

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598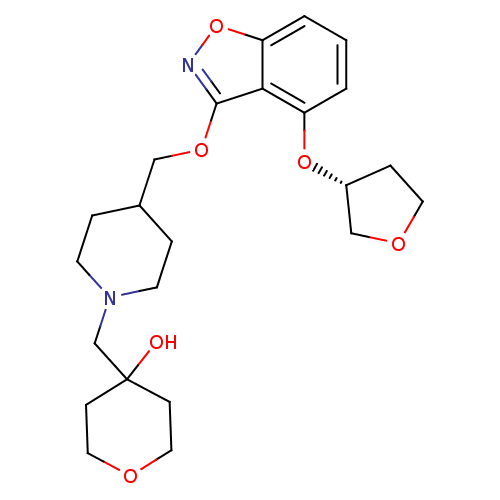 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398597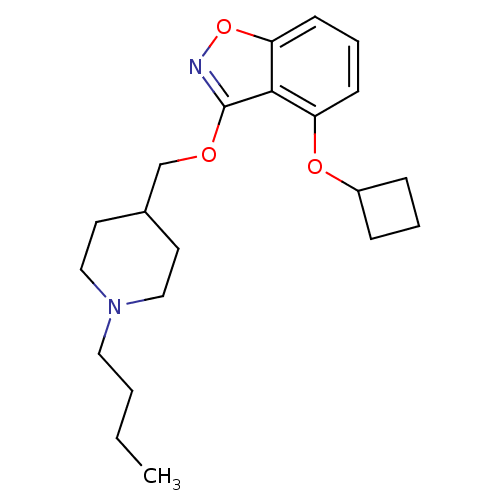 (CHEMBL2179584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398593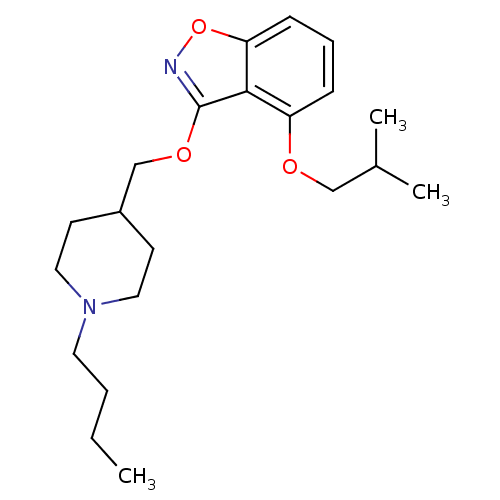 (CHEMBL2179587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044676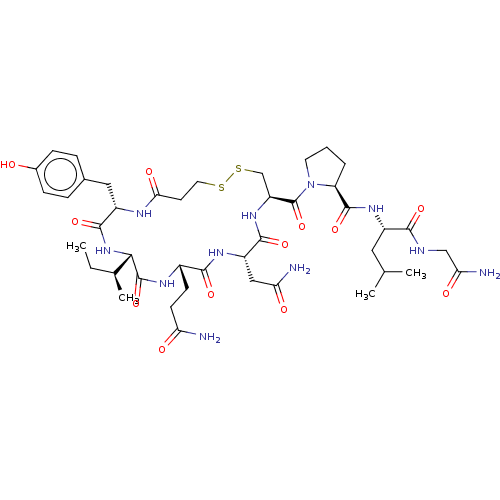 (CHEMBL439044) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398596 (CHEMBL2179589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398599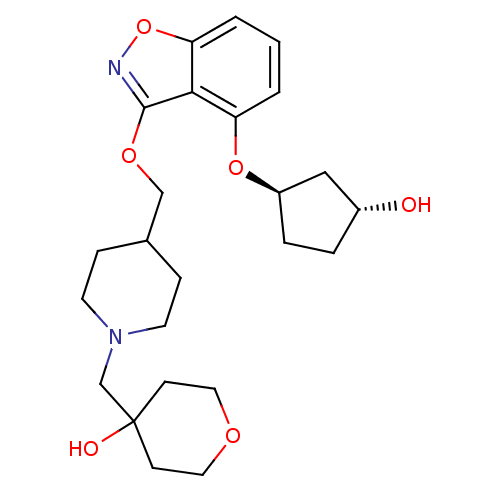 (CHEMBL2179580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398594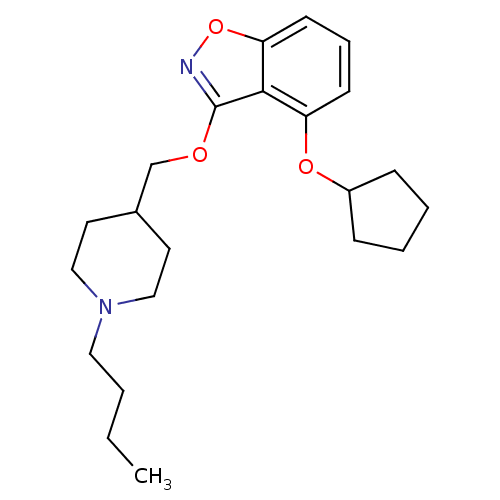 (CHEMBL2179585) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398595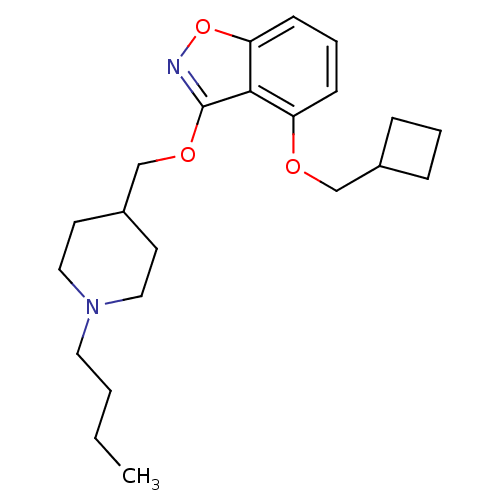 (CHEMBL2179586) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180014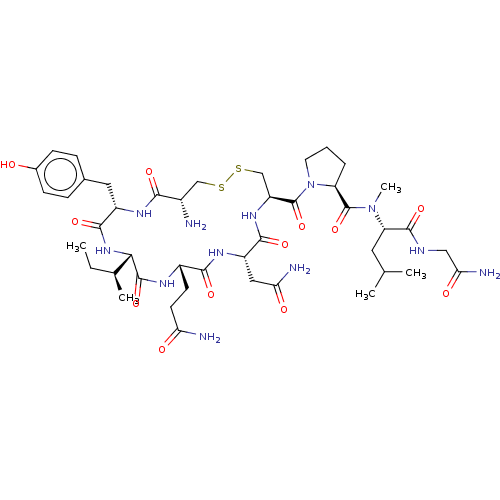 (CHEMBL3814744) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180155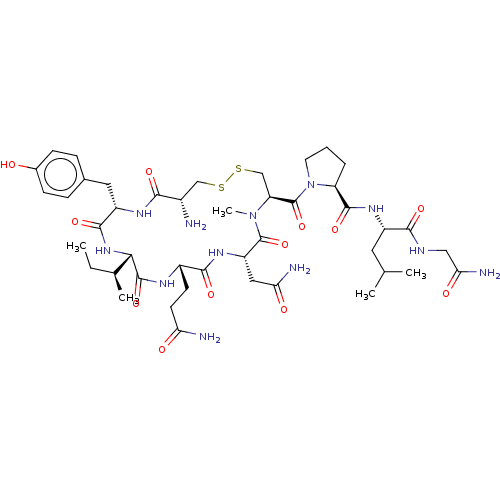 (CHEMBL3813894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180132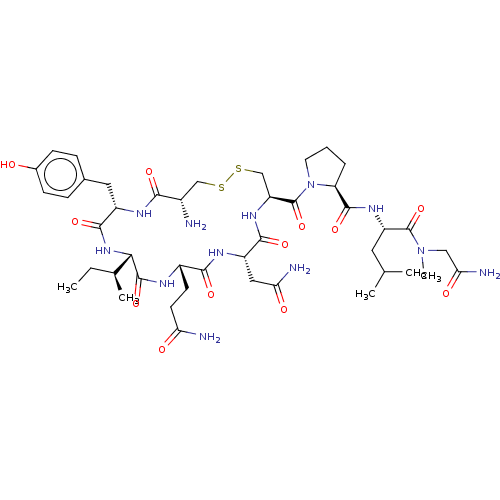 (CHEMBL3814633) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398590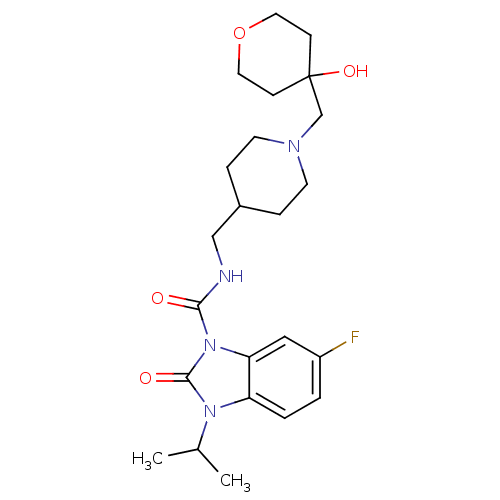 (CHEMBL2179583) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588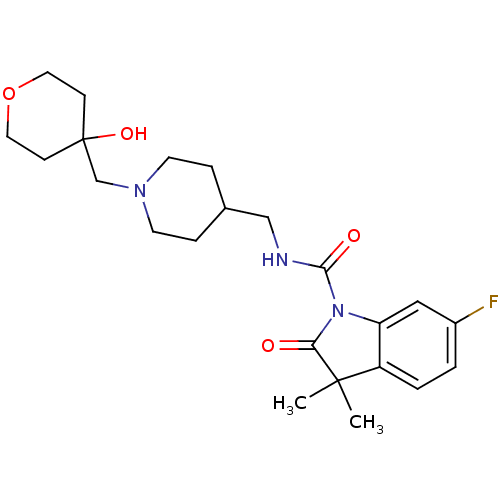 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398592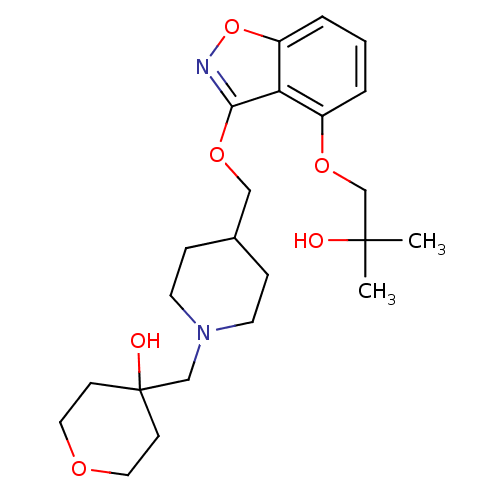 (CHEMBL2179590) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180160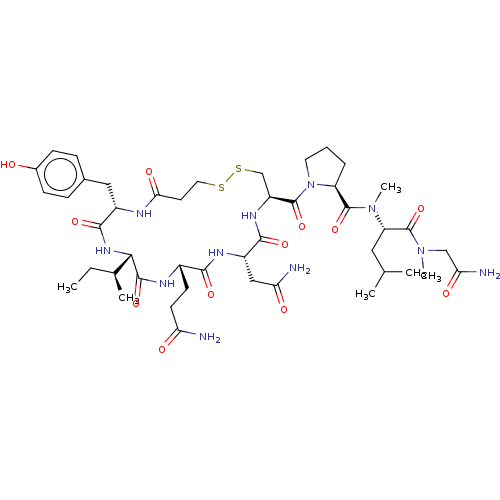 (CHEMBL3815099) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398591 (CHEMBL2179581) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398589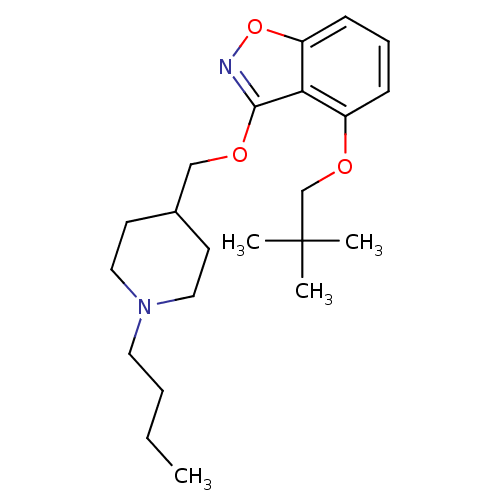 (CHEMBL2179588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179992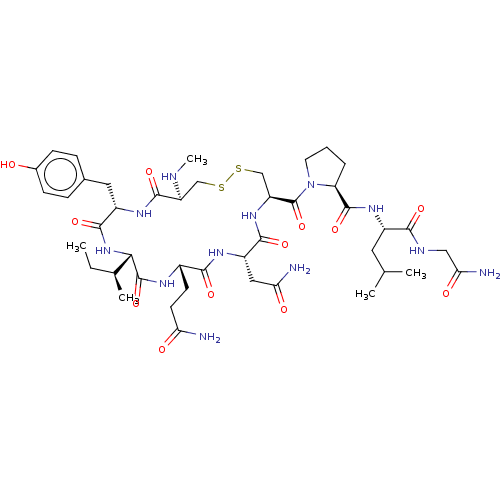 (CHEMBL3814395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179993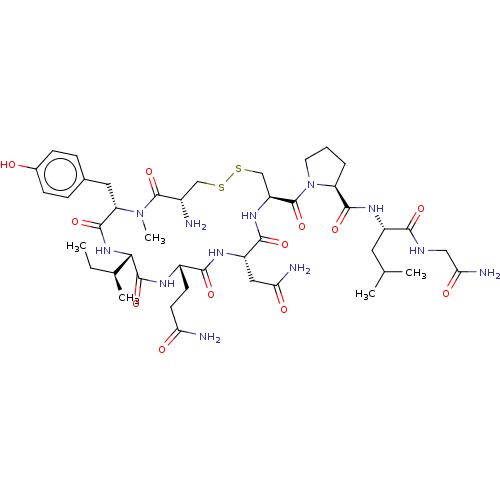 (CHEMBL3814165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180165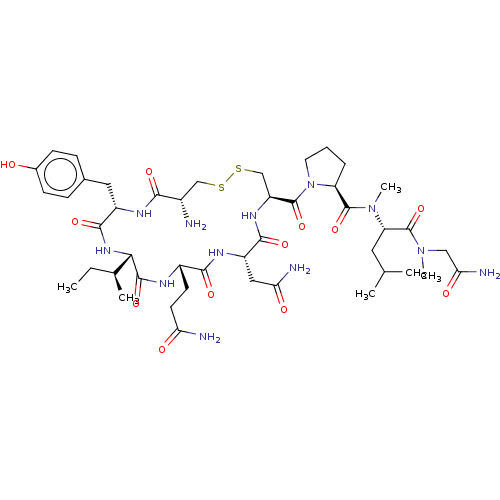 (CHEMBL3813722) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180154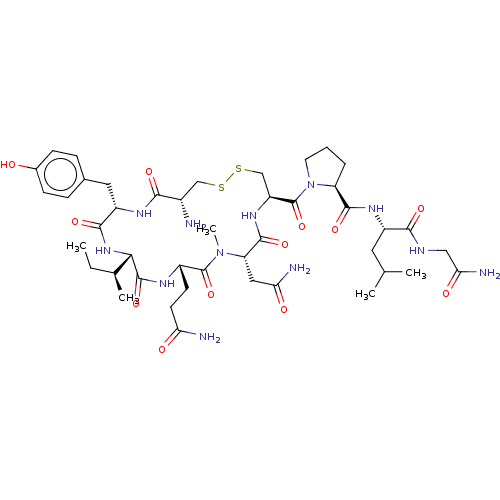 (CHEMBL3814617) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180164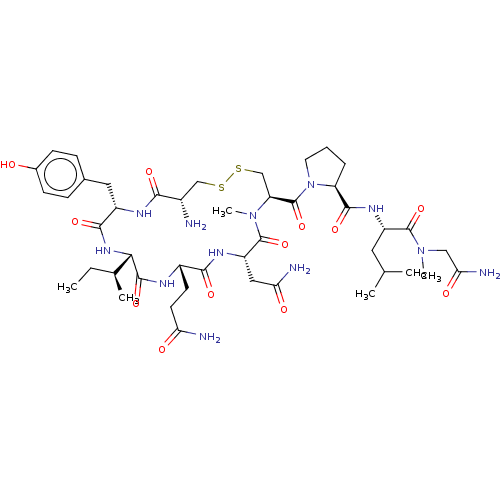 (CHEMBL3814120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180158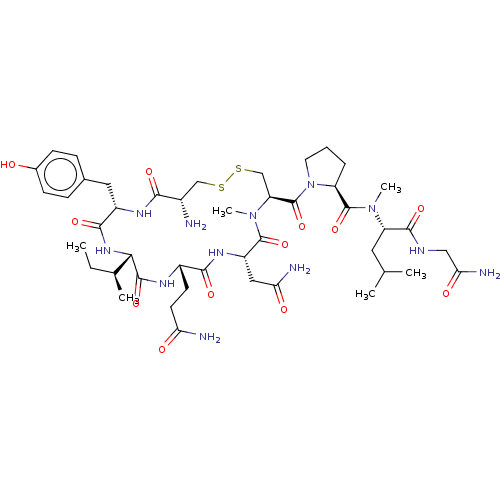 (CHEMBL3813858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180159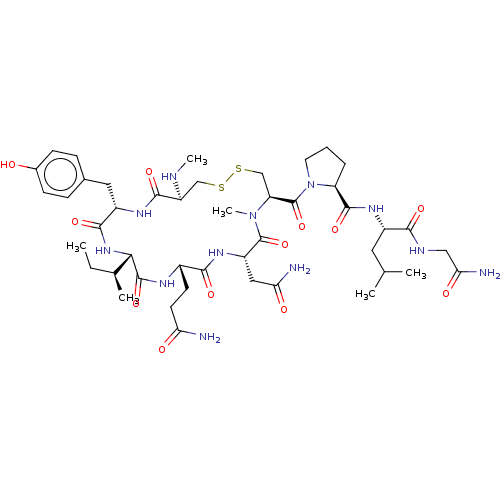 (CHEMBL3814232) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179994 (CHEMBL3814126) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180013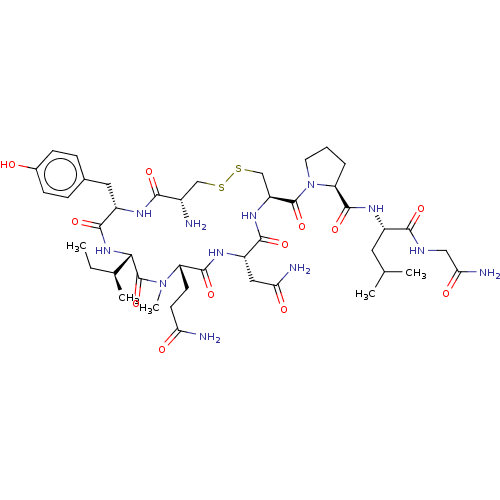 (CHEMBL3814904) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat 5HT4L receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat 5HT4L receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4D receptor expressed in HEK293 cells assessed as cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4D receptor expressed in HEK293 cells assessed as cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat 5HT4L receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4B receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4B receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4B receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4E receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4E receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat 5HT4E receptor expressed in HEK293 cells assessed as increase in cAMP production after 30 mins by HTRF assay | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |