 Found 156 hits with Last Name = 'bibi' and Initial = 'm'
Found 156 hits with Last Name = 'bibi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50229885
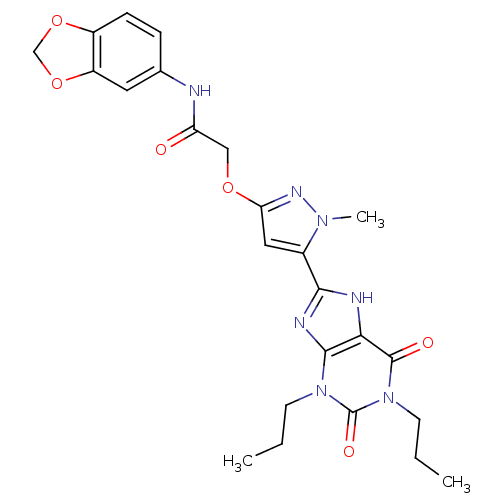
(CHEMBL260331 | CHEMBL506685 | N-(benzo[d][1,3]diox...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1cc(OCC(=O)Nc2ccc3OCOc3c2)nn1C Show InChI InChI=1S/C24H27N7O6/c1-4-8-30-22-20(23(33)31(9-5-2)24(30)34)26-21(27-22)15-11-19(28-29(15)3)35-12-18(32)25-14-6-7-16-17(10-14)37-13-36-16/h6-7,10-11H,4-5,8-9,12-13H2,1-3H3,(H,25,32)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Isfahan
Curated by ChEMBL
| Assay Description
Binding affinity towards human adenosine A1 receptor expressed in CHO cells using [3H]-DPCPX |
Bioorg Med Chem Lett 14: 3611-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.110
BindingDB Entry DOI: 10.7270/Q2QF8SBN |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50398388
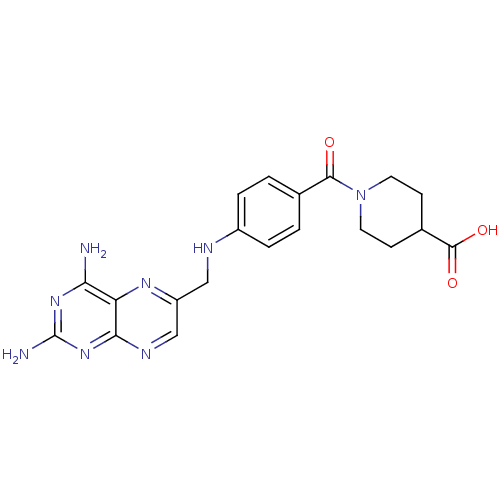
(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398388

(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562452

(CHEMBL4776197)Show SMILES Nc1cc(cc2nc(c(Nc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)nc12)-c1ccccc1)C(F)(F)F | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562453
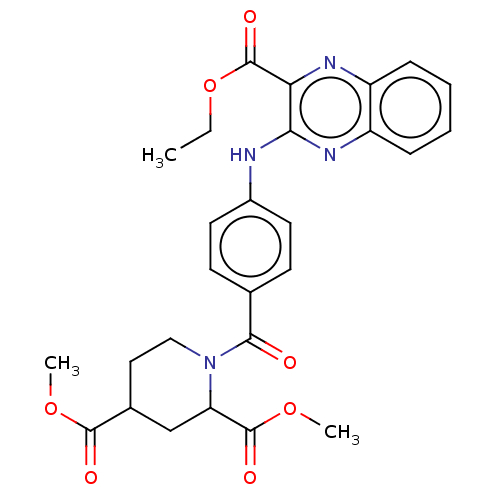
(CHEMBL4783402)Show SMILES CCOC(=O)c1nc2ccccc2nc1Nc1ccc(cc1)C(=O)N1CCC(CC1C(=O)OC)C(=O)OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562451
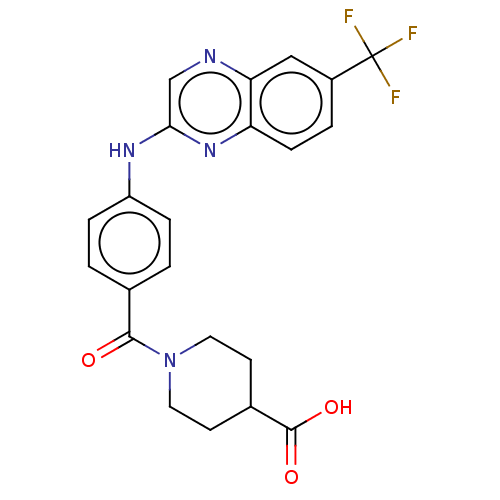
(CHEMBL4763597)Show SMILES OC(=O)C1CCN(CC1)C(=O)c1ccc(Nc2cnc3cc(ccc3n2)C(F)(F)F)cc1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562452

(CHEMBL4776197)Show SMILES Nc1cc(cc2nc(c(Nc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)nc12)-c1ccccc1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562451

(CHEMBL4763597)Show SMILES OC(=O)C1CCN(CC1)C(=O)c1ccc(Nc2cnc3cc(ccc3n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562453

(CHEMBL4783402)Show SMILES CCOC(=O)c1nc2ccccc2nc1Nc1ccc(cc1)C(=O)N1CCC(CC1C(=O)OC)C(=O)OC | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072672
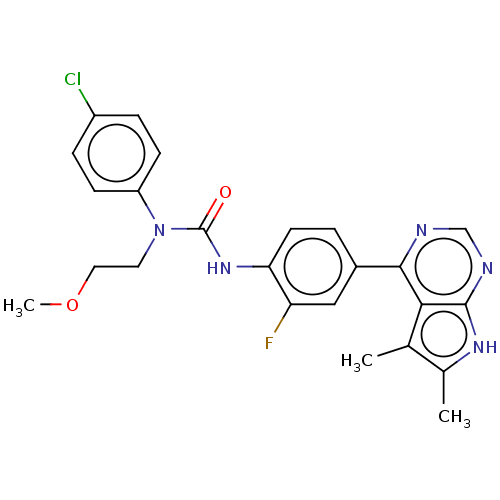
(CHEMBL3410056)Show SMILES COCCN(C(=O)Nc1ccc(cc1F)-c1ncnc2[nH]c(C)c(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H23ClFN5O2/c1-14-15(2)29-23-21(14)22(27-13-28-23)16-4-9-20(19(26)12-16)30-24(32)31(10-11-33-3)18-7-5-17(25)6-8-18/h4-9,12-13H,10-11H2,1-3H3,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562445

(CHEMBL4748139)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072662
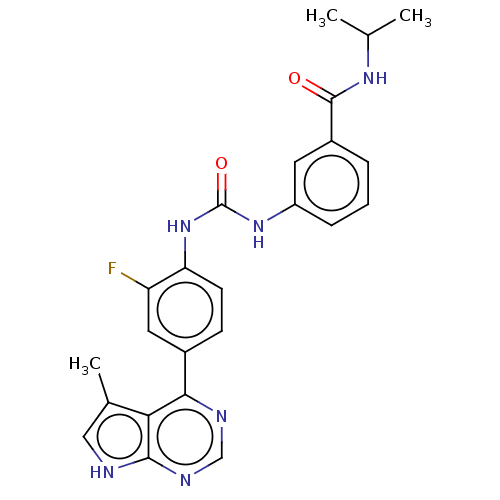
(CHEMBL3407526)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2F)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C24H23FN6O2/c1-13(2)29-23(32)16-5-4-6-17(9-16)30-24(33)31-19-8-7-15(10-18(19)25)21-20-14(3)11-26-22(20)28-12-27-21/h4-13H,1-3H3,(H,29,32)(H,26,27,28)(H2,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072671
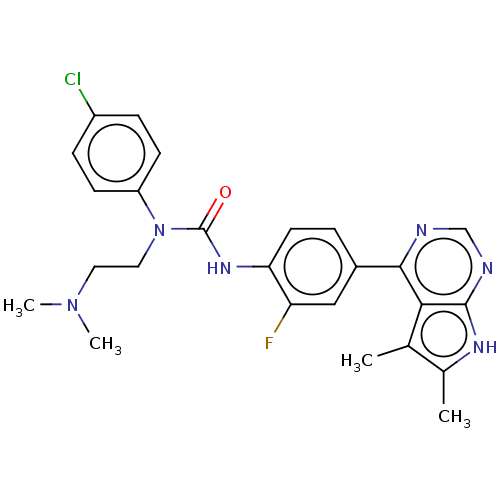
(CHEMBL3410055)Show SMILES CN(C)CCN(C(=O)Nc1ccc(cc1F)-c1ncnc2[nH]c(C)c(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H26ClFN6O/c1-15-16(2)30-24-22(15)23(28-14-29-24)17-5-10-21(20(27)13-17)31-25(34)33(12-11-32(3)4)19-8-6-18(26)7-9-19/h5-10,13-14H,11-12H2,1-4H3,(H,31,34)(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
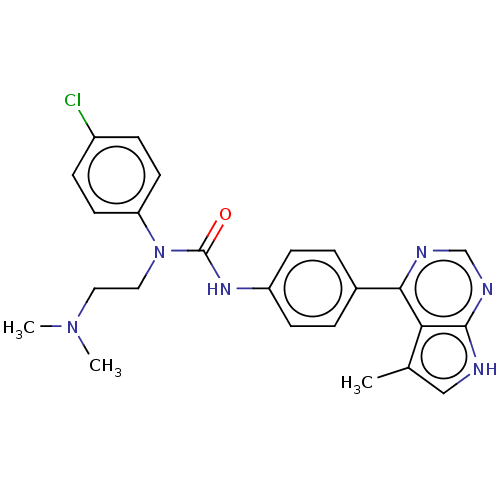
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072728
