 Found 240 hits with Last Name = 'klein' and Initial = 'mg'
Found 240 hits with Last Name = 'klein' and Initial = 'mg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50166588
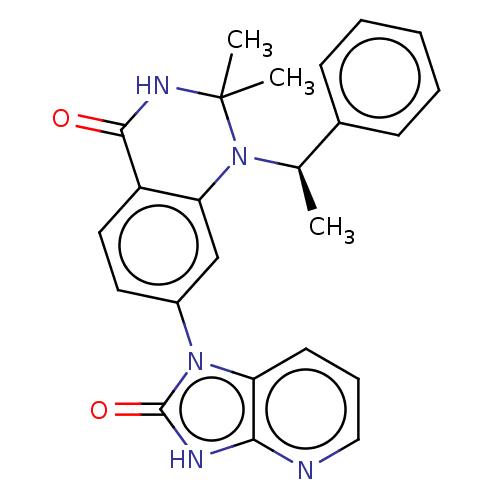
(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50166601
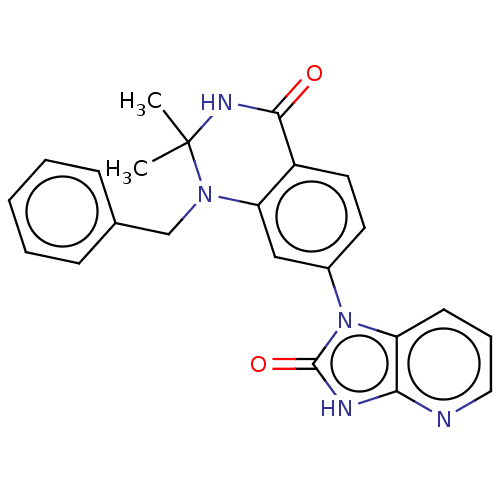
(CHEMBL3798679)Show SMILES CC1(C)NC(=O)c2ccc(cc2N1Cc1ccccc1)-n1c2cccnc2[nH]c1=O Show InChI InChI=1S/C23H21N5O2/c1-23(2)26-21(29)17-11-10-16(28-18-9-6-12-24-20(18)25-22(28)30)13-19(17)27(23)14-15-7-4-3-5-8-15/h3-13H,14H2,1-2H3,(H,26,29)(H,24,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50166602
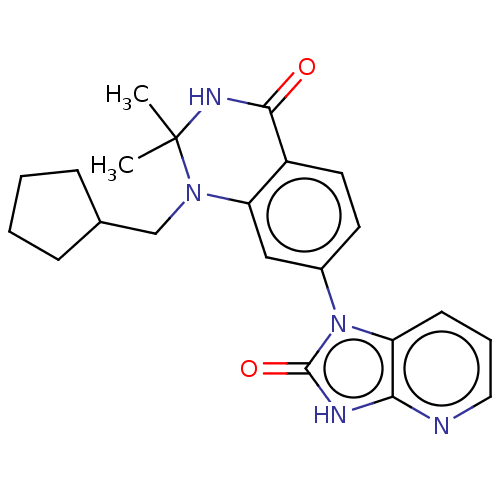
(CHEMBL3800310)Show SMILES CC1(C)NC(=O)c2ccc(cc2N1CC1CCCC1)-n1c2cccnc2[nH]c1=O Show InChI InChI=1S/C22H25N5O2/c1-22(2)25-20(28)16-10-9-15(12-18(16)26(22)13-14-6-3-4-7-14)27-17-8-5-11-23-19(17)24-21(27)29/h5,8-12,14H,3-4,6-7,13H2,1-2H3,(H,25,28)(H,23,24,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50367855
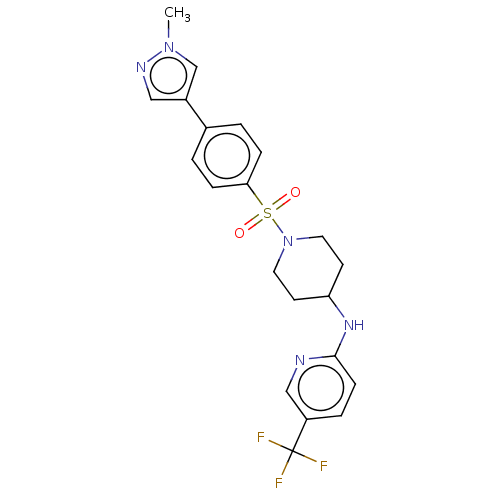
(CHEMBL4175929)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)N1CCC(CC1)Nc1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C21H22F3N5O2S/c1-28-14-16(12-26-28)15-2-5-19(6-3-15)32(30,31)29-10-8-18(9-11-29)27-20-7-4-17(13-25-20)21(22,23)24/h2-7,12-14,18H,8-11H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612294
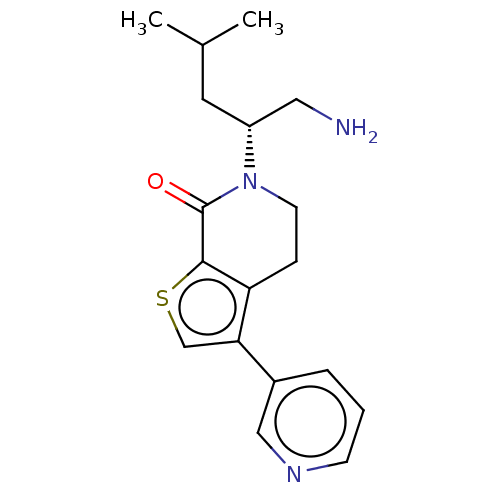
(CHEMBL5281395)Show SMILES CC(C)C[C@H](CN)N1CCc2c(csc2C1=O)-c1cccnc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged GSK-3beta (unknown origin) expressed in baculovirus preincubated for 5 mins using GSK3 substrate peptide measured... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human TrkA expressed in baculovirus preincubated for 5 mins measured after 10 mins in presence of ATP by AlphaScr... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CLK (unknown origin) |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCalpha (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCdelta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCbeta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measur... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50367676
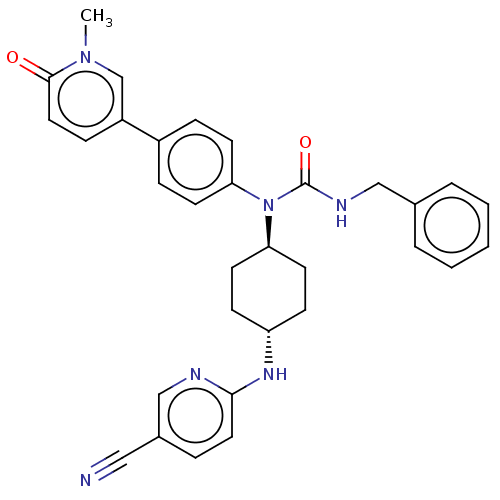
(CHEMBL4160662)Show SMILES Cn1cc(ccc1=O)-c1ccc(cc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)C(=O)NCc1ccccc1 |r,wU:15.16,wD:18.23,(17.35,-24.38,;18.11,-25.71,;19.66,-25.71,;20.43,-27.05,;19.66,-28.38,;18.11,-28.38,;17.35,-27.05,;15.8,-27.05,;21.97,-27.05,;22.74,-28.38,;24.28,-28.38,;25.05,-27.05,;24.28,-25.71,;22.74,-25.71,;26.6,-27.05,;27.37,-25.71,;26.6,-24.38,;27.37,-23.04,;28.91,-23.04,;29.68,-24.38,;28.91,-25.71,;29.68,-21.71,;31.22,-21.71,;31.99,-20.37,;33.54,-20.37,;34.3,-21.71,;33.54,-23.04,;31.99,-23.04,;35.85,-21.71,;37.39,-21.71,;27.37,-28.38,;26.6,-29.71,;28.91,-28.38,;29.68,-29.71,;31.22,-29.71,;31.99,-28.38,;33.54,-28.38,;34.3,-29.71,;33.54,-31.05,;31.99,-31.05,)| Show InChI InChI=1S/C32H32N6O2/c1-37-22-26(10-18-31(37)39)25-8-13-28(14-9-25)38(32(40)35-20-23-5-3-2-4-6-23)29-15-11-27(12-16-29)36-30-17-7-24(19-33)21-34-30/h2-10,13-14,17-18,21-22,27,29H,11-12,15-16,20H2,1H3,(H,34,36)(H,35,40)/t27-,29- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50367737

(CHEMBL4173631)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)N1CCC(CC1)Nc1ccc(cn1)C#N Show InChI InChI=1S/C21H22N6O2S/c1-26-15-18(14-24-26)17-3-5-20(6-4-17)30(28,29)27-10-8-19(9-11-27)25-21-7-2-16(12-22)13-23-21/h2-7,13-15,19H,8-11H2,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612293
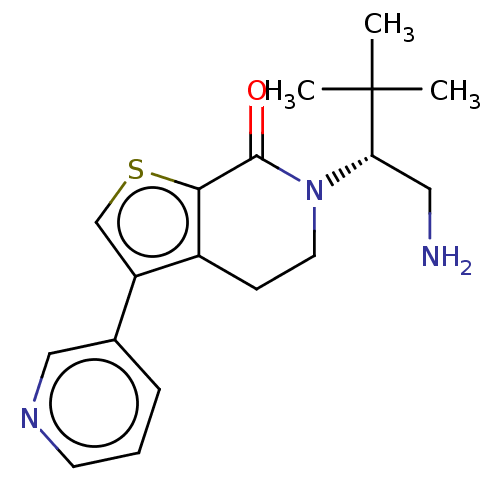
(CHEMBL5265996)Show SMILES CC(C)(C)[C@H](CN)N1CCc2c(csc2C1=O)-c1cccnc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta in human Jurkat cells after overnight incubation by luminescence analysis |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612290
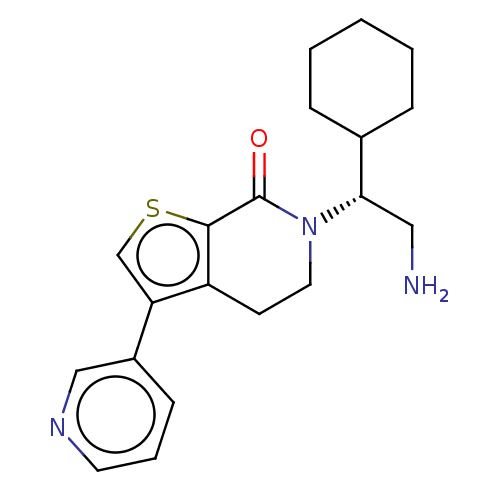
(CHEMBL5288132)Show SMILES NC[C@@H](C1CCCCC1)N1CCc2c(csc2C1=O)-c1cccnc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50163919
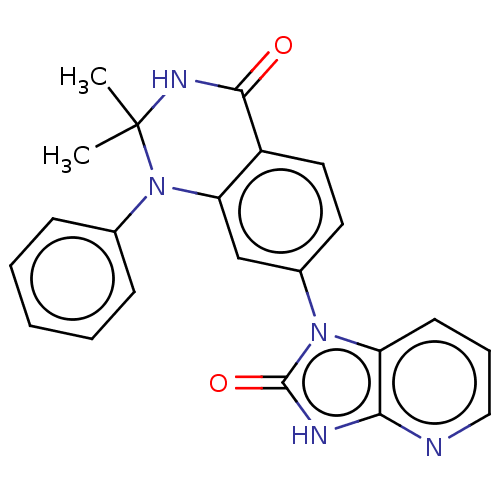
(CHEMBL3798266)Show SMILES CC1(C)NC(=O)c2ccc(cc2N1c1ccccc1)-n1c2cccnc2[nH]c1=O Show InChI InChI=1S/C22H19N5O2/c1-22(2)25-20(28)16-11-10-15(13-18(16)27(22)14-7-4-3-5-8-14)26-17-9-6-12-23-19(17)24-21(26)29/h3-13H,1-2H3,(H,25,28)(H,23,24,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Mus musculus) | BDBM50266936
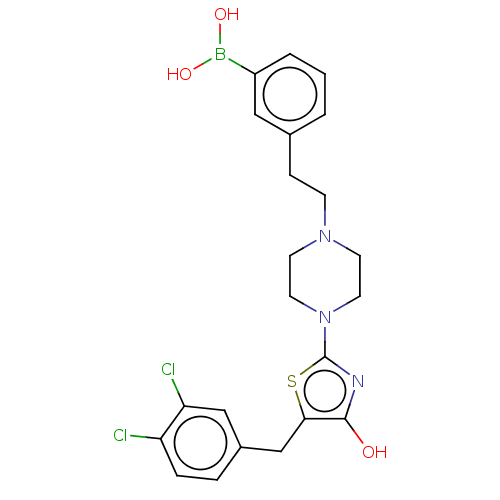
(CHEMBL4078601)Show SMILES OB(O)c1cccc(CCN2CCN(CC2)c2nc(O)c(Cc3ccc(Cl)c(Cl)c3)s2)c1 Show InChI InChI=1S/C22H24BCl2N3O3S/c24-18-5-4-16(13-19(18)25)14-20-21(29)26-22(32-20)28-10-8-27(9-11-28)7-6-15-2-1-3-17(12-15)23(30)31/h1-5,12-13,29-31H,6-11,14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ATX using TG-mTMP as substrate after 2 hrs by fluorometric analysis |
J Med Chem 60: 5209-5215 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01224
BindingDB Entry DOI: 10.7270/Q2319ZCT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50367857
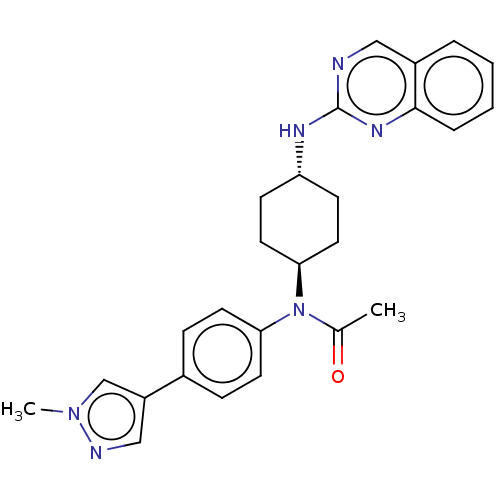
(CHEMBL4159417)Show SMILES CC(=O)N([C@H]1CC[C@@H](CC1)Nc1ncc2ccccc2n1)c1ccc(cc1)-c1cnn(C)c1 |r,wU:4.3,wD:7.10,(61.19,-8.94,;59.85,-9.71,;58.52,-8.94,;59.85,-11.25,;58.51,-12.02,;58.51,-13.56,;57.18,-14.32,;55.86,-13.55,;55.83,-12.02,;57.18,-11.24,;54.52,-14.32,;53.19,-13.56,;51.85,-14.33,;50.51,-13.56,;50.51,-12.01,;49.19,-11.25,;49.18,-9.72,;50.51,-8.95,;51.84,-9.71,;51.84,-11.24,;53.18,-12.01,;61.19,-12.03,;61.17,-13.56,;62.51,-14.33,;63.84,-13.57,;63.84,-12.02,;62.51,-11.25,;65.18,-14.34,;65.34,-15.87,;66.85,-16.19,;67.62,-14.85,;69.15,-14.69,;66.59,-13.71,)| Show InChI InChI=1S/C26H28N6O/c1-18(33)32(23-11-7-19(8-12-23)21-16-28-31(2)17-21)24-13-9-22(10-14-24)29-26-27-15-20-5-3-4-6-25(20)30-26/h3-8,11-12,15-17,22,24H,9-10,13-14H2,1-2H3,(H,27,29,30)/t22-,24- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612285

(CHEMBL5269564)Show SMILES NC[C@H](N1CCc2c(csc2C1=O)-c1cccnc1)c1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CDK9/CycT1 (04 to 110 residues) assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide p... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-C
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CDK8/CycC (04 to 109 residues) using kinase tracer 236 probe incubated for 60 mins by TR-FRET assay |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50166601

(CHEMBL3798679)Show SMILES CC1(C)NC(=O)c2ccc(cc2N1Cc1ccccc1)-n1c2cccnc2[nH]c1=O Show InChI InChI=1S/C23H21N5O2/c1-23(2)26-21(29)17-11-10-16(28-18-9-6-12-24-20(18)25-22(28)30)13-19(17)27(23)14-15-7-4-3-5-8-15/h3-13H,14H2,1-2H3,(H,26,29)(H,24,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta in human Jurkat cells after overnight incubation by luminescence analysis |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612287
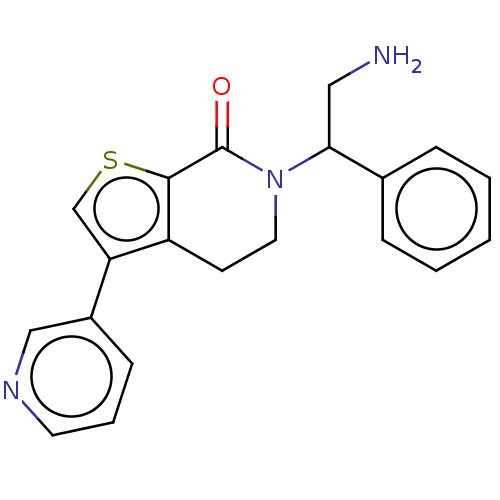
(CHEMBL5274333) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Mus musculus) | BDBM50266937
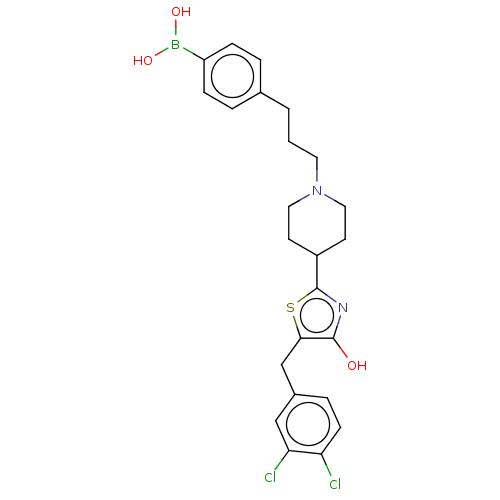
(CHEMBL4095125)Show SMILES OB(O)c1ccc(CCCN2CCC(CC2)c2nc(O)c(Cc3ccc(Cl)c(Cl)c3)s2)cc1 Show InChI InChI=1S/C24H27BCl2N2O3S/c26-20-8-5-17(14-21(20)27)15-22-23(30)28-24(33-22)18-9-12-29(13-10-18)11-1-2-16-3-6-19(7-4-16)25(31)32/h3-8,14,18,30-32H,1-2,9-13,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ATX using TG-mTMP as substrate after 2 hrs by fluorometric analysis |
J Med Chem 60: 5209-5215 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01224
