 Found 50 hits with Last Name = 'araki' and Initial = 'm'
Found 50 hits with Last Name = 'araki' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054109

(CHEMBL3319301)Show SMILES CC(C)(C)C(=O)NC1=NN(C(=O)C(C)(C)C)C(CNS(C)(=O)=O)(S1)c1ccccc1 |t:7| Show InChI InChI=1S/C20H30N4O4S2/c1-18(2,3)15(25)22-17-23-24(16(26)19(4,5)6)20(29-17,13-21-30(7,27)28)14-11-9-8-10-12-14/h8-12,21H,13H2,1-7H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054192

(CHEMBL3319304)Show SMILES CC(C)C(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:6| Show InChI InChI=1S/C20H30N4O4S2/c1-14(2)16(25)24-20(12-13-21-30(6,27)28,15-10-8-7-9-11-15)29-18(23-24)22-17(26)19(3,4)5/h7-11,14,21H,12-13H2,1-6H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054192

(CHEMBL3319304)Show SMILES CC(C)C(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:6| Show InChI InChI=1S/C20H30N4O4S2/c1-14(2)16(25)24-20(12-13-21-30(6,27)28,15-10-8-7-9-11-15)29-18(23-24)22-17(26)19(3,4)5/h7-11,14,21H,12-13H2,1-6H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054187
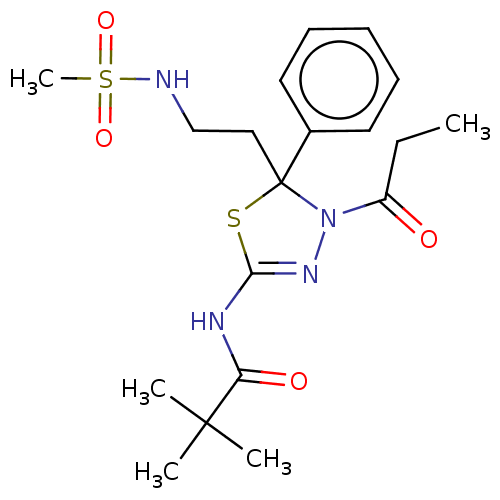
(CHEMBL3319303)Show SMILES CCC(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:5| Show InChI InChI=1S/C19H28N4O4S2/c1-6-15(24)23-19(12-13-20-29(5,26)27,14-10-8-7-9-11-14)28-17(22-23)21-16(25)18(2,3)4/h7-11,20H,6,12-13H2,1-5H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054187

(CHEMBL3319303)Show SMILES CCC(=O)N1N=C(NC(=O)C(C)(C)C)SC1(CCNS(C)(=O)=O)c1ccccc1 |t:5| Show InChI InChI=1S/C19H28N4O4S2/c1-6-15(24)23-19(12-13-20-29(5,26)27,14-10-8-7-9-11-14)28-17(22-23)21-16(25)18(2,3)4/h7-11,20H,6,12-13H2,1-5H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054136

(CHEMBL3319302)Show SMILES CC(C)(C)C(=O)NC1=NN(C(=O)C(C)(C)C)C(CCNS(C)(=O)=O)(S1)c1ccccc1 |t:7| Show InChI InChI=1S/C21H32N4O4S2/c1-19(2,3)16(26)23-18-24-25(17(27)20(4,5)6)21(30-18,13-14-22-31(7,28)29)15-11-9-8-10-12-15/h8-12,22H,13-14H2,1-7H3,(H,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158866
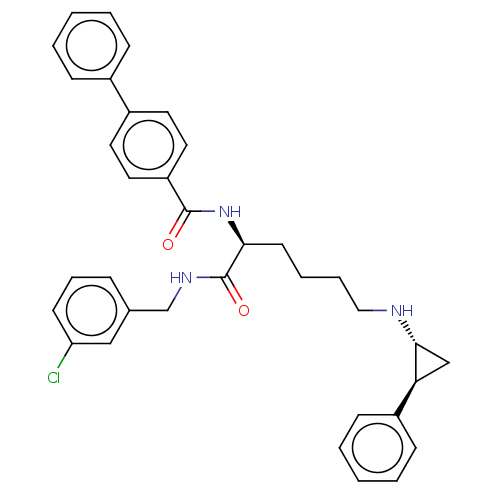
(CHEMBL3785355)Show SMILES Clc1cccc(CNC(=O)[C@H](CCCCN[C@@H]2C[C@H]2c2ccccc2)NC(=O)c2ccc(cc2)-c2ccccc2)c1 |r| Show InChI InChI=1S/C35H36ClN3O2/c36-30-15-9-10-25(22-30)24-38-35(41)32(16-7-8-21-37-33-23-31(33)28-13-5-2-6-14-28)39-34(40)29-19-17-27(18-20-29)26-11-3-1-4-12-26/h1-6,9-15,17-20,22,31-33,37H,7-8,16,21,23-24H2,(H,38,41)(H,39,40)/t31-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LSD1 using H3K4me2 peptide as substrate measured after 30 mins by fluorescence assay |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50054108
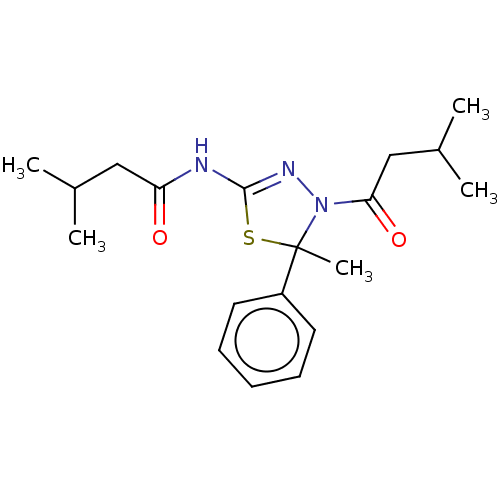
(CHEMBL3356912)Show SMILES CC(C)CC(=O)NC1=NN(C(=O)CC(C)C)C(C)(S1)c1ccccc1 |t:7| Show InChI InChI=1S/C19H27N3O2S/c1-13(2)11-16(23)20-18-21-22(17(24)12-14(3)4)19(5,25-18)15-9-7-6-8-10-15/h6-10,13-14H,11-12H2,1-5H3,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50460073
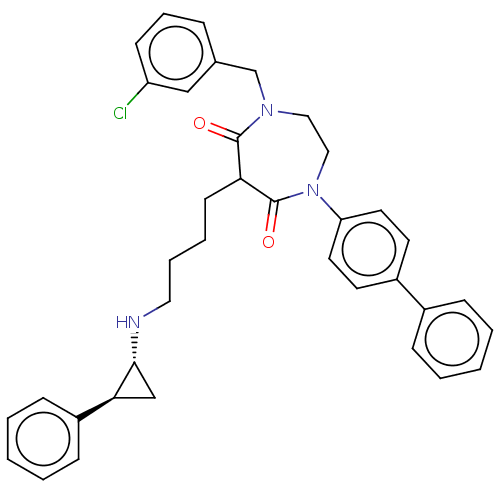
(CHEMBL4227224)Show SMILES Clc1cccc(CN2CCN(c3ccc(cc3)-c3ccccc3)C(=O)C(CCCCN[C@@H]3C[C@H]3c3ccccc3)C2=O)c1 |r| Show InChI InChI=1S/C37H38ClN3O2/c38-31-15-9-10-27(24-31)26-40-22-23-41(32-19-17-29(18-20-32)28-11-3-1-4-12-28)37(43)33(36(40)42)16-7-8-21-39-35-25-34(35)30-13-5-2-6-14-30/h1-6,9-15,17-20,24,33-35,39H,7-8,16,21-23,25-26H2/t33?,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LSD1 using H3K4me2 peptide as substrate measured after 30 mins by fluorescence assay |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50431886

(CHEMBL1413473)Show InChI InChI=1S/C13H15N3O2S/c1-9(17)14-12-15-16(10(2)18)13(3,19-12)11-7-5-4-6-8-11/h4-8H,1-3H3,(H,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kirin Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Eg5 (unknown origin) |
Bioorg Med Chem Lett 24: 3961-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.034
BindingDB Entry DOI: 10.7270/Q2DF6SVT |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazol... |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazol... |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009200

(CHEMBL3238374)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-23(15-20-17-33-27(35-28(20)36)29(40)34-21(16-32)7-5-6-14-31)18-38-26(39)19-37(30(38)41)22-10-12-25(13-11-22)42-24-8-3-2-4-9-24/h2-4,8-13,15,17,21H,5-7,14,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093550
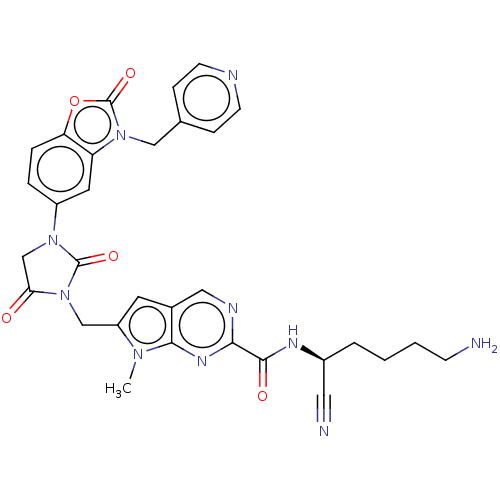
(CHEMBL3585737)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc3oc(=O)n(Cc4ccncc4)c3c2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H30N10O5/c1-38-23(12-20-15-35-27(37-28(20)38)29(43)36-21(14-33)4-2-3-9-32)17-41-26(42)18-39(30(41)44)22-5-6-25-24(13-22)40(31(45)46-25)16-19-7-10-34-11-8-19/h5-8,10-13,15,21H,2-4,9,16-18,32H2,1H3,(H,36,43)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093544
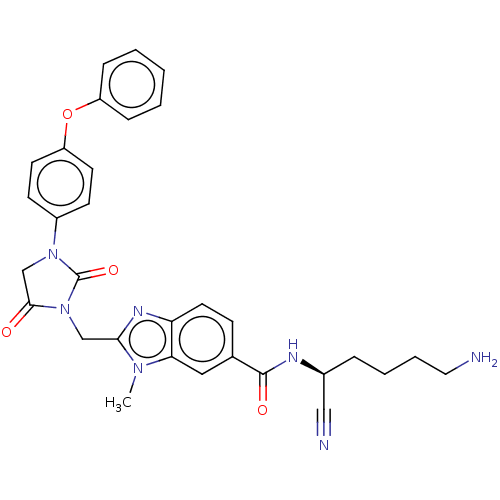
(CHEMBL3585744)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H31N7O4/c1-36-27-17-21(30(40)34-22(18-33)7-5-6-16-32)10-15-26(27)35-28(36)19-38-29(39)20-37(31(38)41)23-11-13-25(14-12-23)42-24-8-3-2-4-9-24/h2-4,8-15,17,22H,5-7,16,19-20,32H2,1H3,(H,34,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093552
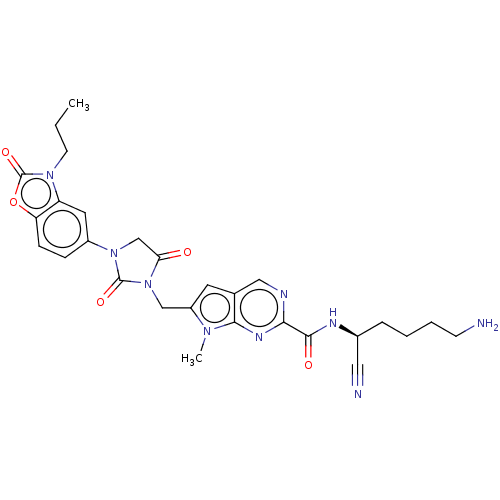
(CHEMBL3585735)Show SMILES CCCn1c2cc(ccc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C28H31N9O5/c1-3-10-35-21-12-19(7-8-22(21)42-28(35)41)36-16-23(38)37(27(36)40)15-20-11-17-14-31-24(33-25(17)34(20)2)26(39)32-18(13-30)6-4-5-9-29/h7-8,11-12,14,18H,3-6,9-10,15-16,29H2,1-2H3,(H,32,39)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093551
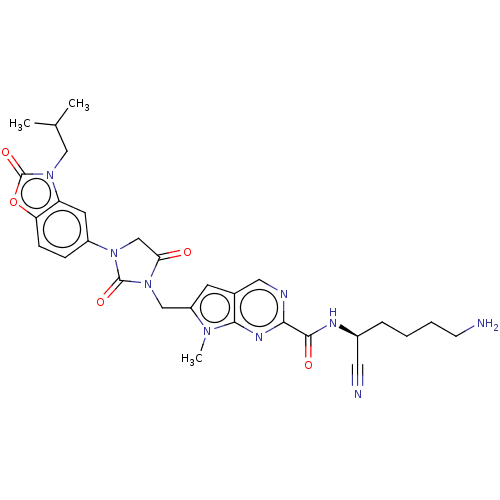
(CHEMBL3585736)Show SMILES CC(C)Cn1c2cc(ccc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C29H33N9O5/c1-17(2)14-37-22-11-20(7-8-23(22)43-29(37)42)36-16-24(39)38(28(36)41)15-21-10-18-13-32-25(34-26(18)35(21)3)27(40)33-19(12-31)6-4-5-9-30/h7-8,10-11,13,17,19H,4-6,9,14-16,30H2,1-3H3,(H,33,40)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009171
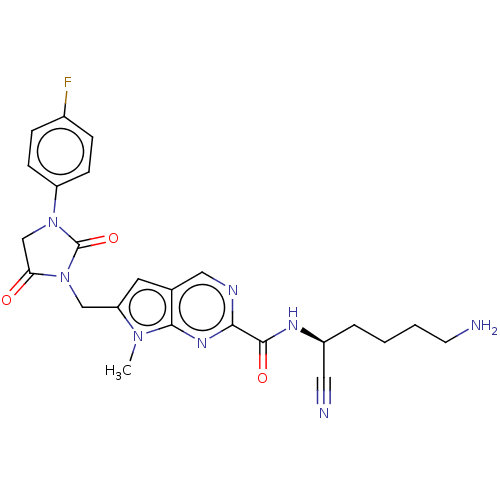
(CHEMBL3238362)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H25FN8O3/c1-31-19(13-33-20(34)14-32(24(33)36)18-7-5-16(25)6-8-18)10-15-12-28-21(30-22(15)31)23(35)29-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093553
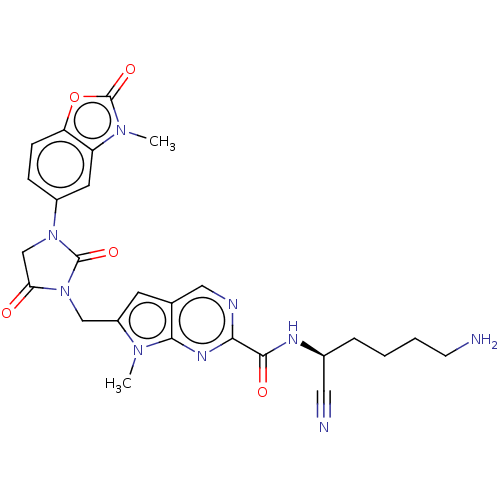
(CHEMBL3585734)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc3oc(=O)n(C)c3c2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H27N9O5/c1-32-18(9-15-12-29-22(31-23(15)32)24(37)30-16(11-28)5-3-4-8-27)13-35-21(36)14-34(25(35)38)17-6-7-20-19(10-17)33(2)26(39)40-20/h6-7,9-10,12,16H,3-5,8,13-14,27H2,1-2H3,(H,30,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093540
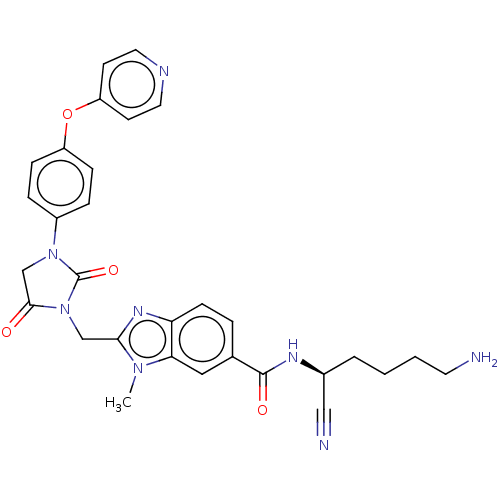
(CHEMBL3585746)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-26-16-20(29(40)34-21(17-32)4-2-3-13-31)5-10-25(26)35-27(36)18-38-28(39)19-37(30(38)41)22-6-8-23(9-7-22)42-24-11-14-33-15-12-24/h5-12,14-16,21H,2-4,13,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093541
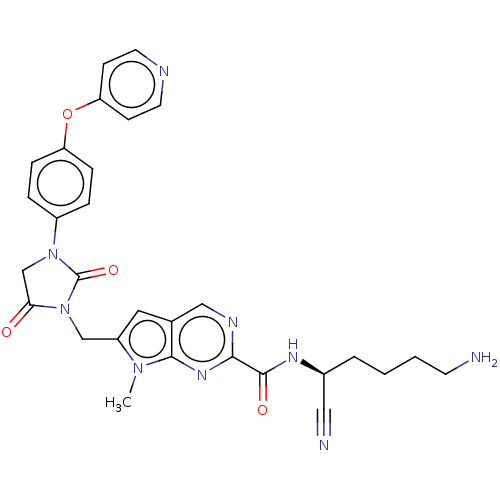
(CHEMBL3585745)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C29H29N9O4/c1-36-22(14-19-16-33-26(35-27(19)36)28(40)34-20(15-31)4-2-3-11-30)17-38-25(39)18-37(29(38)41)21-5-7-23(8-6-21)42-24-9-12-32-13-10-24/h5-10,12-14,16,20H,2-4,11,17-18,30H2,1H3,(H,34,40)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LSD1 using H3K4me2 peptide as substrate measured after 30 mins by fluorescence assay |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093542
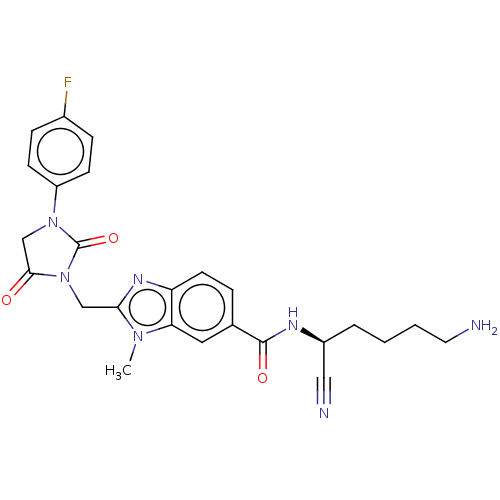
(CHEMBL3585742)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H26FN7O3/c1-31-21-12-16(24(35)29-18(13-28)4-2-3-11-27)5-10-20(21)30-22(31)14-33-23(34)15-32(25(33)36)19-8-6-17(26)7-9-19/h5-10,12,18H,2-4,11,14-15,27H2,1H3,(H,29,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50158866