(CHEMBL3410052)Show SMILES CN(C)CCN(C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H25ClN6O/c1-16-14-26-23-21(16)22(27-15-28-23)17-4-8-19(9-5-17)29-24(32)31(13-12-30(2)3)20-10-6-18(25)7-11-20/h4-11,14-15H,12-13H2,1-3H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072727
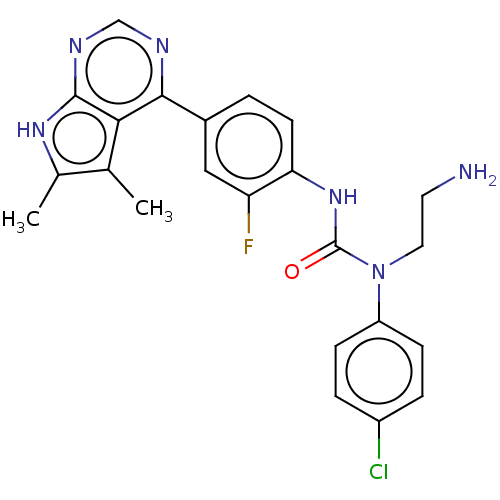
(CHEMBL3410054)Show SMILES Cc1[nH]c2ncnc(-c3ccc(NC(=O)N(CCN)c4ccc(Cl)cc4)c(F)c3)c2c1C Show InChI InChI=1S/C23H22ClFN6O/c1-13-14(2)29-22-20(13)21(27-12-28-22)15-3-8-19(18(25)11-15)30-23(32)31(10-9-26)17-6-4-16(24)5-7-17/h3-8,11-12H,9-10,26H2,1-2H3,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072670
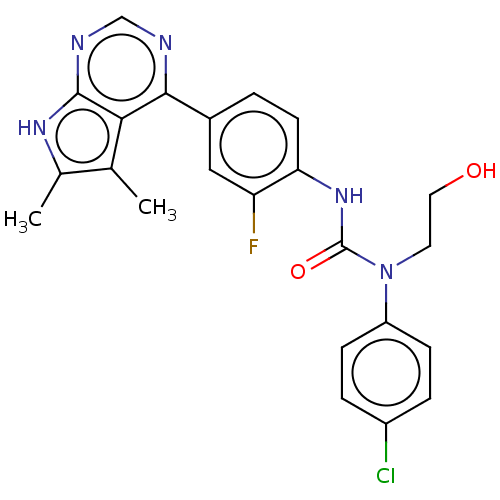
(CHEMBL3410053)Show SMILES Cc1[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(Cl)cc4)c(F)c3)c2c1C Show InChI InChI=1S/C23H21ClFN5O2/c1-13-14(2)28-22-20(13)21(26-12-27-22)15-3-8-19(18(25)11-15)29-23(32)30(9-10-31)17-6-4-16(24)5-7-17/h3-8,11-12,31H,9-10H2,1-2H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
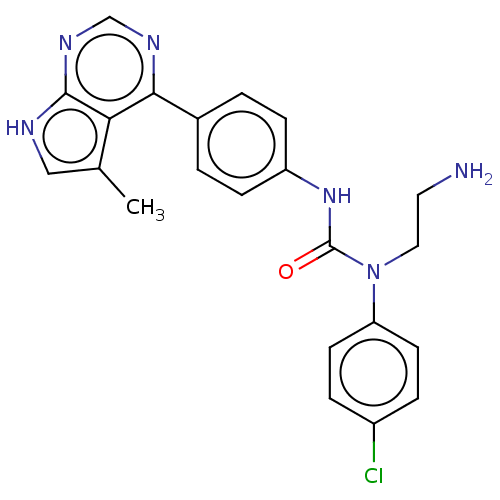
(Homo sapiens (Human)) | BDBM50072730
(CHEMBL3410050)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCN)c4ccc(Cl)cc4)cc3)c12 Show InChI InChI=1S/C22H21ClN6O/c1-14-12-25-21-19(14)20(26-13-27-21)15-2-6-17(7-3-15)28-22(30)29(11-10-24)18-8-4-16(23)5-9-18/h2-9,12-13H,10-11,24H2,1H3,(H,28,30)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562454

(CHEMBL533684 | TCMDC-141974) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072667
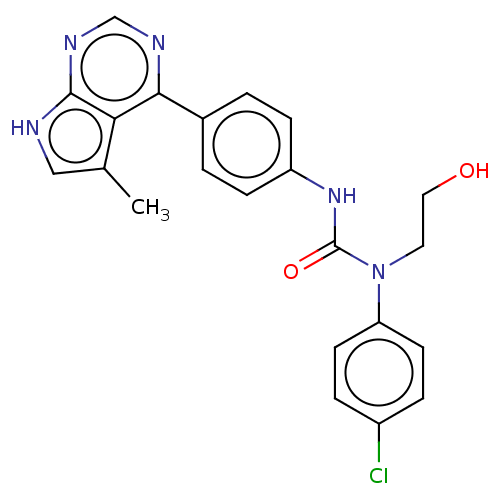
(CHEMBL3410042)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(Cl)cc4)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-2-6-17(7-3-15)27-22(30)28(10-11-29)18-8-4-16(23)5-9-18/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072731
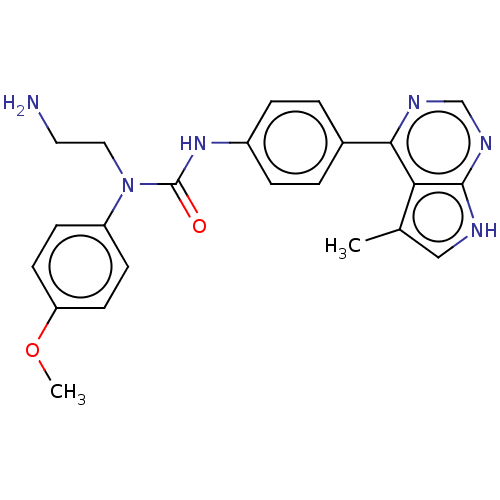
(CHEMBL3410049)Show SMILES COc1ccc(cc1)N(CCN)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C23H24N6O2/c1-15-13-25-22-20(15)21(26-14-27-22)16-3-5-17(6-4-16)28-23(30)29(12-11-24)18-7-9-19(31-2)10-8-18/h3-10,13-14H,11-12,24H2,1-2H3,(H,28,30)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562450
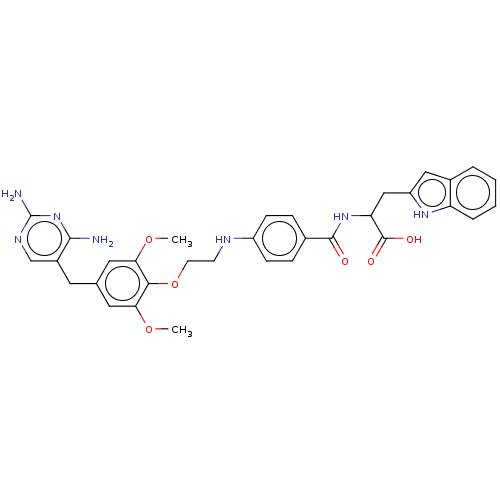
(CHEMBL4757974)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(Cc1cc2ccccc2[nH]1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072723
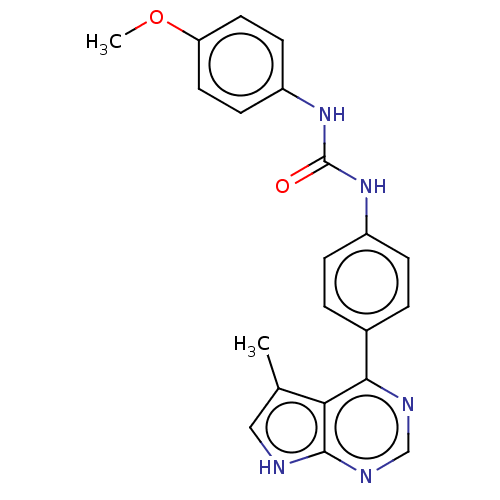
(CHEMBL3410032)Show SMILES COc1ccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]cc(C)c23)cc1 Show InChI InChI=1S/C21H19N5O2/c1-13-11-22-20-18(13)19(23-12-24-20)14-3-5-15(6-4-14)25-21(27)26-16-7-9-17(28-2)10-8-16/h3-12H,1-2H3,(H,22,23,24)(H2,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072668
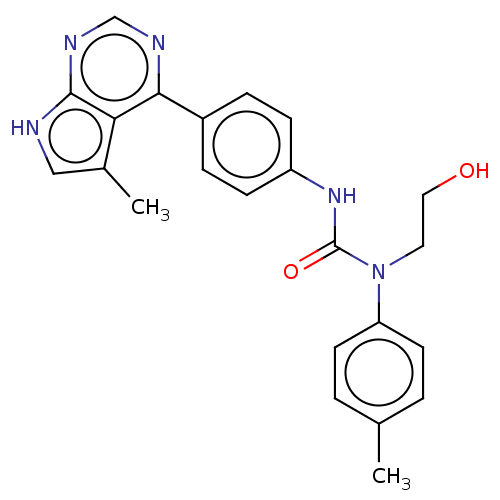
(CHEMBL3410045)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(C)cc4)cc3)c12 Show InChI InChI=1S/C23H23N5O2/c1-15-3-9-19(10-4-15)28(11-12-29)23(30)27-18-7-5-17(6-8-18)21-20-16(2)13-24-22(20)26-14-25-21/h3-10,13-14,29H,11-12H2,1-2H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562443

(CHEMBL4759800)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(C(C)C)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072664
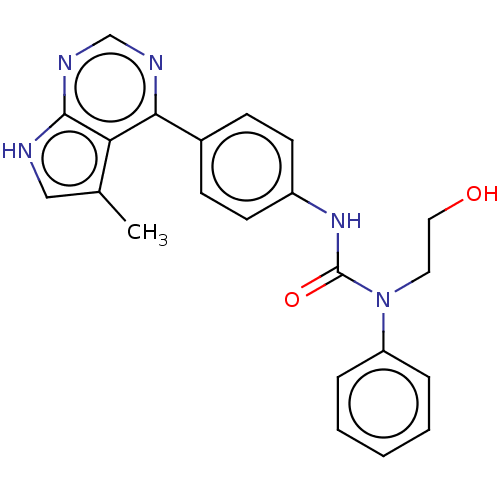
(CHEMBL3410036)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccccc4)cc3)c12 Show InChI InChI=1S/C22H21N5O2/c1-15-13-23-21-19(15)20(24-14-25-21)16-7-9-17(10-8-16)26-22(29)27(11-12-28)18-5-3-2-4-6-18/h2-10,13-14,28H,11-12H2,1H3,(H,26,29)(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50072675
