

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484842 (CHEMBL1958482 | GRL-0249A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483336 (CHEMBL1651153 | GRL-0476) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484847 (CHEMBL1958483 | GRL-0289A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483338 (CHEMBL1651155) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484190 (CHEMBL1817686) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484193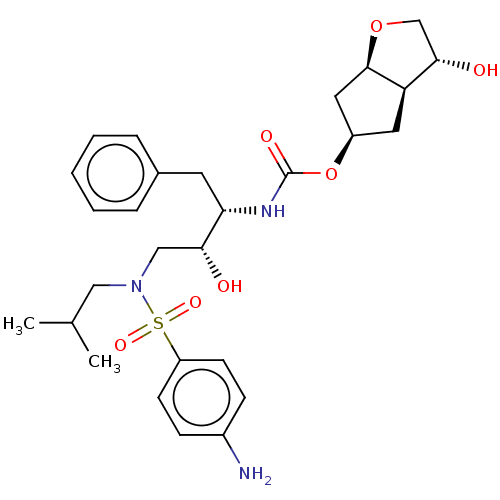 (CHEMBL1819294) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484191 (CHEMBL1819295) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484845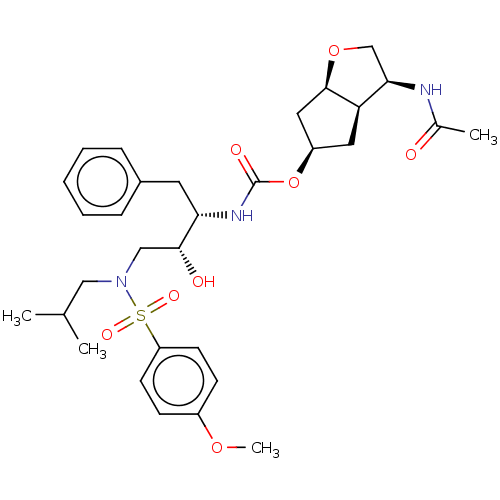 (CHEMBL1958480) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484841 (CHEMBL1958481) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483334 (CHEMBL1651160) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484196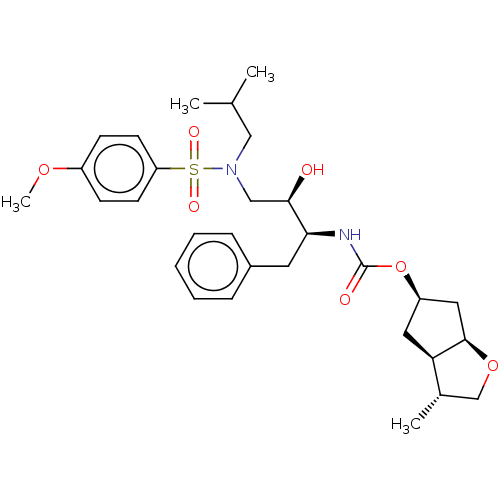 (CHEMBL1819297) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484844 (CHEMBL1958486) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484843 (CHEMBL1958484) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484189 (CHEMBL1819292) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483337 (CHEMBL1651154) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484197 (CHEMBL1819290) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484188 (CHEMBL1819291) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483335 (CHEMBL1651161) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483341 (CHEMBL1651159) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484198 (CHEMBL1819296) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484846 (CHEMBL1958485) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50437405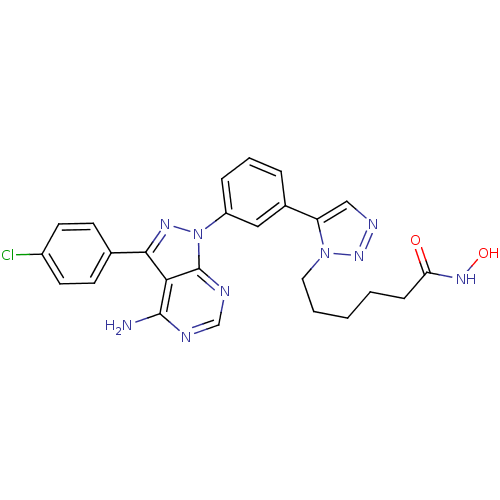 (CHEMBL2408778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) by Fluor de Lys based assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50437405 (CHEMBL2408778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484195 (CHEMBL1819293) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005012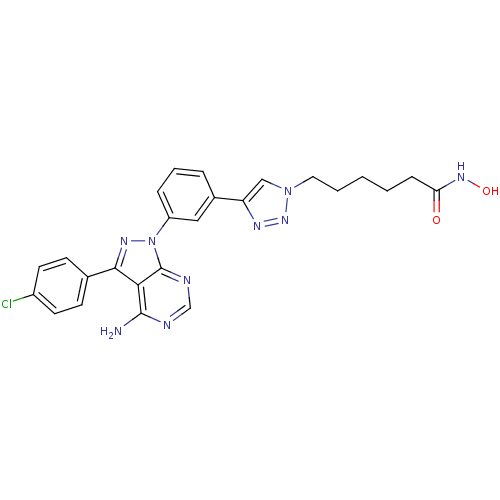 (CHEMBL2408777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) by Fluor de Lys based assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005013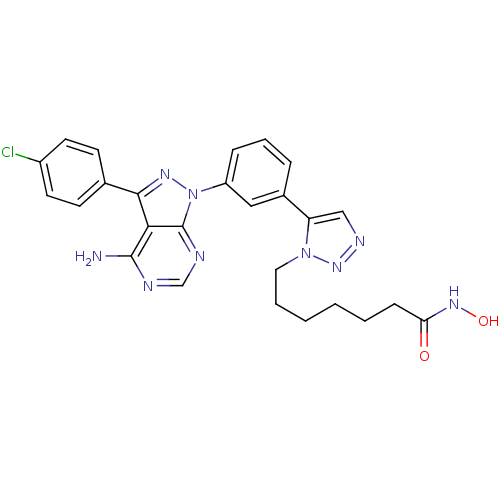 (CHEMBL2408779) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484194 (CHEMBL1819289) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483342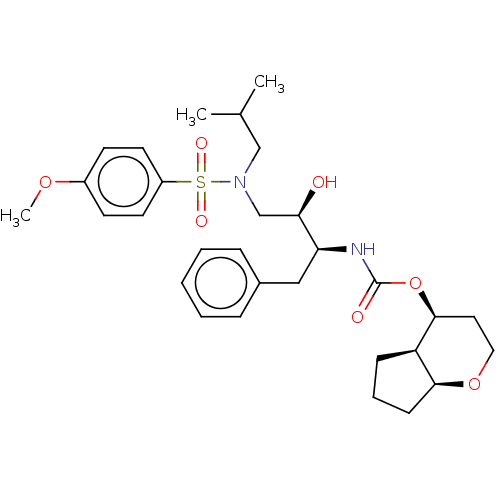 (CHEMBL1651156) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483339 (CHEMBL1651158) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483340 (CHEMBL1651157) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005015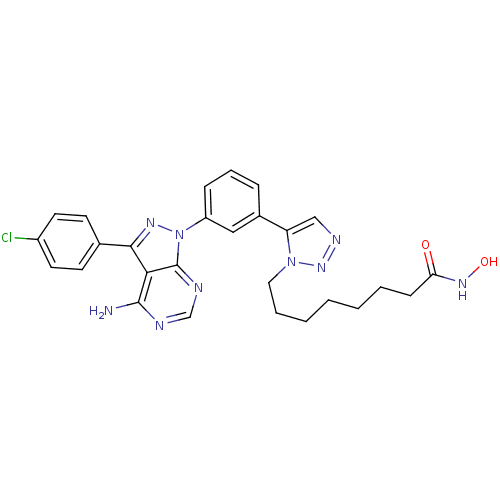 (CHEMBL2408780) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484192 (CHEMBL1819298) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005016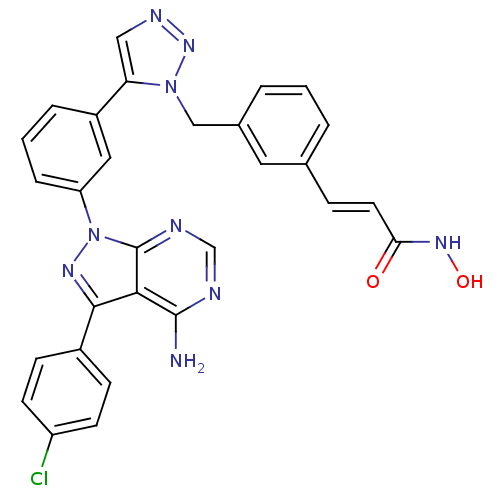 (CHEMBL2408782) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005014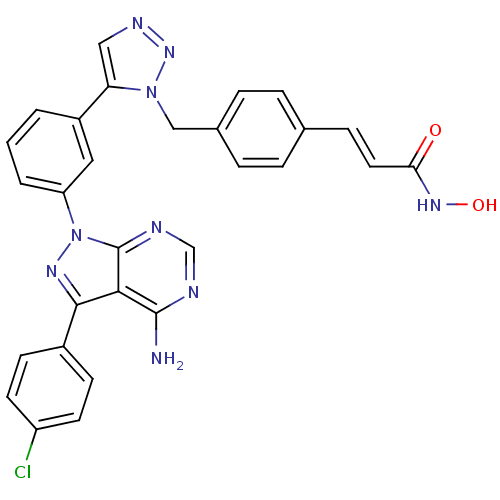 (CHEMBL2408781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005018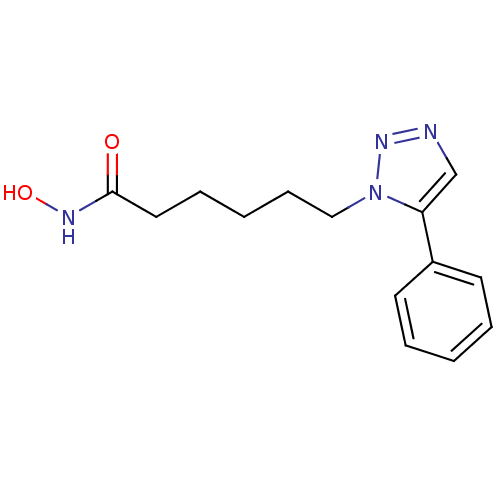 (CHEMBL2407108) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) by Fluor de Lys based assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119568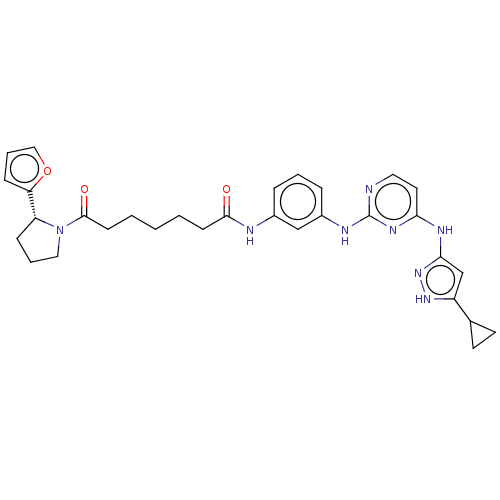 (CHEMBL3617738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119569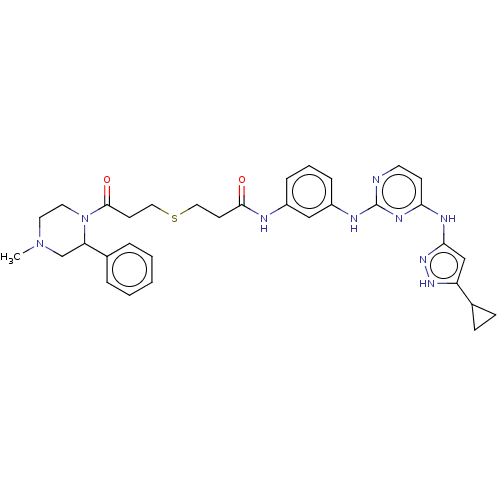 (CHEMBL3617733) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119569 (CHEMBL3617733) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src C277S, C483S, S496S mutant (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119567 (CHEMBL3617740) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50437405 (CHEMBL2408778) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) by fluorescence assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50437405 (CHEMBL2408778) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) after 10 mins by fluorimetric assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119578 (CHEMBL3617734) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50005013 (CHEMBL2408779) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) after 10 mins by fluorimetric assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119570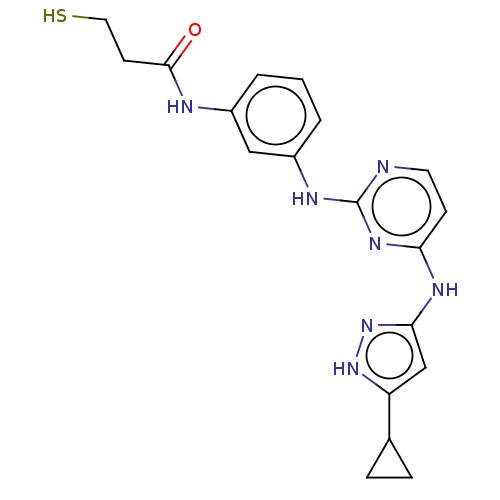 (CHEMBL3617731) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src C277S, C483S, S496S mutant (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119576 (CHEMBL3617736) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119568 (CHEMBL3617738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src E280G mutant (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119575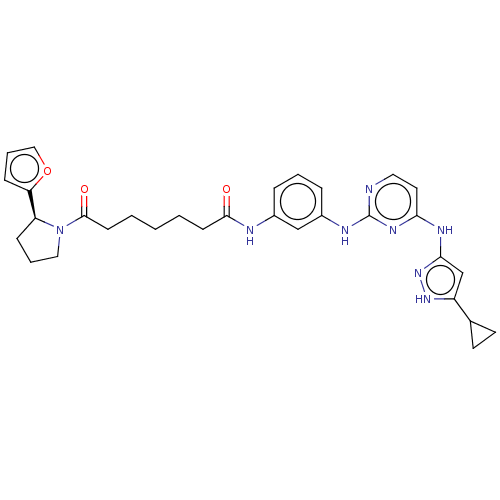 (CHEMBL3617737) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119579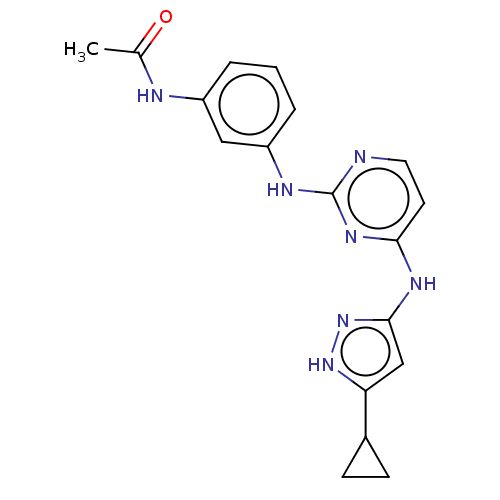 (CHEMBL3617730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50119574 (CHEMBL3617739) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | ACS Med Chem Lett 6: 898-901 (2015) Article DOI: 10.1021/acsmedchemlett.5b00167 BindingDB Entry DOI: 10.7270/Q2833TTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50005012 (CHEMBL2408777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 371 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) by fluorescence assay | ACS Med Chem Lett 4: 779-783 (2013) Article DOI: 10.1021/ml400175d BindingDB Entry DOI: 10.7270/Q2CZ38KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |