

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginase (Leishmania amazonensis) | BDBM23417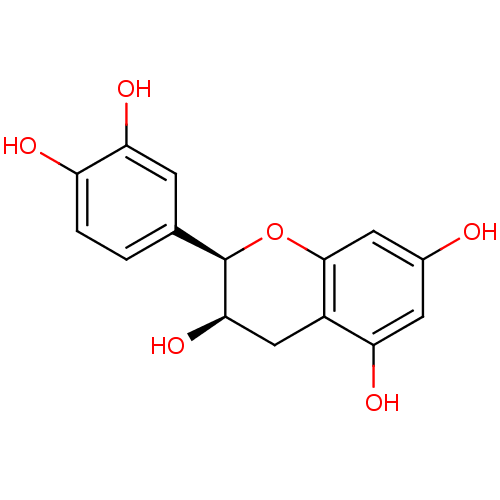 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM23416 (α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446567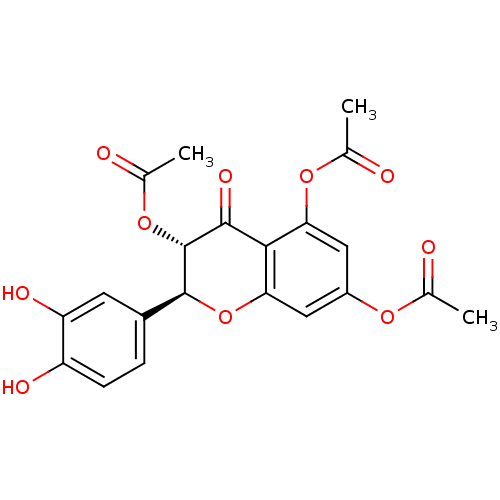 (CHEMBL3109443) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446568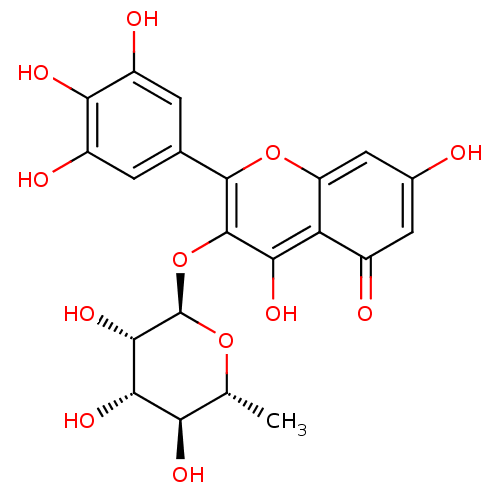 (CHEMBL3109439) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM84978 (Quercitrin | cid_5280459) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50329614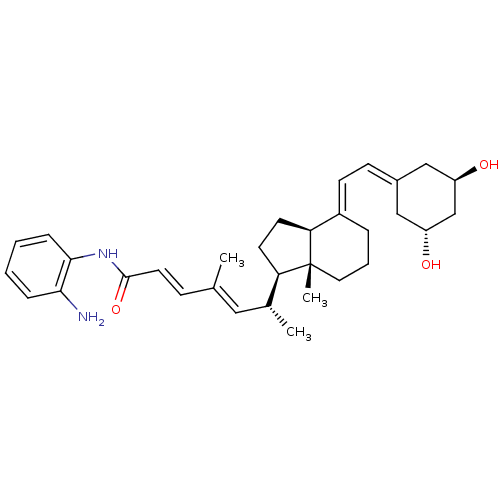 ((6R)-N-(2-aminophenyl)-6-((1R,7aR)-4-(2-((3R,5S)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR-LBD by fluorescence polarization competition assay | J Med Chem 53: 7461-5 (2010) Article DOI: 10.1021/jm1007159 BindingDB Entry DOI: 10.7270/Q2V9889V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320737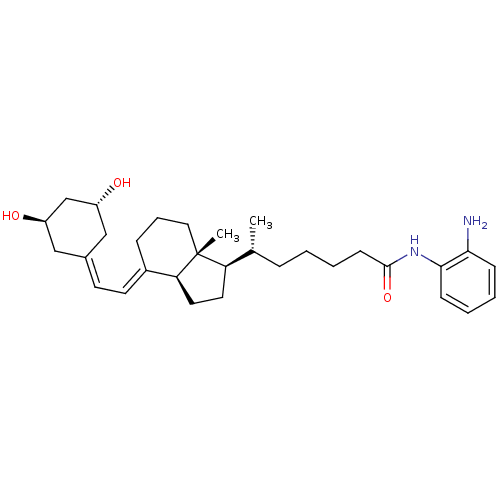 ((R)-N-(2-Aminophenyl)-6-((1R,3aS,7aR,E)-4-(2-((3R,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR-LBD by fluorescence polarization competition assay | J Med Chem 53: 7461-5 (2010) Article DOI: 10.1021/jm1007159 BindingDB Entry DOI: 10.7270/Q2V9889V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446567 (CHEMBL3109443) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032157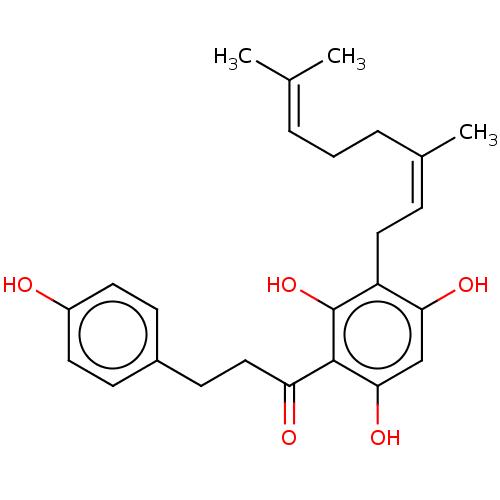 (CHEMBL3360923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM23417 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM23416 (α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50241354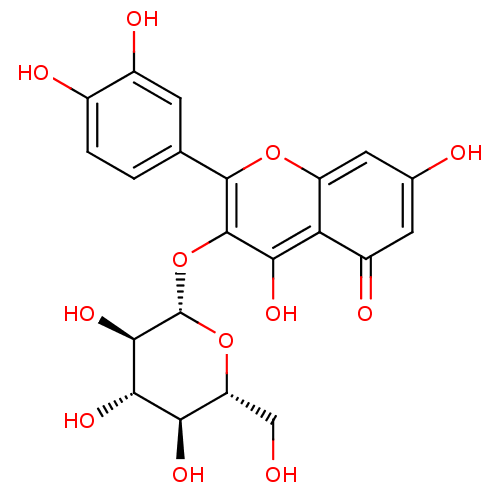 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446568 (CHEMBL3109439) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032226 (CHEMBL3352911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446571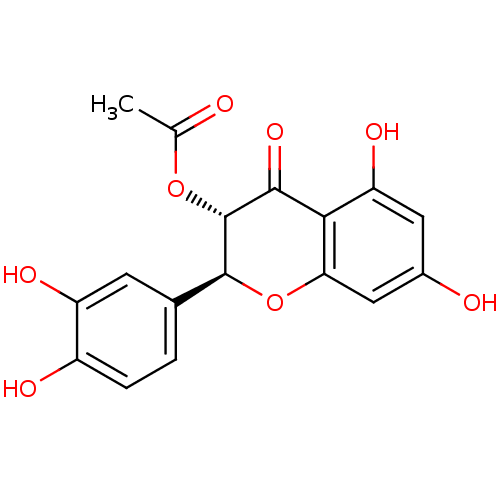 (CHEMBL3109442) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446570 (CHEMBL3109444) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032157 (CHEMBL3360923) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032159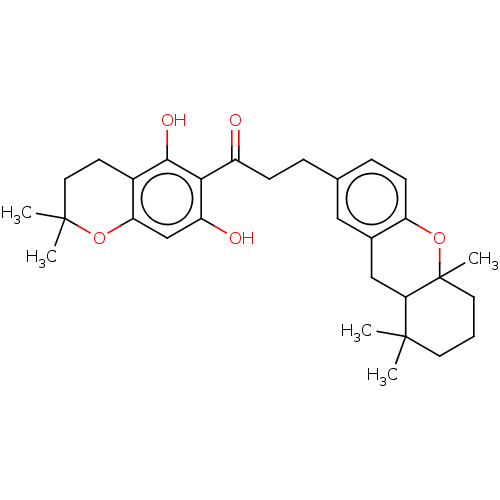 (CHEMBL3352912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032159 (CHEMBL3352912) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032226 (CHEMBL3352911) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM84978 (Quercitrin | cid_5280459) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50349826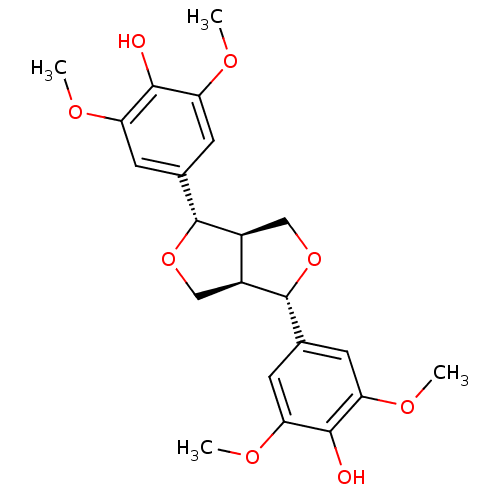 (SYRINGARESINOL) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032240 (CHEMBL3352910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446569 (Trigonostemone) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032240 (CHEMBL3352910) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446572 (CHEMBL3109441) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50320737 ((R)-N-(2-Aminophenyl)-6-((1R,3aS,7aR,E)-4-(2-((3R,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 by fluorescence assay | J Med Chem 53: 7461-5 (2010) Article DOI: 10.1021/jm1007159 BindingDB Entry DOI: 10.7270/Q2V9889V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446575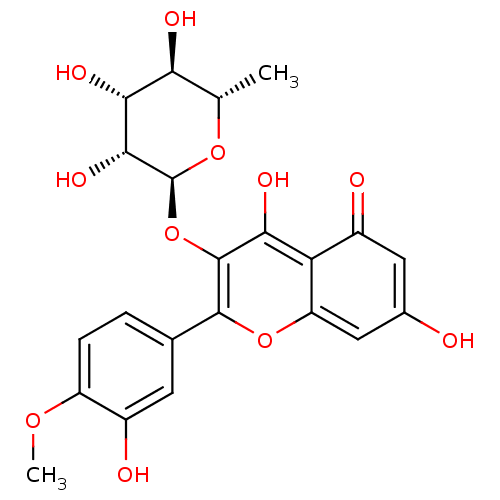 (CHEMBL3109437) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032157 (CHEMBL3360923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032159 (CHEMBL3352912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032240 (CHEMBL3352910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032226 (CHEMBL3352911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50404746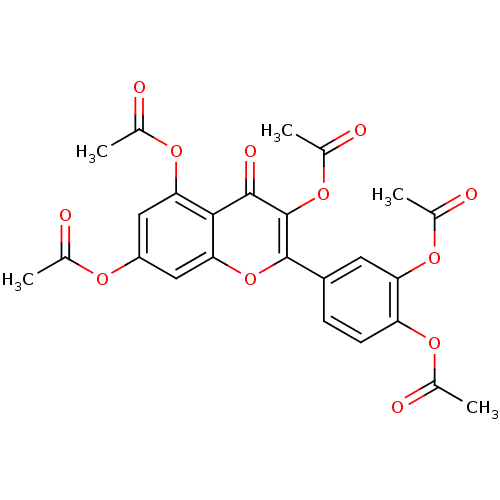 (NSC-115919 | PENTAACETYLQUERCETIN) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446573 (CHEMBL3109440) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446574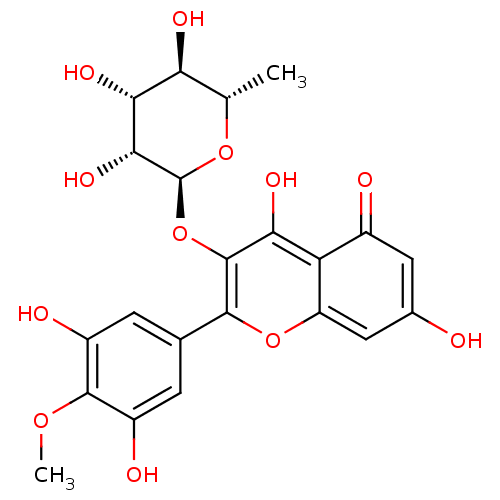 (CHEMBL3109438) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||