(CHEMBL3785355)Show SMILES Clc1cccc(CNC(=O)[C@H](CCCCN[C@@H]2C[C@H]2c2ccccc2)NC(=O)c2ccc(cc2)-c2ccccc2)c1 |r| Show InChI InChI=1S/C35H36ClN3O2/c36-30-15-9-10-25(22-30)24-38-35(41)32(16-7-8-21-37-33-23-31(33)28-13-5-2-6-14-28)39-34(40)29-19-17-27(18-20-29)26-11-3-1-4-12-26/h1-6,9-15,17-20,22,31-33,37H,7-8,16,21,23-24H2,(H,38,41)(H,39,40)/t31-,32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazol... |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50460073

(CHEMBL4227224)Show SMILES Clc1cccc(CN2CCN(c3ccc(cc3)-c3ccccc3)C(=O)C(CCCCN[C@@H]3C[C@H]3c3ccccc3)C2=O)c1 |r| Show InChI InChI=1S/C37H38ClN3O2/c38-31-15-9-10-27(24-31)26-40-22-23-41(32-19-17-29(18-20-32)28-11-3-1-4-12-28)37(43)33(36(40)42)16-7-8-21-39-35-25-34(35)30-13-5-2-6-14-30/h1-6,9-15,17-20,24,33-35,39H,7-8,16,21-23,25-26H2/t33?,34-,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazol... |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50158866

(CHEMBL3785355)Show SMILES Clc1cccc(CNC(=O)[C@H](CCCCN[C@@H]2C[C@H]2c2ccccc2)NC(=O)c2ccc(cc2)-c2ccccc2)c1 |r| Show InChI InChI=1S/C35H36ClN3O2/c36-30-15-9-10-25(22-30)24-38-35(41)32(16-7-8-21-37-33-23-31(33)28-13-5-2-6-14-28)39-34(40)29-19-17-27(18-20-29)26-11-3-1-4-12-26/h1-6,9-15,17-20,22,31-33,37H,7-8,16,21,23-24H2,(H,38,41)(H,39,40)/t31-,32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazol... |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50460073

(CHEMBL4227224)Show SMILES Clc1cccc(CN2CCN(c3ccc(cc3)-c3ccccc3)C(=O)C(CCCCN[C@@H]3C[C@H]3c3ccccc3)C2=O)c1 |r| Show InChI InChI=1S/C37H38ClN3O2/c38-31-15-9-10-27(24-31)26-40-22-23-41(32-19-17-29(18-20-32)28-11-3-1-4-12-28)37(43)33(36(40)42)16-7-8-21-39-35-25-34(35)30-13-5-2-6-14-30/h1-6,9-15,17-20,24,33-35,39H,7-8,16,21-23,25-26H2/t33?,34-,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazol... |
Bioorg Med Chem 26: 775-785 (2018)
Article DOI: 10.1016/j.bmc.2017.12.045
BindingDB Entry DOI: 10.7270/Q28W3GX3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093549
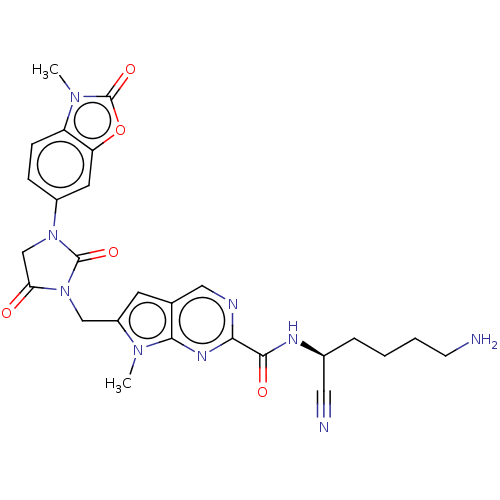
(CHEMBL3585738)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc3n(C)c(=O)oc3c2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H27N9O5/c1-32-18(9-15-12-29-22(31-23(15)32)24(37)30-16(11-28)5-3-4-8-27)13-35-21(36)14-34(25(35)38)17-6-7-19-20(10-17)40-26(39)33(19)2/h6-7,9-10,12,16H,3-5,8,13-14,27H2,1-2H3,(H,30,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093548
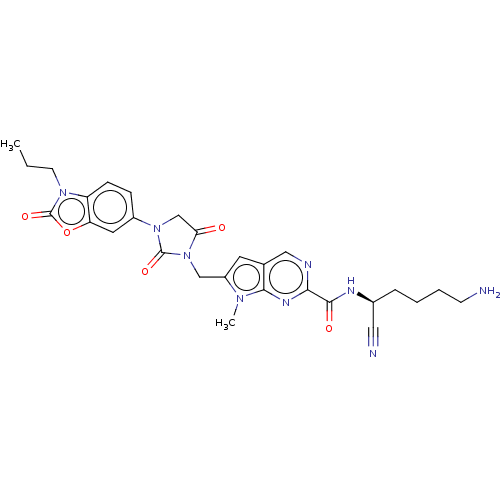
(CHEMBL3585739)Show SMILES CCCn1c2ccc(cc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C28H31N9O5/c1-3-10-35-21-8-7-19(12-22(21)42-28(35)41)36-16-23(38)37(27(36)40)15-20-11-17-14-31-24(33-25(17)34(20)2)26(39)32-18(13-30)6-4-5-9-29/h7-8,11-12,14,18H,3-6,9-10,15-16,29H2,1-2H3,(H,32,39)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50093541

(CHEMBL3585745)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C29H29N9O4/c1-36-22(14-19-16-33-26(35-27(19)36)28(40)34-20(15-31)4-2-3-11-30)17-38-25(39)18-37(29(38)41)21-5-7-23(8-6-21)42-24-9-12-32-13-10-24/h5-10,12-14,16,20H,2-4,11,17-18,30H2,1H3,(H,34,40)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50093540

(CHEMBL3585746)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-26-16-20(29(40)34-21(17-32)4-2-3-13-31)5-10-25(26)35-27(36)18-38-28(39)19-37(30(38)41)22-6-8-23(9-7-22)42-24-11-14-33-15-12-24/h5-12,14-16,21H,2-4,13,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009200

(CHEMBL3238374)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-23(15-20-17-33-27(35-28(20)36)29(40)34-21(16-32)7-5-6-14-31)18-38-26(39)19-37(30(38)41)22-10-12-25(13-11-22)42-24-8-3-2-4-9-24/h2-4,8-13,15,17,21H,5-7,14,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50093542

(CHEMBL3585742)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H26FN7O3/c1-31-21-12-16(24(35)29-18(13-28)4-2-3-11-27)5-10-20(21)30-22(31)14-33-23(34)15-32(25(33)36)19-8-6-17(26)7-9-19/h5-10,12,18H,2-4,11,14-15,27H2,1H3,(H,29,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093547
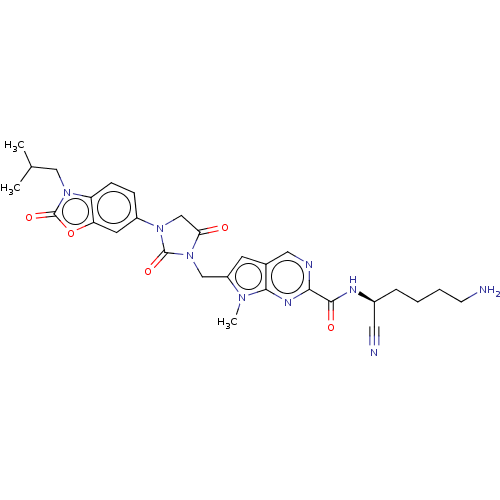
(CHEMBL3585740)Show SMILES CC(C)Cn1c2ccc(cc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C29H33N9O5/c1-17(2)14-37-22-8-7-20(11-23(22)43-29(37)42)36-16-24(39)38(28(36)41)15-21-10-18-13-32-25(34-26(18)35(21)3)27(40)33-19(12-31)6-4-5-9-30/h7-8,10-11,13,17,19H,4-6,9,14-16,30H2,1-3H3,(H,33,40)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093545
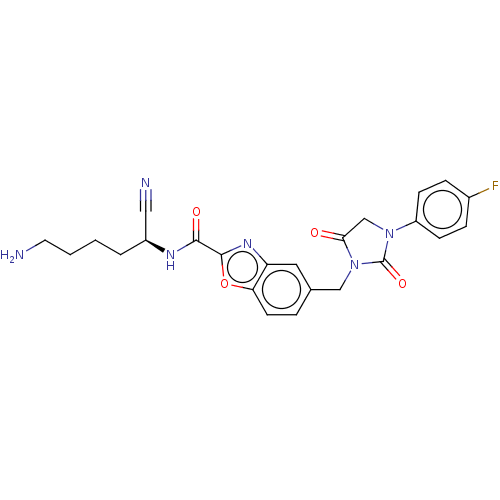
(CHEMBL3585743)Show SMILES NCCCC[C@H](NC(=O)c1nc2cc(CN3C(=O)CN(C3=O)c3ccc(F)cc3)ccc2o1)C#N |r| Show InChI InChI=1S/C24H23FN6O4/c25-16-5-7-18(8-6-16)30-14-21(32)31(24(30)34)13-15-4-9-20-19(11-15)29-23(35-20)22(33)28-17(12-27)3-1-2-10-26/h4-9,11,17H,1-3,10,13-14,26H2,(H,28,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093546
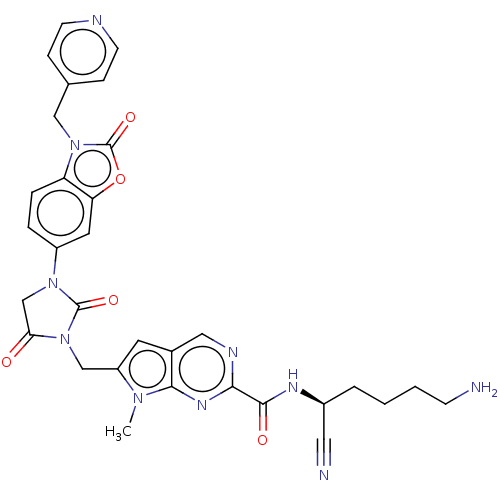
(CHEMBL3585741)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc3n(Cc4ccncc4)c(=O)oc3c2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H30N10O5/c1-38-23(12-20-15-35-27(37-28(20)38)29(43)36-21(14-33)4-2-3-9-32)17-41-26(42)18-39(30(41)44)22-5-6-24-25(13-22)46-31(45)40(24)16-19-7-10-34-11-8-19/h5-8,10-13,15,21H,2-4,9,16-18,32H2,1H3,(H,36,43)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093543
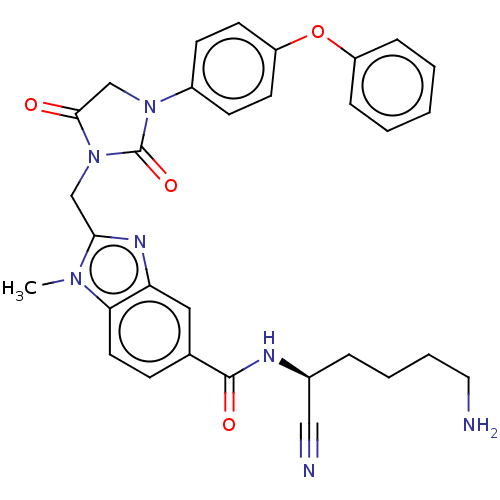
(CHEMBL3585747)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)nc2cc(ccc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H31N7O4/c1-36-27-15-10-21(30(40)34-22(18-33)7-5-6-16-32)17-26(27)35-28(36)19-38-29(39)20-37(31(38)41)23-11-13-25(14-12-23)42-24-8-3-2-4-9-24/h2-4,8-15,17,22H,5-7,16,19-20,32H2,1H3,(H,34,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009171

(CHEMBL3238362)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H25FN8O3/c1-31-19(13-33-20(34)14-32(24(33)36)18-7-5-16(25)6-8-18)10-15-12-28-21(30-22(15)31)23(35)29-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50203072
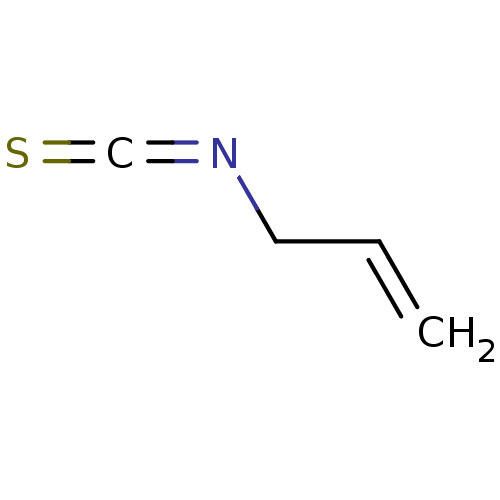
(3-isothiocyanatoprop-1-ene | Allyl isothiocyanate ...) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.12E+3 | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Agonist activity at TRPA1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127142
BindingDB Entry DOI: 10.7270/Q29C720P |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50548720
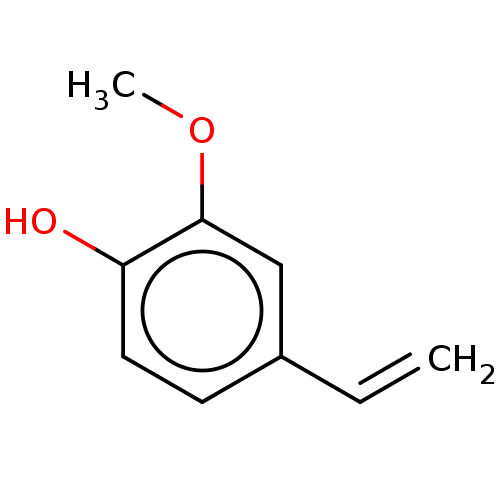
(CHEBI:42438 | CHEMBL1232595) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.28E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM36301
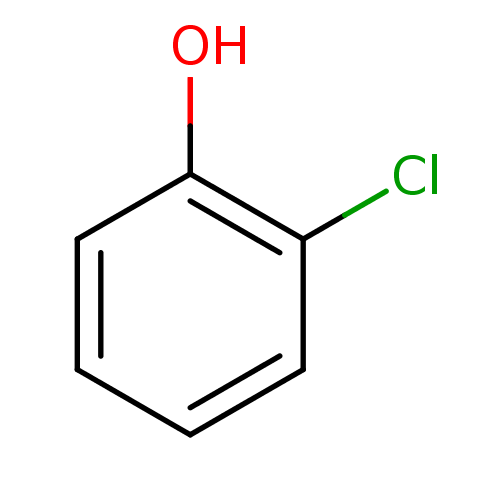
(2-Chlorophenol (2-CP) | 2-chlorophenol)Show InChI InChI=1S/C6H5ClO/c7-5-3-1-2-4-6(5)8/h1-4,8H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.01E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50548721

(CHEMBL3186900) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50548722
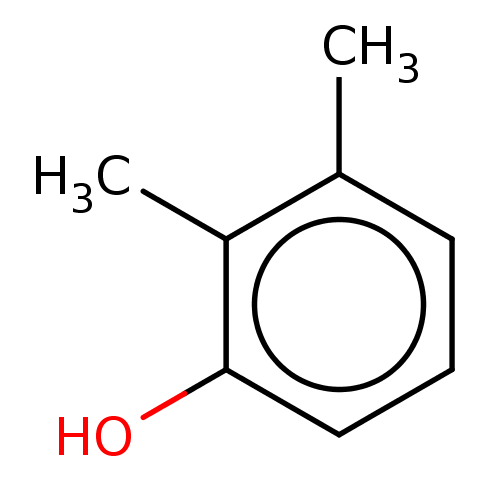
(CHEMBL1490403) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50548725
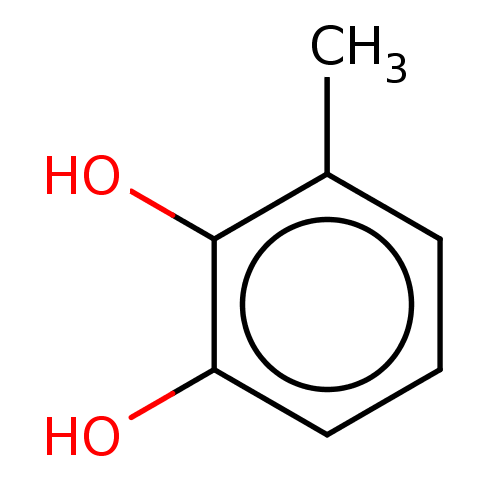
(3-Methylcatechol | CHEBI:18404 | CHEMBL1173328) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.83E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50548723
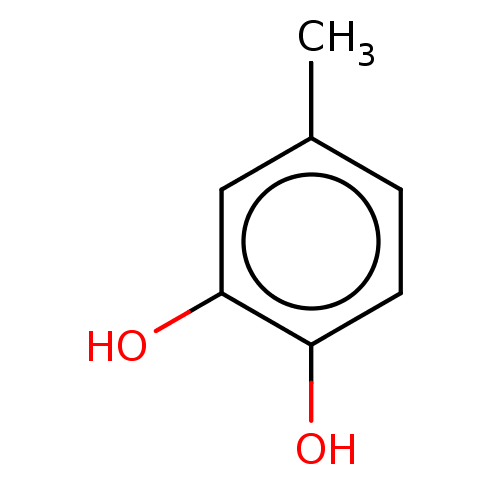
(CHEBI:17254 | CHEMBL158766) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50094702
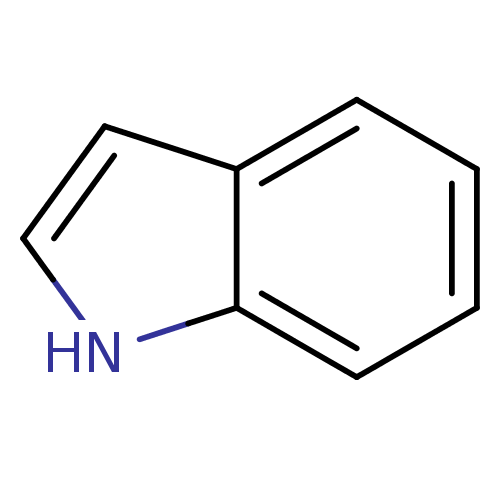
(1H-indole | CHEMBL15844 | Indol | Indole, 7 | indo...)Show InChI InChI=1S/C8H7N/c1-2-4-8-7(3-1)5-6-9-8/h1-6,9H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.52E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50164168
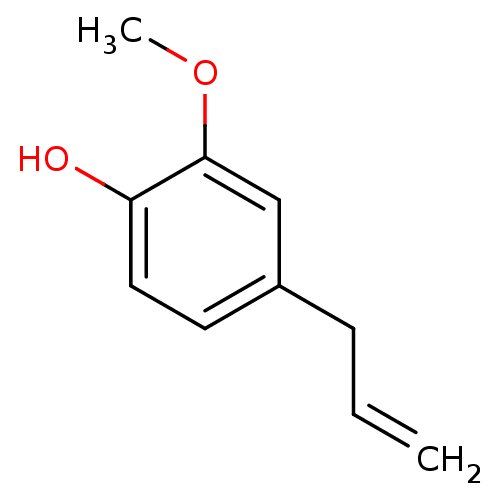
(1,3,4-Eugenol | 1-Hydroxy-2-methoxy-4-allylbenzene...)Show InChI InChI=1S/C10H12O2/c1-3-4-8-5-6-9(11)10(7-8)12-2/h3,5-7,11H,1,4H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.16E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50548724
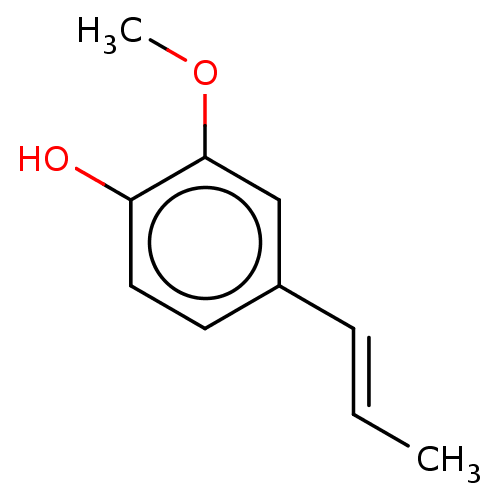
(CHEBI:50545 | CHEMBL193598) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50240432

(1-hydroxy-5-methyl-2-isopropylbenzene | 2-isopropy...)Show InChI InChI=1S/C10H14O/c1-7(2)9-5-4-8(3)6-10(9)11/h4-7,11H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.69E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50483042

(CHEMBL1276241)Show InChI InChI=1S/C8H10O2/c1-2-6-3-4-7(9)8(10)5-6/h3-5,9-10H,2H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.69E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TRPA1 (unknown origin) by calcium imaging assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127639
BindingDB Entry DOI: 10.7270/Q2S46WKS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data