

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53-Ser15 phosphorylation in human HCT116 cells after 24 hrs | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50025671 (CHEMBL583413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53-Ser15 phosphorylation in human HCT116 cells after 24 hrs | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50025671 (CHEMBL583413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299820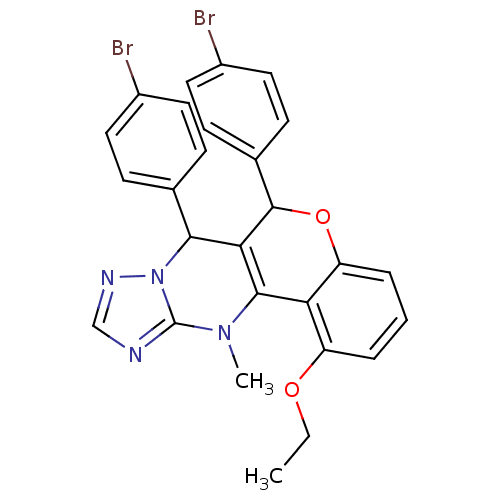 ((+/-)-syn-6,7-Bis(4-bromophenyl)-1-ethoxy-12-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299822 ((+/-)-syn-6,7-Bis(4-chlorophenyl)-12-methyl-7,12-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299818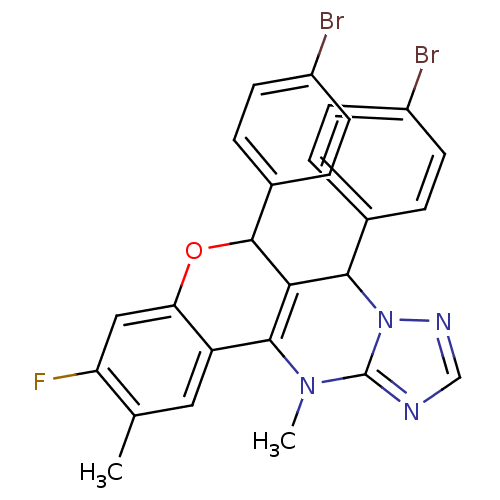 ((+/-)-syn-6,7-Bis(4-bromophenyl)-3-fluoro-2,12-dim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299821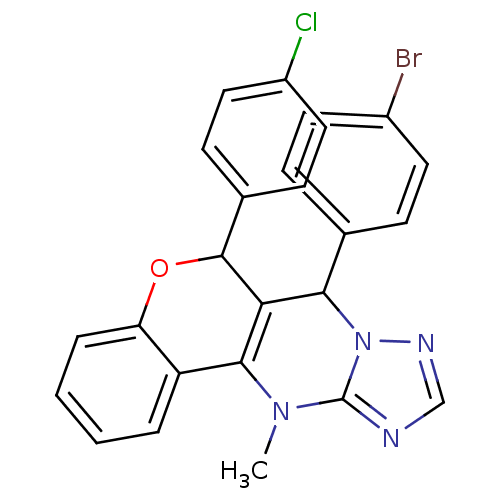 ((+/-)-syn-7-(4-Bromophenyl)-6-(4-chlorophenyl)-12-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299819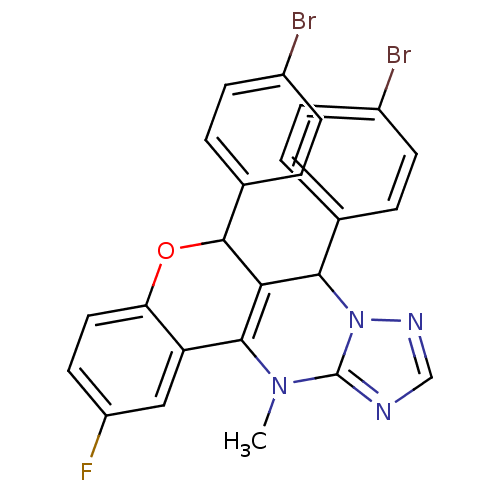 ((+/-)-syn-6,7-Bis(4-bromophenyl)-2-fluoro-12-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50299823 ((6R,7S)-6,7-bis(4-bromophenyl)-7,11-dihydro-6H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of ligase activity of human MDM2 | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||