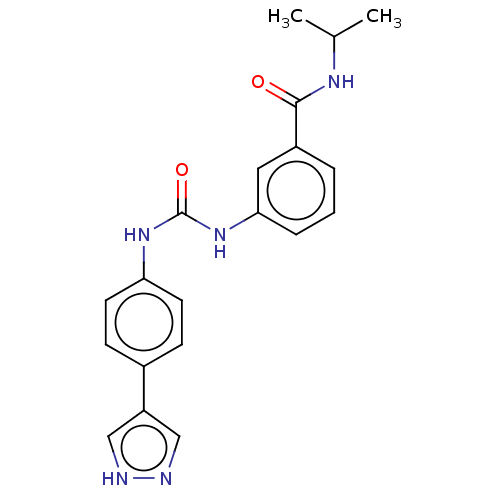
(CHEMBL3410022)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C20H21N5O2/c1-13(2)23-19(26)15-4-3-5-18(10-15)25-20(27)24-17-8-6-14(7-9-17)16-11-21-22-12-16/h3-13H,1-2H3,(H,21,22)(H,23,26)(H2,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK2 using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK peptide substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072729
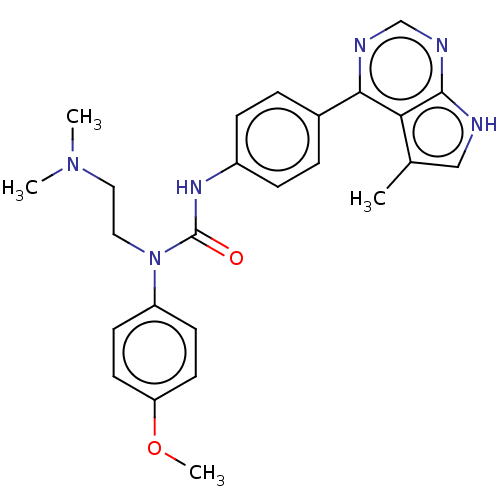
(CHEMBL3410051)Show SMILES COc1ccc(cc1)N(CCN(C)C)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C25H28N6O2/c1-17-15-26-24-22(17)23(27-16-28-24)18-5-7-19(8-6-18)29-25(32)31(14-13-30(2)3)20-9-11-21(33-4)12-10-20/h5-12,15-16H,13-14H2,1-4H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072669
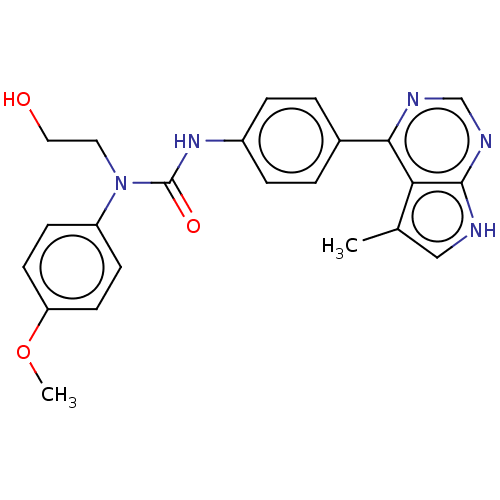
(CHEMBL3410048)Show SMILES COc1ccc(cc1)N(CCO)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C23H23N5O3/c1-15-13-24-22-20(15)21(25-14-26-22)16-3-5-17(6-4-16)27-23(30)28(11-12-29)18-7-9-19(31-2)10-8-18/h3-10,13-14,29H,11-12H2,1-2H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072665
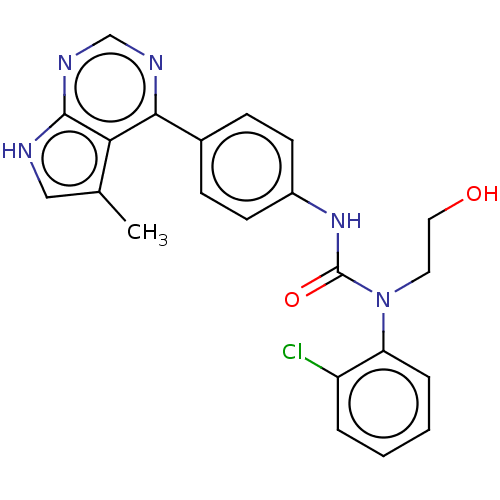
(CHEMBL3410040)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccccc4Cl)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-6-8-16(9-7-15)27-22(30)28(10-11-29)18-5-3-2-4-17(18)23/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072619
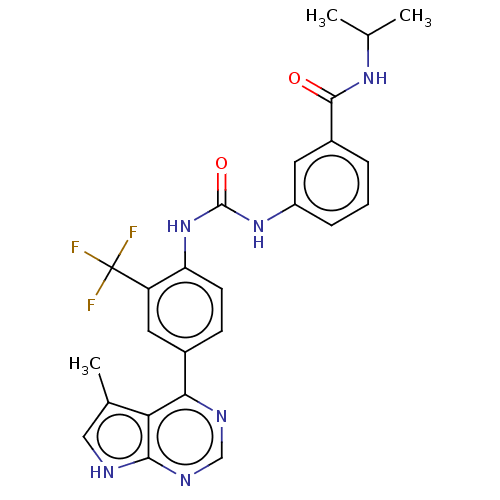
(CHEMBL3410026)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2C(F)(F)F)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C25H23F3N6O2/c1-13(2)32-23(35)16-5-4-6-17(9-16)33-24(36)34-19-8-7-15(10-18(19)25(26,27)28)21-20-14(3)11-29-22(20)31-12-30-21/h4-13H,1-3H3,(H,32,35)(H,29,30,31)(H2,33,34,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50364649
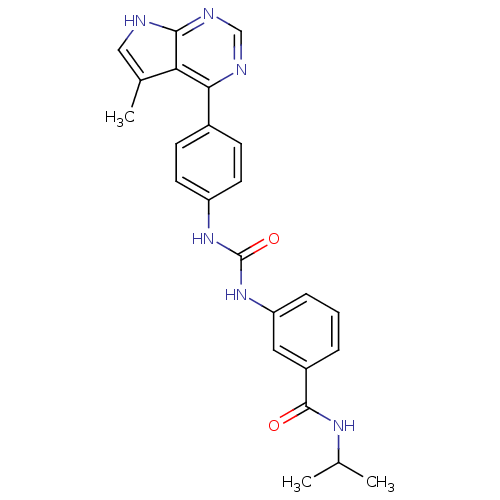
(CHEMBL1951346)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C24H24N6O2/c1-14(2)28-23(31)17-5-4-6-19(11-17)30-24(32)29-18-9-7-16(8-10-18)21-20-15(3)12-25-22(20)27-13-26-21/h4-14H,1-3H3,(H,28,31)(H,25,26,27)(H2,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072666
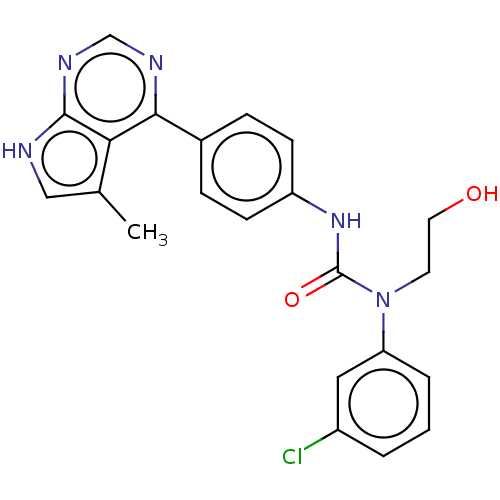
(CHEMBL3410041)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4cccc(Cl)c4)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-5-7-17(8-6-15)27-22(30)28(9-10-29)18-4-2-3-16(23)11-18/h2-8,11-13,29H,9-10H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072703
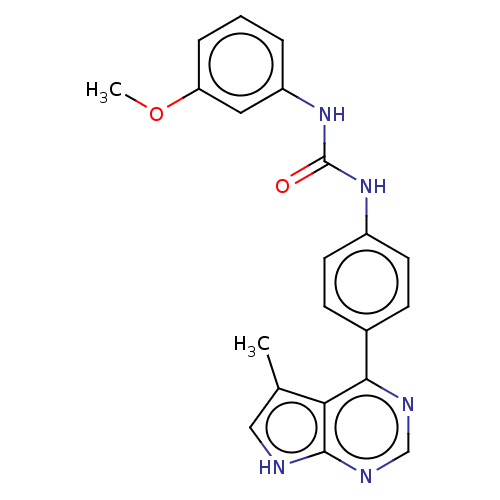
(CHEMBL3410031)Show SMILES COc1cccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C21H19N5O2/c1-13-11-22-20-18(13)19(23-12-24-20)14-6-8-15(9-7-14)25-21(27)26-16-4-3-5-17(10-16)28-2/h3-12H,1-2H3,(H,22,23,24)(H2,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072616
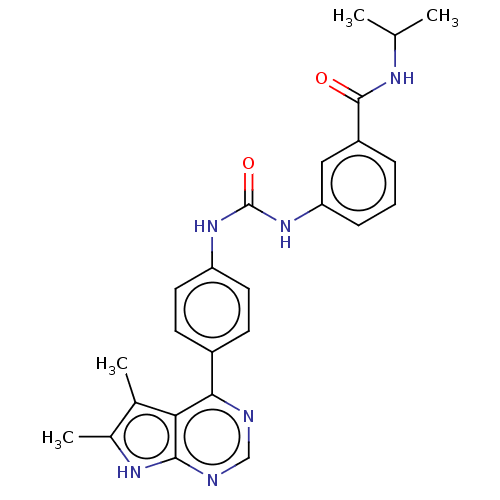
(CHEMBL3410025)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]c(C)c(C)c23)c1 Show InChI InChI=1S/C25H26N6O2/c1-14(2)28-24(32)18-6-5-7-20(12-18)31-25(33)30-19-10-8-17(9-11-19)22-21-15(3)16(4)29-23(21)27-13-26-22/h5-14H,1-4H3,(H,28,32)(H,26,27,29)(H2,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072615
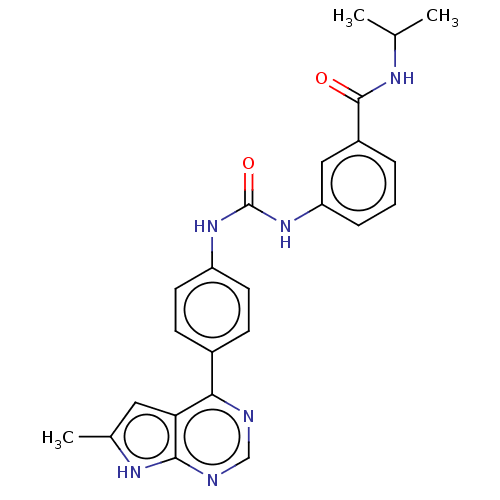
(CHEMBL3410024)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]c(C)cc23)c1 Show InChI InChI=1S/C24H24N6O2/c1-14(2)27-23(31)17-5-4-6-19(12-17)30-24(32)29-18-9-7-16(8-10-18)21-20-11-15(3)28-22(20)26-13-25-21/h4-14H,1-3H3,(H,27,31)(H,25,26,28)(H2,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072829
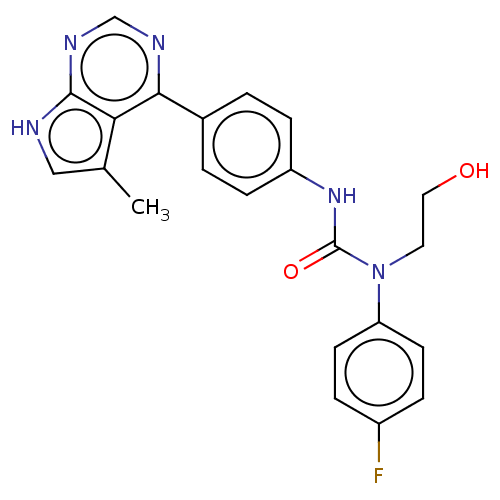
(CHEMBL3410039)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(F)cc4)cc3)c12 Show InChI InChI=1S/C22H20FN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-2-6-17(7-3-15)27-22(30)28(10-11-29)18-8-4-16(23)5-9-18/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50364653
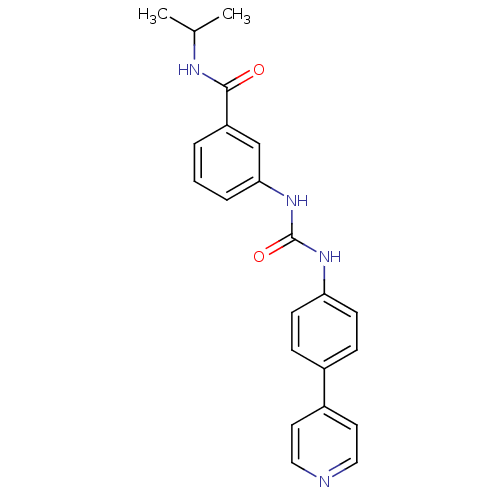
(CHEMBL1951443)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2ccncc2)c1 Show InChI InChI=1S/C22H22N4O2/c1-15(2)24-21(27)18-4-3-5-20(14-18)26-22(28)25-19-8-6-16(7-9-19)17-10-12-23-13-11-17/h3-15H,1-2H3,(H,24,27)(H2,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK2 using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK peptide substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562444

(CHEMBL4783671)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(Cc1ccccc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562449
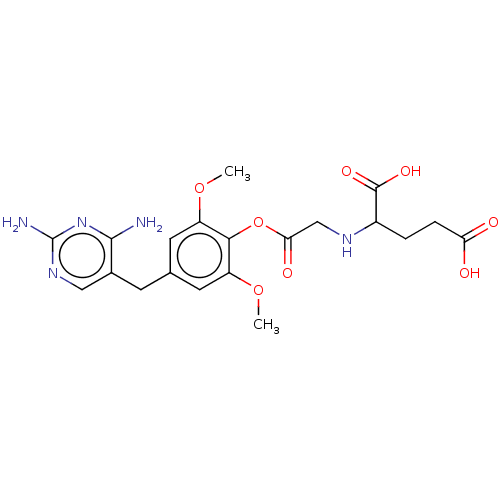
(CHEMBL4745475)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CCC(O)=O)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072732
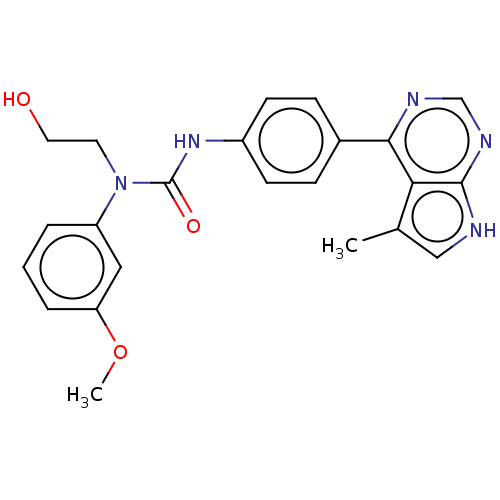
(CHEMBL3410047)Show SMILES COc1cccc(c1)N(CCO)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C23H23N5O3/c1-15-13-24-22-20(15)21(25-14-26-22)16-6-8-17(9-7-16)27-23(30)28(10-11-29)18-4-3-5-19(12-18)31-2/h3-9,12-14,29H,10-11H2,1-2H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072830
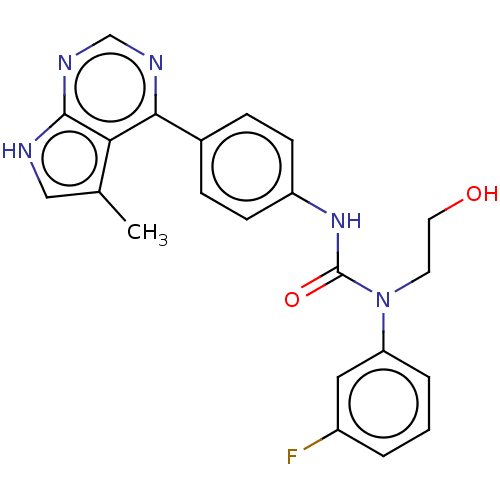
(CHEMBL3410038)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4cccc(F)c4)cc3)c12 Show InChI InChI=1S/C22H20FN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-5-7-17(8-6-15)27-22(30)28(9-10-29)18-4-2-3-16(23)11-18/h2-8,11-13,29H,9-10H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Rattus norvegicus) | BDBM50072667

(CHEMBL3410042)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(Cl)cc4)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-2-6-17(7-3-15)27-22(30)28(10-11-29)18-8-4-16(23)5-9-18/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Limk1 in rat A7r5 cells assessed as reduction in cofilin phosphorylation by Western blot method |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562446
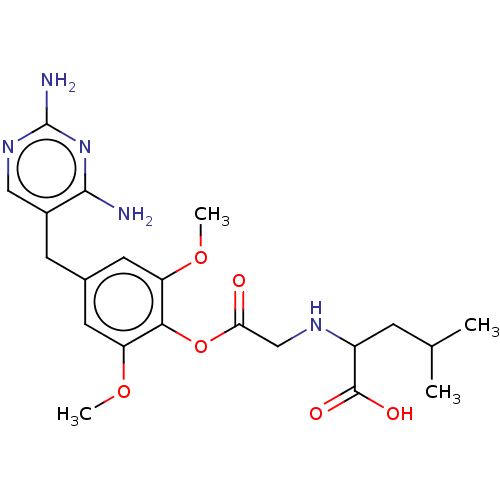
(CHEMBL4752301)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CC(C)C)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50072677
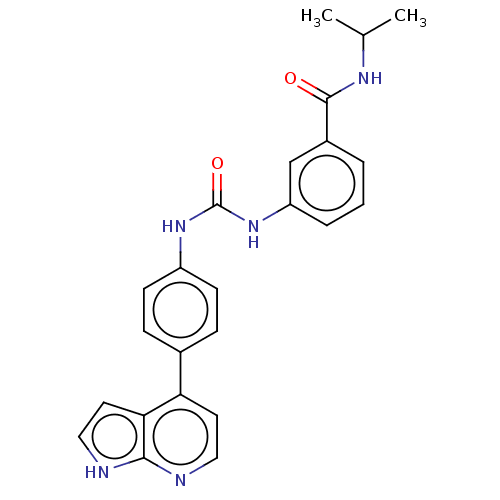
(CHEMBL1951349)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2ccnc3[nH]ccc23)c1 Show InChI InChI=1S/C24H23N5O2/c1-15(2)27-23(30)17-4-3-5-19(14-17)29-24(31)28-18-8-6-16(7-9-18)20-10-12-25-22-21(20)11-13-26-22/h3-15H,1-2H3,(H,25,26)(H,27,30)(H2,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK2 using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK peptide substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072831
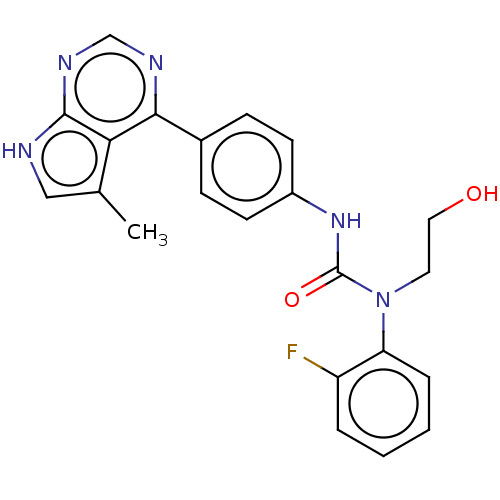
(CHEMBL3410037)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccccc4F)cc3)c12 Show InChI InChI=1S/C22H20FN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-6-8-16(9-7-15)27-22(30)28(10-11-29)18-5-3-2-4-17(18)23/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562448

(CHEMBL4779765)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(Cc1ccccc1)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data