BindingDB Entry DOI: 10.7270/Q2319ZCT |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50367857

(CHEMBL4159417)Show SMILES CC(=O)N([C@H]1CC[C@@H](CC1)Nc1ncc2ccccc2n1)c1ccc(cc1)-c1cnn(C)c1 |r,wU:4.3,wD:7.10,(61.19,-8.94,;59.85,-9.71,;58.52,-8.94,;59.85,-11.25,;58.51,-12.02,;58.51,-13.56,;57.18,-14.32,;55.86,-13.55,;55.83,-12.02,;57.18,-11.24,;54.52,-14.32,;53.19,-13.56,;51.85,-14.33,;50.51,-13.56,;50.51,-12.01,;49.19,-11.25,;49.18,-9.72,;50.51,-8.95,;51.84,-9.71,;51.84,-11.24,;53.18,-12.01,;61.19,-12.03,;61.17,-13.56,;62.51,-14.33,;63.84,-13.57,;63.84,-12.02,;62.51,-11.25,;65.18,-14.34,;65.34,-15.87,;66.85,-16.19,;67.62,-14.85,;69.15,-14.69,;66.59,-13.71,)| Show InChI InChI=1S/C26H28N6O/c1-18(33)32(23-11-7-19(8-12-23)21-16-28-31(2)17-21)24-13-9-22(10-14-24)29-26-27-15-20-5-3-4-6-25(20)30-26/h3-8,11-12,15-17,22,24H,9-10,13-14H2,1-2H3,(H,27,29,30)/t22-,24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612292
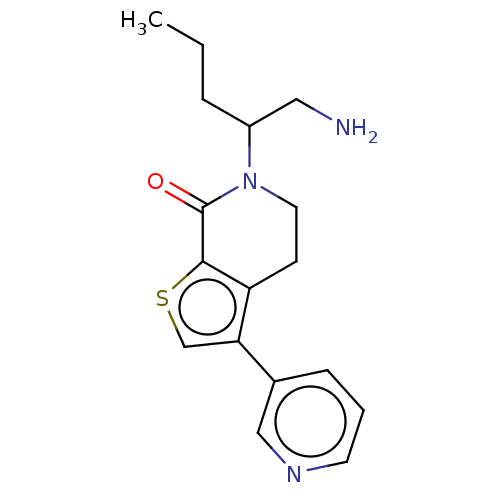
(CHEMBL5268272) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612295
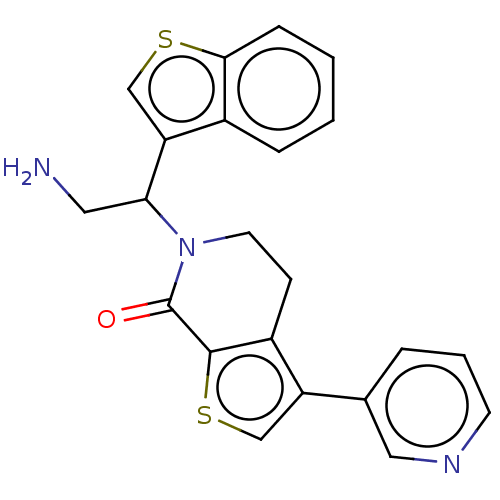
(CHEMBL5267547)Show SMILES NCC(N1CCc2c(csc2C1=O)-c1cccnc1)c1csc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612291

(CHEMBL5278471) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50367955
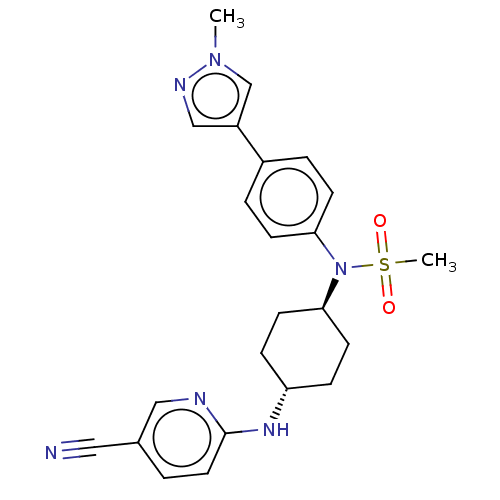
(CHEMBL4174716)Show SMILES Cn1cc(cn1)-c1ccc(cc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)S(C)(=O)=O |r,wU:13.14,wD:16.21,(31.03,-25.96,;29.49,-26.06,;28.51,-24.88,;27.08,-25.45,;27.17,-26.98,;28.67,-27.36,;25.77,-24.62,;25.84,-23.08,;24.54,-22.26,;23.17,-22.98,;23.11,-24.52,;24.41,-25.34,;21.86,-22.15,;20.54,-22.94,;20.56,-24.48,;19.23,-25.27,;17.89,-24.52,;17.87,-22.98,;19.19,-22.19,;16.57,-25.3,;15.22,-24.55,;13.9,-25.34,;12.55,-24.58,;12.53,-23.04,;13.86,-22.26,;15.2,-23.01,;11.19,-22.29,;9.84,-21.53,;21.93,-20.61,;23.25,-19.82,;20.58,-19.86,;21.53,-19.11,)| Show InChI InChI=1S/C23H26N6O2S/c1-28-16-19(15-26-28)18-4-8-21(9-5-18)29(32(2,30)31)22-10-6-20(7-11-22)27-23-12-3-17(13-24)14-25-23/h3-5,8-9,12,14-16,20,22H,6-7,10-11H2,1-2H3,(H,25,27)/t20-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50367873

(CHEMBL4169139)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)N1CCC(CC1)Nc1cc(ccn1)C#N Show InChI InChI=1S/C21H22N6O2S/c1-26-15-18(14-24-26)17-2-4-20(5-3-17)30(28,29)27-10-7-19(8-11-27)25-21-12-16(13-22)6-9-23-21/h2-6,9,12,14-15,19H,7-8,10-11H2,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50367957
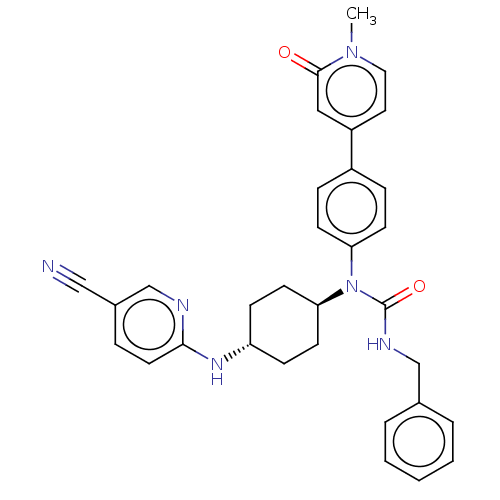
(CHEMBL4174715)Show SMILES Cn1ccc(cc1=O)-c1ccc(cc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)C(=O)NCc1ccccc1 |r,wU:15.16,wD:18.23,(13.41,-28.38,;14.96,-28.38,;15.73,-29.72,;17.27,-29.72,;18.04,-28.38,;17.27,-27.05,;15.73,-27.05,;14.96,-25.71,;19.58,-28.38,;20.35,-29.72,;21.9,-29.72,;22.67,-28.38,;21.9,-27.05,;20.35,-27.05,;24.21,-28.38,;24.98,-27.05,;24.21,-25.71,;24.98,-24.38,;26.52,-24.38,;27.29,-25.71,;26.52,-27.05,;27.29,-23.04,;28.84,-23.04,;29.6,-21.71,;31.15,-21.71,;31.92,-23.04,;31.15,-24.38,;29.6,-24.38,;33.46,-23.04,;35.01,-23.04,;24.98,-29.72,;24.21,-31.05,;26.52,-29.72,;27.29,-31.05,;28.84,-31.05,;29.6,-29.72,;31.15,-29.72,;31.92,-31.05,;31.15,-32.38,;29.6,-32.38,)| Show InChI InChI=1S/C32H32N6O2/c1-37-18-17-26(19-31(37)39)25-8-12-28(13-9-25)38(32(40)35-21-23-5-3-2-4-6-23)29-14-10-27(11-15-29)36-30-16-7-24(20-33)22-34-30/h2-9,12-13,16-19,22,27,29H,10-11,14-15,21H2,1H3,(H,34,36)(H,35,40)/t27-,29- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50164188
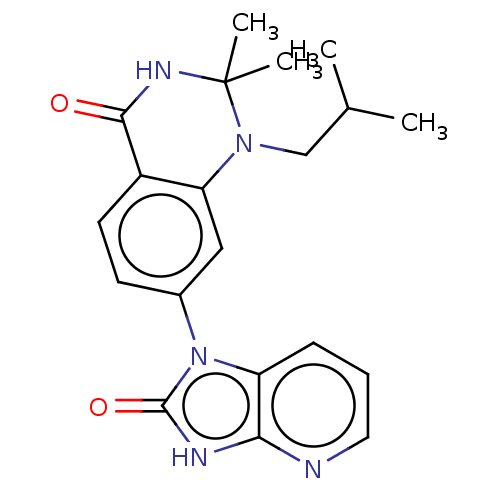
(CHEMBL3799399)Show SMILES CC(C)CN1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O Show InChI InChI=1S/C20H23N5O2/c1-12(2)11-24-16-10-13(7-8-14(16)18(26)23-20(24,3)4)25-15-6-5-9-21-17(15)22-19(25)27/h5-10,12H,11H2,1-4H3,(H,23,26)(H,21,22,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Cyclin-K/Cyclin-dependent kinase 12
(Homo sapiens (Human)) | BDBM50367676

(CHEMBL4160662)Show SMILES Cn1cc(ccc1=O)-c1ccc(cc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)C(=O)NCc1ccccc1 |r,wU:15.16,wD:18.23,(17.35,-24.38,;18.11,-25.71,;19.66,-25.71,;20.43,-27.05,;19.66,-28.38,;18.11,-28.38,;17.35,-27.05,;15.8,-27.05,;21.97,-27.05,;22.74,-28.38,;24.28,-28.38,;25.05,-27.05,;24.28,-25.71,;22.74,-25.71,;26.6,-27.05,;27.37,-25.71,;26.6,-24.38,;27.37,-23.04,;28.91,-23.04,;29.68,-24.38,;28.91,-25.71,;29.68,-21.71,;31.22,-21.71,;31.99,-20.37,;33.54,-20.37,;34.3,-21.71,;33.54,-23.04,;31.99,-23.04,;35.85,-21.71,;37.39,-21.71,;27.37,-28.38,;26.6,-29.71,;28.91,-28.38,;29.68,-29.71,;31.22,-29.71,;31.99,-28.38,;33.54,-28.38,;34.3,-29.71,;33.54,-31.05,;31.99,-31.05,)| Show InChI InChI=1S/C32H32N6O2/c1-37-22-26(10-18-31(37)39)25-8-13-28(14-9-25)38(32(40)35-20-23-5-3-2-4-6-23)29-15-11-27(12-16-29)36-30-17-7-24(19-33)21-34-30/h2-10,13-14,17-18,21-22,27,29H,11-12,15-16,20H2,1H3,(H,34,36)(H,35,40)/t27-,29- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50367685

(CHEMBL4172363)Show SMILES O=C(NCc1ccccc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)c1ccc(cc1)-c1cccnc1 |r,wU:11.11,wD:14.18,(23.87,-32.62,;24.64,-31.28,;26.18,-31.28,;26.95,-32.62,;28.49,-32.62,;29.26,-31.28,;30.81,-31.28,;31.58,-32.62,;30.81,-33.95,;29.26,-33.95,;23.87,-29.95,;24.64,-28.61,;23.87,-27.28,;24.64,-25.95,;26.18,-25.95,;26.95,-27.28,;26.18,-28.61,;26.95,-24.61,;28.49,-24.61,;29.26,-23.28,;30.81,-23.28,;31.58,-24.61,;30.81,-25.95,;29.26,-25.95,;33.12,-24.61,;34.66,-24.61,;22.32,-29.95,;21.56,-31.28,;20.01,-31.28,;19.24,-29.95,;20.01,-28.61,;21.56,-28.61,;17.7,-29.95,;16.93,-31.28,;15.39,-31.28,;14.61,-29.95,;15.39,-28.61,;16.93,-28.61,)| Show InChI InChI=1S/C31H30N6O/c32-19-24-8-17-30(34-21-24)36-27-11-15-29(16-12-27)37(31(38)35-20-23-5-2-1-3-6-23)28-13-9-25(10-14-28)26-7-4-18-33-22-26/h1-10,13-14,17-18,21-22,27,29H,11-12,15-16,20H2,(H,34,36)(H,35,38)/t27-,29- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50164176
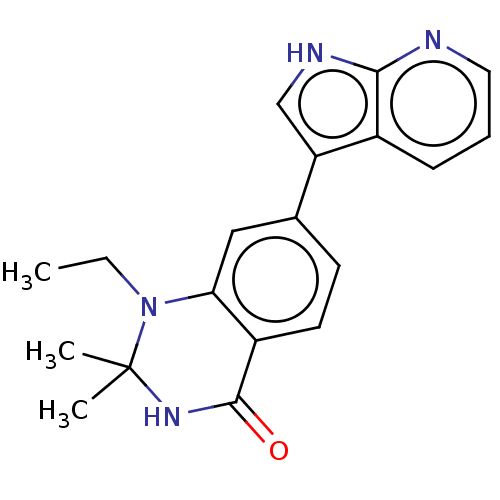
(CHEMBL3798713)Show SMILES CCN1c2cc(ccc2C(=O)NC1(C)C)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C19H20N4O/c1-4-23-16-10-12(7-8-14(16)18(24)22-19(23,2)3)15-11-21-17-13(15)6-5-9-20-17/h5-11H,4H2,1-3H3,(H,20,21)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50514710
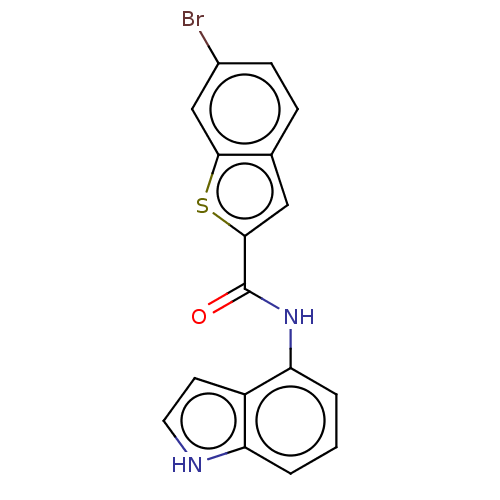
(CHEMBL4555677)Show InChI InChI=1S/C17H11BrN2OS/c18-11-5-4-10-8-16(22-15(10)9-11)17(21)20-14-3-1-2-13-12(14)6-7-19-13/h1-9,19H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50514710

(CHEMBL4555677)Show InChI InChI=1S/C17H11BrN2OS/c18-11-5-4-10-8-16(22-15(10)9-11)17(21)20-14-3-1-2-13-12(14)6-7-19-13/h1-9,19H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged TEV protease site linked DHPS expressed in Escherichia coli BL21 (DE3) assessed as reduction in radiolabel... |
J Med Chem 63: 3215-3226 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01979
BindingDB Entry DOI: 10.7270/Q2K35Z1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50367845
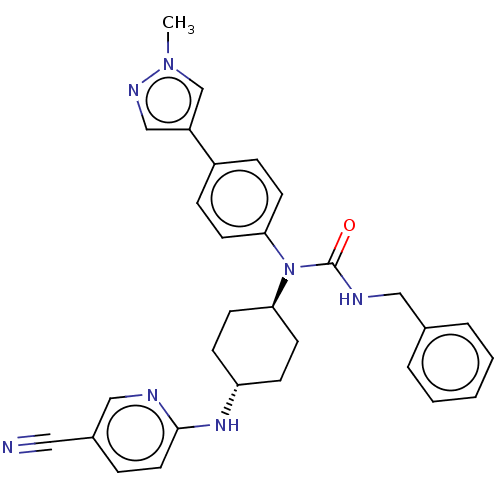
(CHEMBL4173254)Show SMILES Cn1cc(cn1)-c1ccc(cc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)C(=O)NCc1ccccc1 |r,wU:13.14,wD:16.21,(33.38,-26.71,;31.84,-26.8,;30.86,-25.61,;29.43,-26.18,;29.52,-27.71,;31.01,-28.09,;28.14,-25.34,;28.21,-23.81,;26.91,-22.98,;25.54,-23.69,;25.48,-25.23,;26.77,-26.05,;24.24,-22.86,;22.92,-23.64,;22.93,-25.18,;21.6,-25.96,;20.26,-25.2,;20.25,-23.66,;21.58,-22.88,;18.94,-25.98,;17.6,-25.22,;16.28,-26,;14.94,-25.24,;14.93,-23.7,;16.25,-22.92,;17.59,-23.68,;13.59,-22.94,;12.25,-22.18,;24.31,-21.33,;25.64,-20.55,;22.97,-20.56,;21.65,-21.34,;20.3,-20.58,;20.29,-19.03,;18.94,-18.27,;17.61,-19.06,;17.62,-20.61,;18.97,-21.37,)| Show InChI InChI=1S/C30H31N7O/c1-36-21-25(20-34-36)24-8-12-27(13-9-24)37(30(38)33-18-22-5-3-2-4-6-22)28-14-10-26(11-15-28)35-29-16-7-23(17-31)19-32-29/h2-9,12-13,16,19-21,26,28H,10-11,14-15,18H2,1H3,(H,32,35)(H,33,38)/t26-,28- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50367707
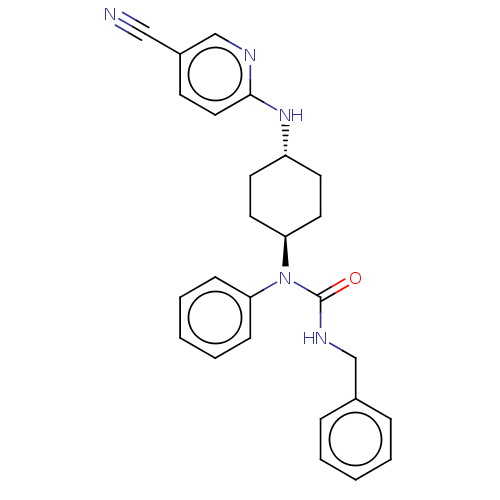
(CHEMBL4167916)Show SMILES O=C(NCc1ccccc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)c1ccccc1 |r,wU:11.11,wD:14.18,(25.33,-38.6,;25.33,-37.05,;26.66,-36.28,;27.99,-37.05,;29.32,-36.28,;29.32,-34.75,;30.66,-33.97,;31.99,-34.75,;31.99,-36.28,;30.66,-37.05,;23.99,-36.28,;22.66,-37.05,;22.66,-38.6,;21.33,-39.36,;20,-38.6,;20,-37.05,;21.33,-36.28,;18.67,-39.36,;17.34,-38.6,;16,-39.36,;14.67,-38.6,;14.67,-37.05,;16,-36.28,;17.34,-37.05,;13.34,-36.28,;12,-35.51,;23.99,-34.75,;22.66,-33.97,;22.66,-32.44,;23.99,-31.67,;25.33,-32.44,;25.33,-33.97,)| Show InChI InChI=1S/C26H27N5O/c27-17-21-11-16-25(28-19-21)30-22-12-14-24(15-13-22)31(23-9-5-2-6-10-23)26(32)29-18-20-7-3-1-4-8-20/h1-11,16,19,22,24H,12-15,18H2,(H,28,30)(H,29,32)/t22-,24- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612294

(CHEMBL5281395)Show SMILES CC(C)C[C@H](CN)N1CCc2c(csc2C1=O)-c1cccnc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
U5 small nuclear ribonucleoprotein 200 kDa helicase
(Homo sapiens (Human)) | BDBM50239869
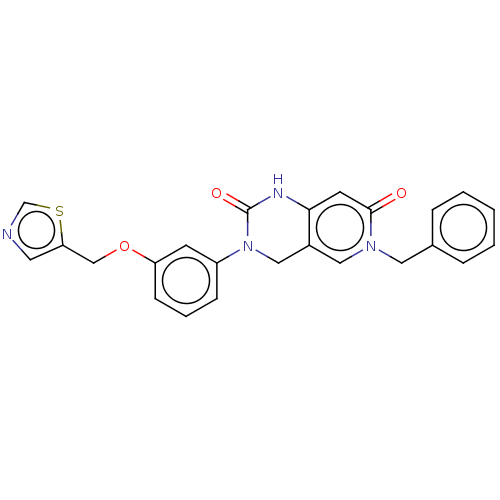
(CHEMBL4084949)Show SMILES O=C1Nc2cc(=O)n(Cc3ccccc3)cc2CN1c1cccc(OCc2cncs2)c1 Show InChI InChI=1S/C24H20N4O3S/c29-23-10-22-18(13-27(23)12-17-5-2-1-3-6-17)14-28(24(30)26-22)19-7-4-8-20(9-19)31-15-21-11-25-16-32-21/h1-11,13,16H,12,14-15H2,(H,26,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His-tagged BRR2 RNA dependent ATPase activity expressed in baculovirus infected Sf9 cells usin... |
J Med Chem 60: 5759-5771 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00461
BindingDB Entry DOI: 10.7270/Q2MC925G |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50514715
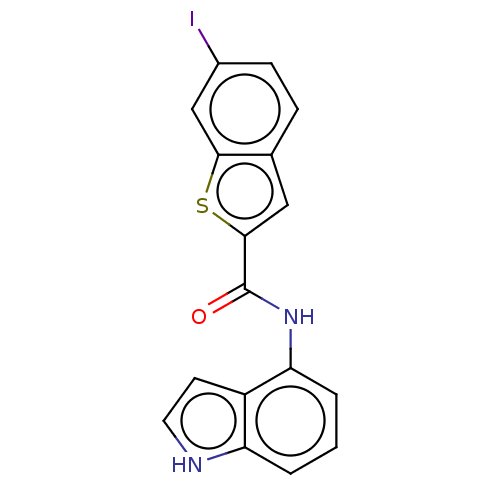
(CHEMBL4543218)Show InChI InChI=1S/C17H11IN2OS/c18-11-5-4-10-8-16(22-15(10)9-11)17(21)20-14-3-1-2-13-12(14)6-7-19-13/h1-9,19H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged TEV protease site linked DHPS expressed in Escherichia coli BL21 (DE3) assessed as reduction in radiolabel... |
J Med Chem 63: 3215-3226 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01979
BindingDB Entry DOI: 10.7270/Q2K35Z1V |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50367684
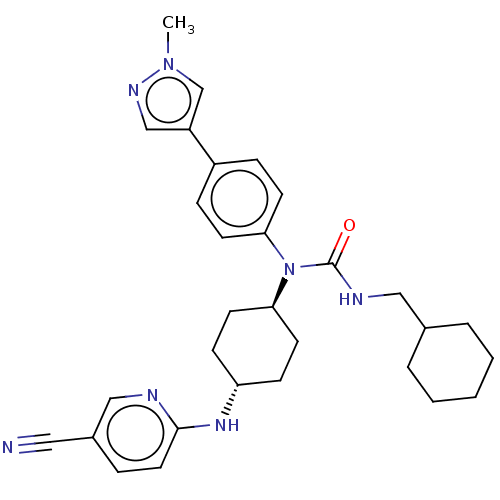
(CHEMBL4162567)Show SMILES Cn1cc(cn1)-c1ccc(cc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)C(=O)NCC1CCCCC1 |r,wU:13.14,wD:16.21,(33.38,-26.71,;31.84,-26.8,;30.86,-25.61,;29.43,-26.18,;29.52,-27.71,;31.01,-28.09,;28.14,-25.34,;28.21,-23.81,;26.91,-22.98,;25.54,-23.69,;25.48,-25.23,;26.77,-26.05,;24.24,-22.86,;22.92,-23.64,;22.93,-25.18,;21.6,-25.96,;20.26,-25.2,;20.25,-23.66,;21.58,-22.88,;18.94,-25.98,;17.6,-25.22,;16.28,-26,;14.94,-25.24,;14.93,-23.7,;16.25,-22.92,;17.59,-23.68,;13.59,-22.94,;12.25,-22.18,;24.31,-21.33,;25.64,-20.55,;22.97,-20.56,;21.65,-21.34,;20.3,-20.58,;20.29,-19.03,;18.94,-18.27,;17.61,-19.06,;17.62,-20.61,;18.97,-21.37,)| Show InChI InChI=1S/C30H37N7O/c1-36-21-25(20-34-36)24-8-12-27(13-9-24)37(30(38)33-18-22-5-3-2-4-6-22)28-14-10-26(11-15-28)35-29-16-7-23(17-31)19-32-29/h7-9,12-13,16,19-22,26,28H,2-6,10-11,14-15,18H2,1H3,(H,32,35)(H,33,38)/t26-,28- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged FAK cytoplasmic domain (unknown origin) expressed in baculovirus preincubated for 5 mins measured after 20 mins ... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50166599
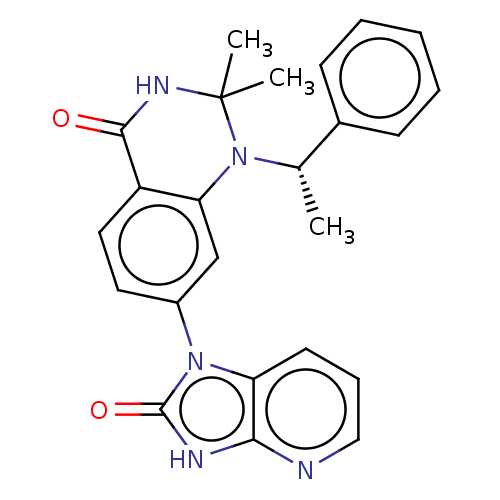
(CHEMBL3800082)Show SMILES C[C@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus preincubated for 5 mins using fluorescein-PKC substrate measu... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant human c-Met preincubated for 5 mins measured after 10 mins in presence of ATP by AlphaScreen assay |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50166588

(CHEMBL3798011)Show SMILES C[C@@H](N1c2cc(ccc2C(=O)NC1(C)C)-n1c2cccnc2[nH]c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-15(16-8-5-4-6-9-16)29-20-14-17(11-12-18(20)22(30)27-24(29,2)3)28-19-10-7-13-25-21(19)26-23(28)31/h4-15H,1-3H3,(H,27,30)(H,25,26,31)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal His-tagged recombinant human SRC preincubated for 5 mins measured after 10 mins in presence of ATP by AlphaScree... |
Bioorg Med Chem 24: 2466-75 (2016)
Article DOI: 10.1016/j.bmc.2016.04.008
BindingDB Entry DOI: 10.7270/Q27W6F29 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data