChI InChI=1S/C10H12N2O2/c1-6-2-3-7-5-8(11)10(13)12(14)9(7)4-6/h2-4,8,14H,5,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503406

(CHEMBL4463538)Show SMILES Cn1cnc2c(cc(cc12)C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C24H25N5O3/c1-3-4-7-29-14-19(17-5-6-25-22(17)24(29)31)18-12-16(13-20-21(18)26-15-27(20)2)23(30)28-8-10-32-11-9-28/h3,5-6,12-15,25H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-tagged ligand from nanoLuc fused N-terminal TAF1 bromodomain 2 (unknown origin) expressed in 293T cells by luciferase repor... |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386303
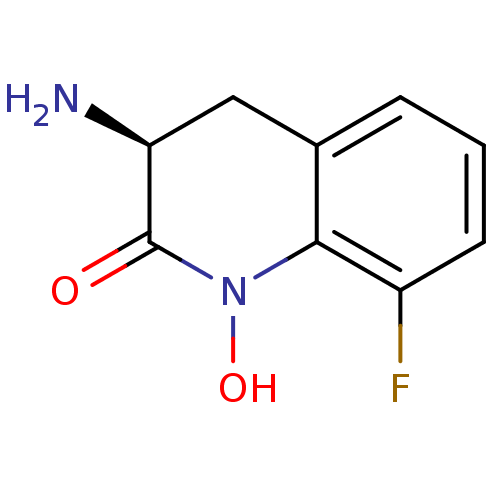
(CHEMBL2047856)Show InChI InChI=1S/C9H9FN2O2/c10-6-3-1-2-5-4-7(11)9(13)12(14)8(5)6/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data