

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50035622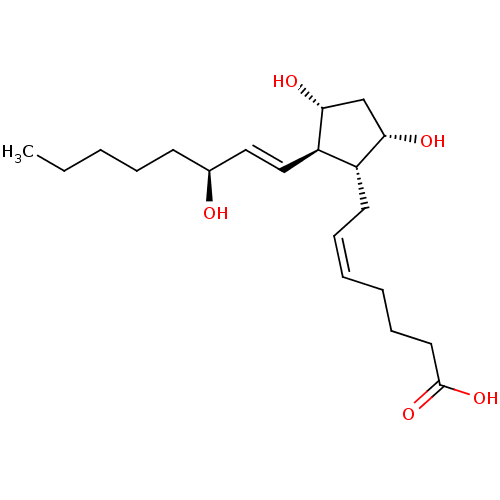 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at TP human prostaglandin receptor using [3H]-SQ-29,548 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human wild type EML4-fused ALK expressed in mouse NIH-3T3 cells assessed as phosphorylated ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106547 (7-[3,5-Dihydroxy-2-(3-hydroxy-4-phenoxy-but-1-enyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]-PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of FER (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of LTK (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]-PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308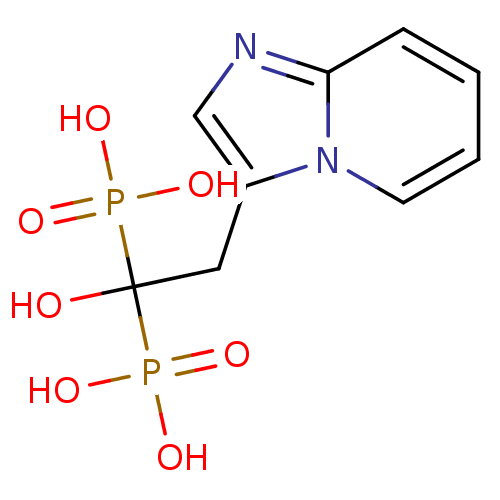 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of human FPPS after 10 mins using [14C]IPP as substrate by liquid scintillation counting | J Med Chem 53: 3454-64 (2010) Checked by Author Article DOI: 10.1021/jm900232u BindingDB Entry DOI: 10.7270/Q21837FJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of NTRK2 (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fes/Fps (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of FES (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PTK2B (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of TNK2 (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PTK2 (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of NTRK1 (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of NTRK3 (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50106547 (7-[3,5-Dihydroxy-2-(3-hydroxy-4-phenoxy-but-1-enyl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1 cells, transiently transfected with human prostaglandin EP1 receptor | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106548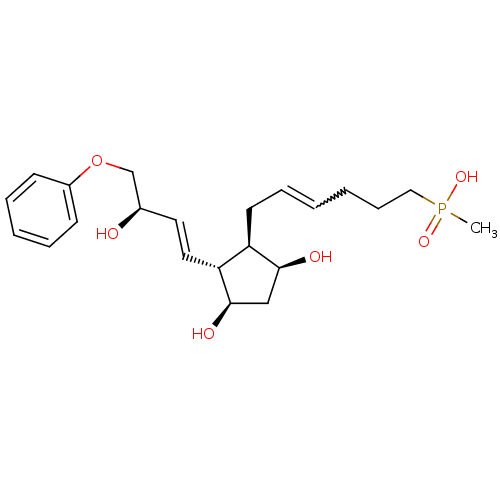 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]-PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human wild type EML4-fused ALK expressed in mouse NIH-3T3 cells assessed as phosphorylated ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP3 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]-PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50106547 (7-[3,5-Dihydroxy-2-(3-hydroxy-4-phenoxy-but-1-enyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1 cells, transiently transfected with human prostaglandin EP3 receptor | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP1 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106549 (CHEMBL336781 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]-PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50106547 (7-[3,5-Dihydroxy-2-(3-hydroxy-4-phenoxy-but-1-enyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1 cells, transiently transfected with human prostaglandin TP receptor | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of GGPPS after 10 mins using [14C]IPP as substrate by liquid scintillation counting | J Med Chem 53: 3454-64 (2010) Checked by Author Article DOI: 10.1021/jm900232u BindingDB Entry DOI: 10.7270/Q21837FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106553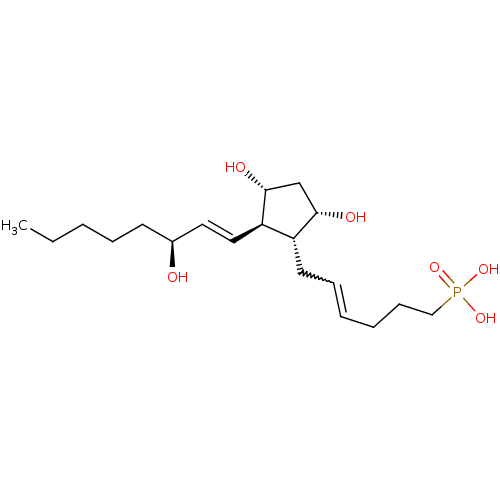 (CHEMBL336543 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]- PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106552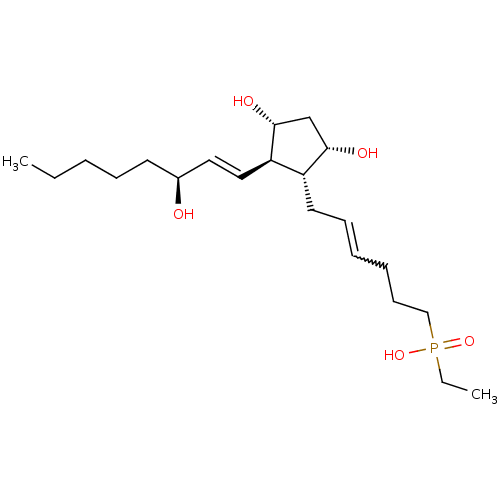 (CHEMBL133945 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]-PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP4 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at DP human prostaglandin receptor using [3H]- PGD-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106551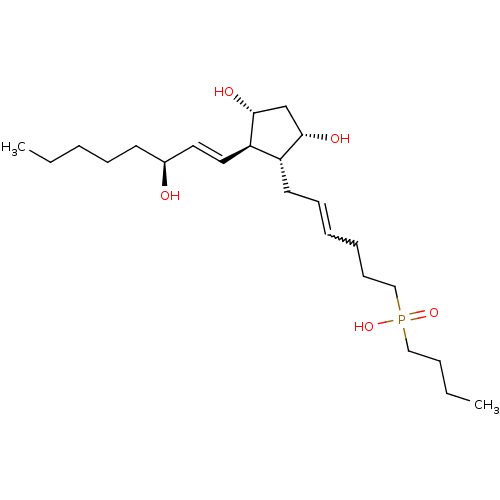 (Butyl-{6-[3,5-dihydroxy-2-(3-hydroxy-oct-1-enyl)-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]-PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at TP human prostaglandin receptor using [3H]-SQ-29,548 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50318032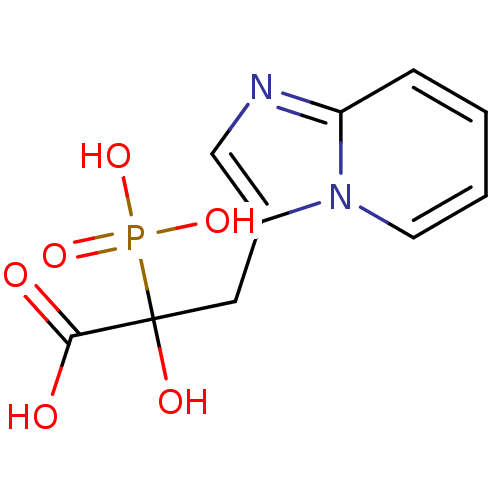 ((+)-2-hydroxy-3-imidazo[1,2-a]pyridin-3-yl-2-phosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of human FPPS after 10 mins using [14C]IPP as substrate by liquid scintillation counting | J Med Chem 53: 3454-64 (2010) Checked by Author Article DOI: 10.1021/jm900232u BindingDB Entry DOI: 10.7270/Q21837FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP4 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50106548 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP4 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP2 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at prostanoid IP receptor using [3H]-Iloprost as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50106548 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at TP human prostaglandin receptor using [3H]-SQ-29,548 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50106548 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP1 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at DP human prostaglandin receptor using [3H]- PGD-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50106550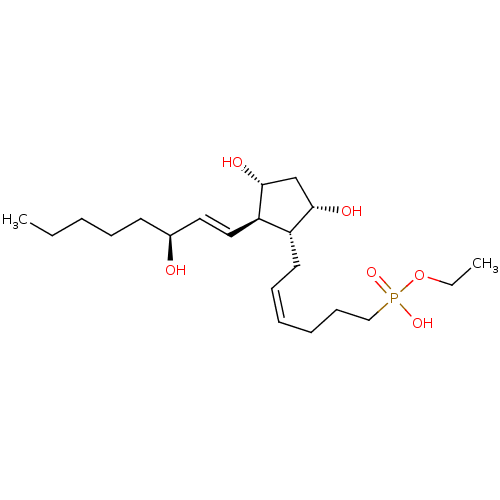 (CHEMBL334810 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at FP human prostaglandin receptor using [3H]- PGF-2 alpha as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50106548 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP3 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50106548 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at DP human prostaglandin receptor using [3H]- PGD-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP3 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50106548 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP2 human prostaglandin receptor | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50106546 (CHEMBL134546 | {6-[3,5-Dihydroxy-2-(3-hydroxy-oct-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP1 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50106548 (CHEMBL440164 | {6-[3,5-Dihydroxy-2-(3-hydroxy-4-ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at prostanoid IP receptor using [3H]-Iloprost as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at EP2 human prostaglandin receptor using [3H]-PGE-2 as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding of the compound at prostanoid IP receptor using [3H]-Iloprost as radioligand | J Med Chem 44: 4157-69 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50318032 ((+)-2-hydroxy-3-imidazo[1,2-a]pyridin-3-yl-2-phosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of GGPPS after 10 mins using [14C]IPP as substrate by liquid scintillation counting | J Med Chem 53: 3454-64 (2010) Checked by Author Article DOI: 10.1021/jm900232u BindingDB Entry DOI: 10.7270/Q21837FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |