

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (BOVINE) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Ability to inhibit the dopamine-stimulated adenylate-cyclase activity in dispersed cells of bovine parathyroid gland | J Med Chem 33: 521-6 (1990) BindingDB Entry DOI: 10.7270/Q22J69VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444496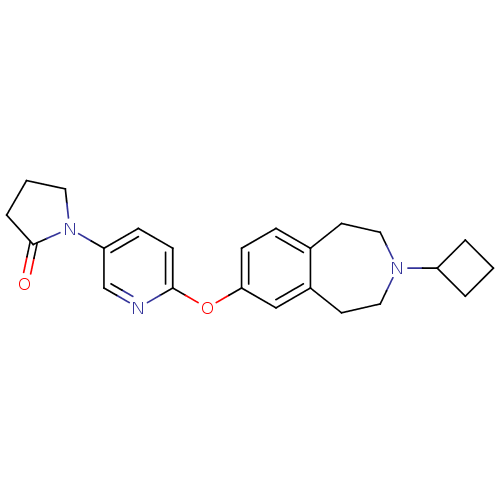 (CHEMBL3092650) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Ability to inhibit [3H]-SCH- 23390 binding to Dopamine receptor D1 of canine striatal membranes | J Med Chem 33: 521-6 (1990) BindingDB Entry DOI: 10.7270/Q22J69VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (BOVINE) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Ability to inhibit [3H]-SCH- 23390 binding to Dopamine receptor D1 of canine striatal membranes | J Med Chem 33: 521-6 (1990) BindingDB Entry DOI: 10.7270/Q22J69VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum | J Med Chem 35: 67-72 (1992) BindingDB Entry DOI: 10.7270/Q2P84CHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444491 (CHEMBL3092823) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010294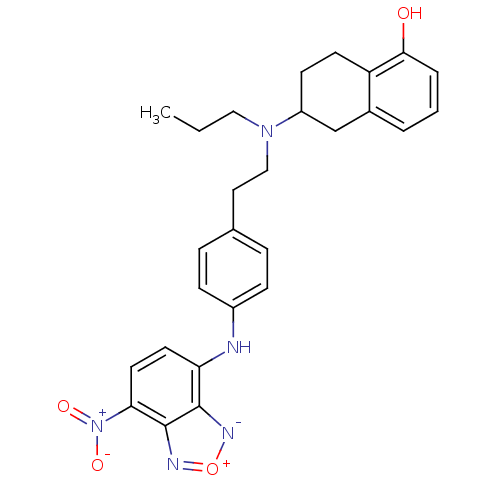 ((R)6-({2-[4-(7-Nitro-benzo[1,2,5]oxadiazol-4-ylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50010301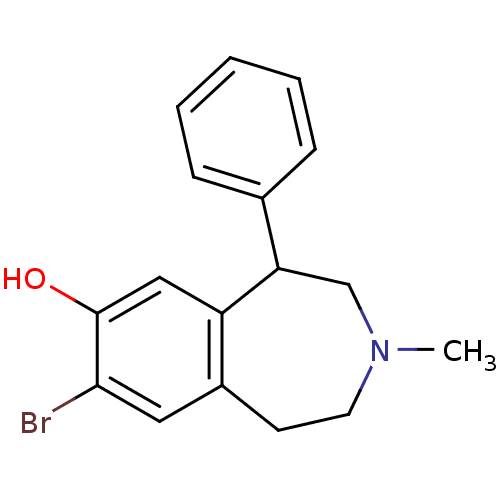 (8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D1 by using [3H]-SCH- 23390 as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444496 (CHEMBL3092650) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010294 ((R)6-({2-[4-(7-Nitro-benzo[1,2,5]oxadiazol-4-ylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444500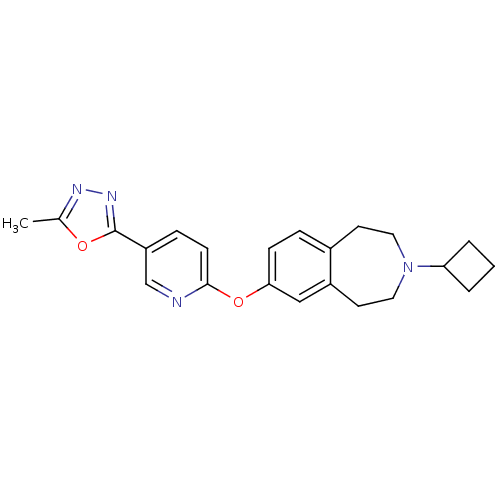 (CHEMBL3092834) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50368313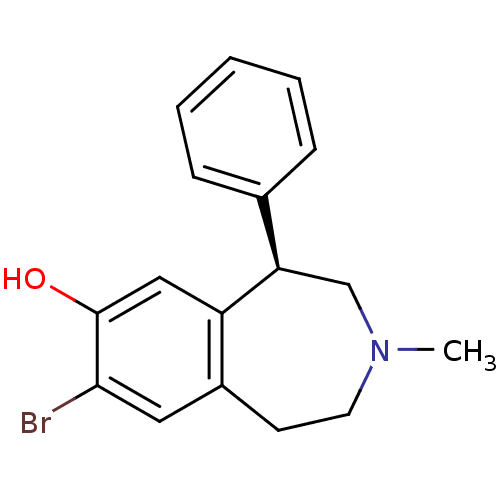 (CHEMBL1744079) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum | J Med Chem 35: 67-72 (1992) BindingDB Entry DOI: 10.7270/Q2P84CHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444509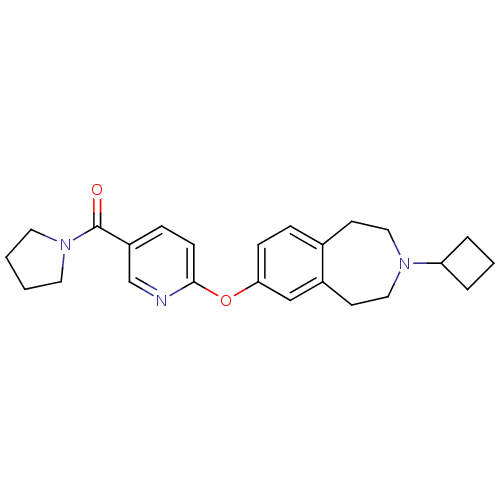 (CHEMBL3092840) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444505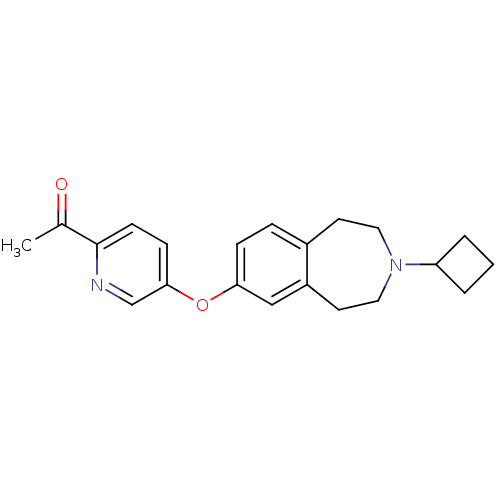 (CHEMBL3092828) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444507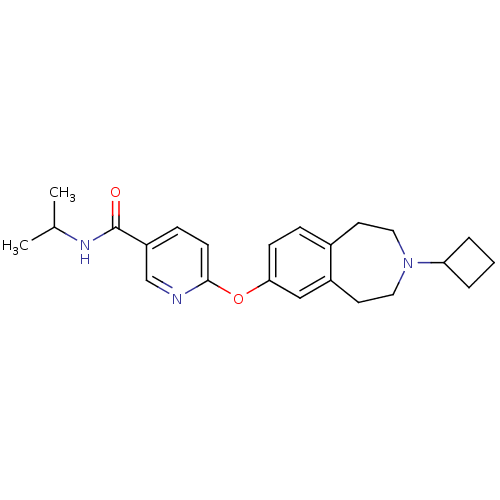 (CHEMBL3092826) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010292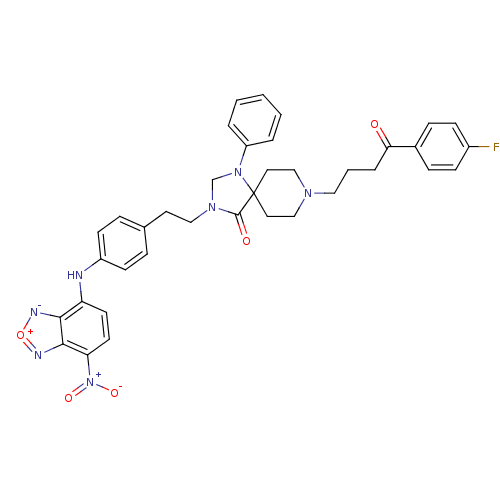 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-{2-[4-(7-nit...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50247054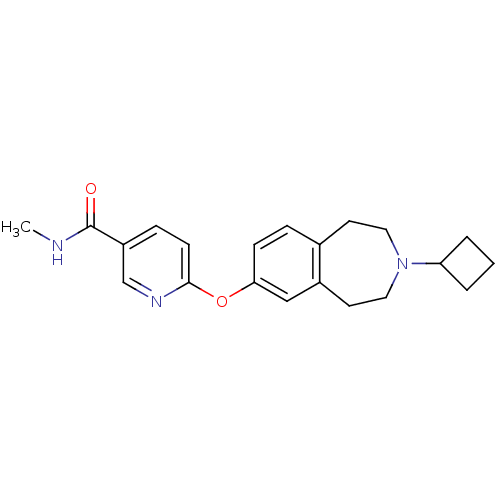 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444489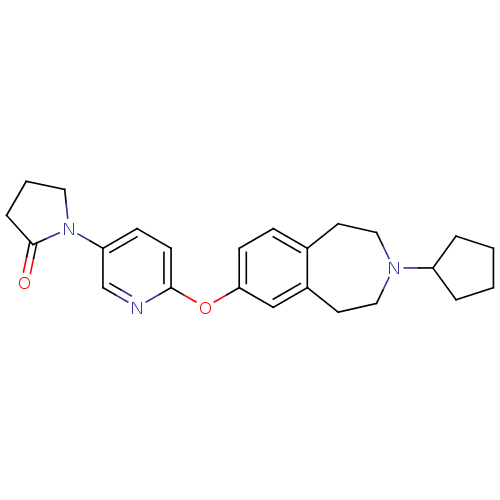 (CHEMBL3092825) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444495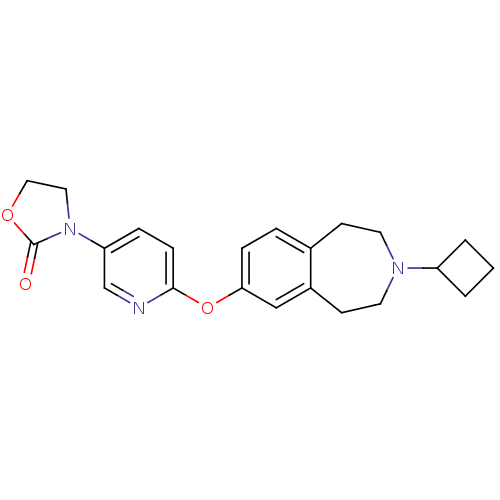 (CHEMBL3092651) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004793 (3-Allyl-8-bromo-5-phenyl-2,3,4,5-tetrahydro-1H-ben...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum | J Med Chem 35: 67-72 (1992) BindingDB Entry DOI: 10.7270/Q2P84CHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50007702 (6-Chloro-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at dopamine receptor D1 | J Med Chem 34: 3366-71 (1992) BindingDB Entry DOI: 10.7270/Q26H4J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444506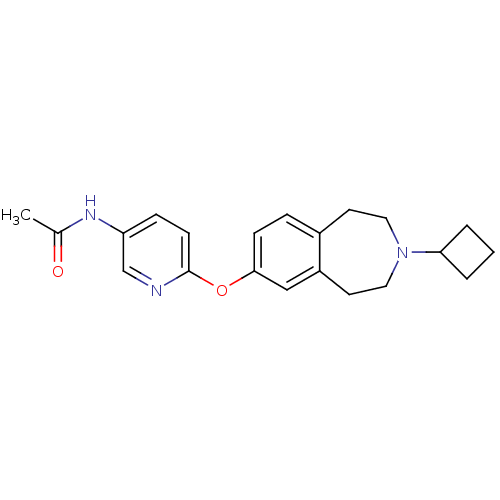 (CHEMBL3092827) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444494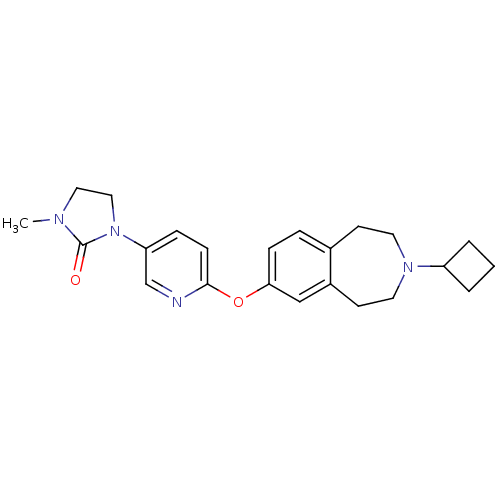 (CHEMBL3092820) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004923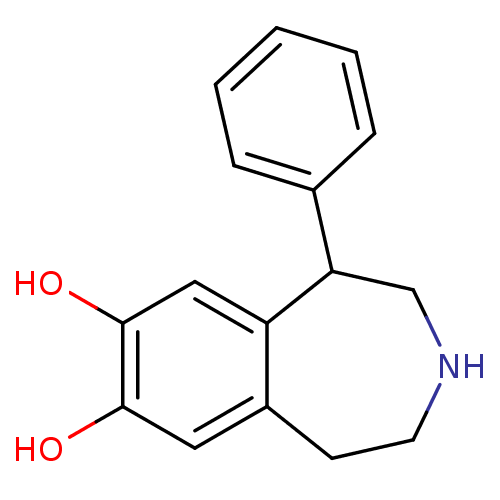 ((+/-)-SKF-38393 | 1-Phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at Dopamine receptor D1 | J Med Chem 34: 3366-71 (1992) BindingDB Entry DOI: 10.7270/Q26H4J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004923 ((+/-)-SKF-38393 | 1-Phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at Dopamine receptor D1 | J Med Chem 34: 3366-71 (1992) BindingDB Entry DOI: 10.7270/Q26H4J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004792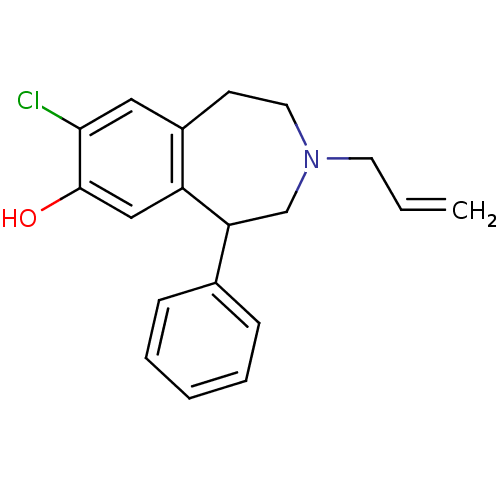 (3-Allyl-8-chloro-5-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum | J Med Chem 35: 67-72 (1992) BindingDB Entry DOI: 10.7270/Q2P84CHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50007700 (6-Bromo-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at dopamine receptor D1 | J Med Chem 34: 3366-71 (1992) BindingDB Entry DOI: 10.7270/Q26H4J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444493 (CHEMBL3092821) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444510 (CHEMBL3092839) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50453025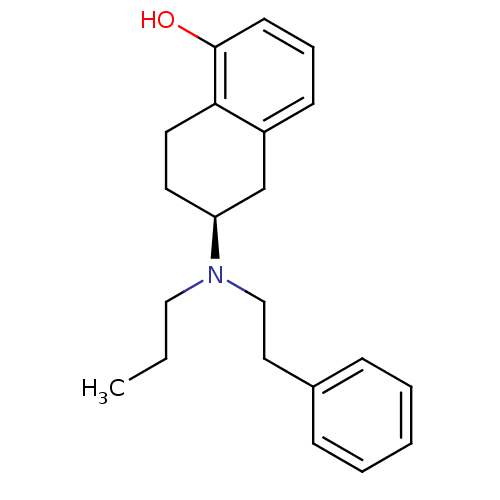 (CHEMBL612083) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50231571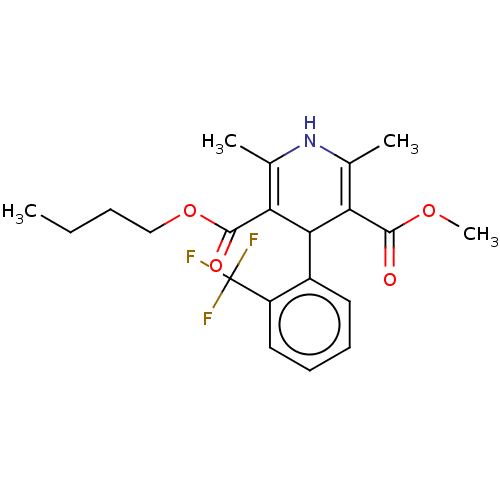 (CHEMBL3350845) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H](+)-PN200-110 binding to L-type calcium channel 1,4-DHP binding site of rat ventricular myocytes | J Med Chem 36: 3743-5 (1994) BindingDB Entry DOI: 10.7270/Q2C24X29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50010290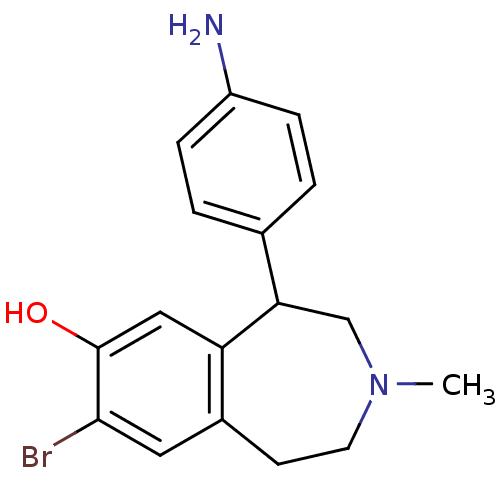 (5-(4-Amino-phenyl)-8-bromo-3-methyl-2,3,4,5-tetrah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D1 by using [3H]-SCH- 23390 as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50231574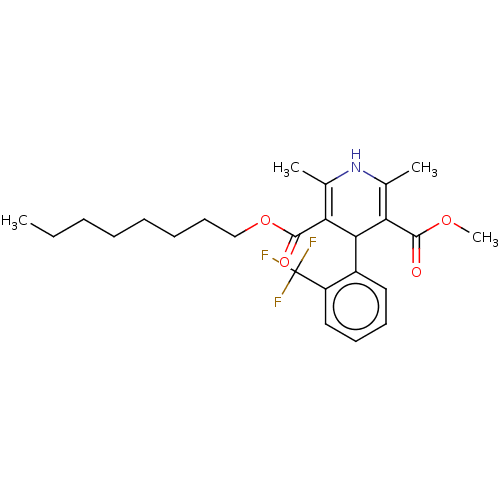 (CHEMBL3350842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H](+)-PN200-110 binding to L-type calcium channel 1,4-DHP binding site of rat ventricular myocytes | J Med Chem 36: 3743-5 (1994) BindingDB Entry DOI: 10.7270/Q2C24X29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444492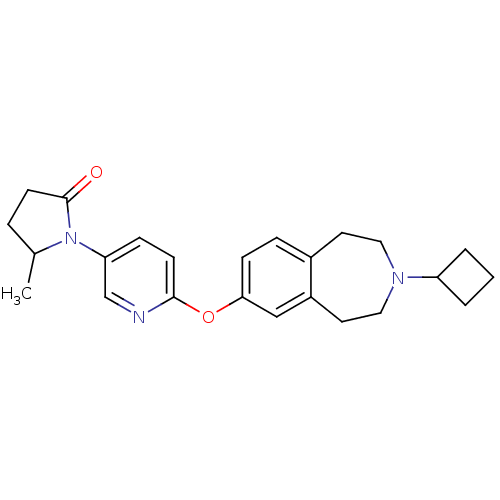 (CHEMBL3092822) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444490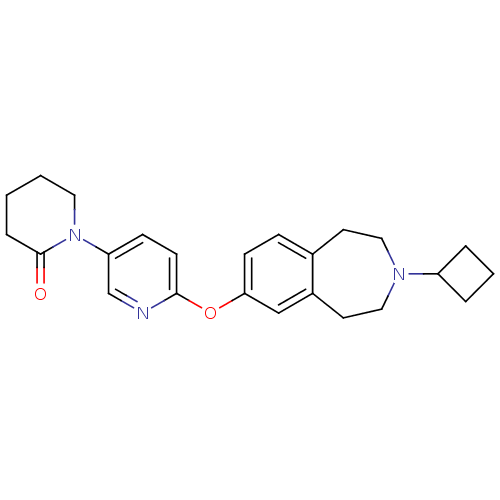 (CHEMBL3092824) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444497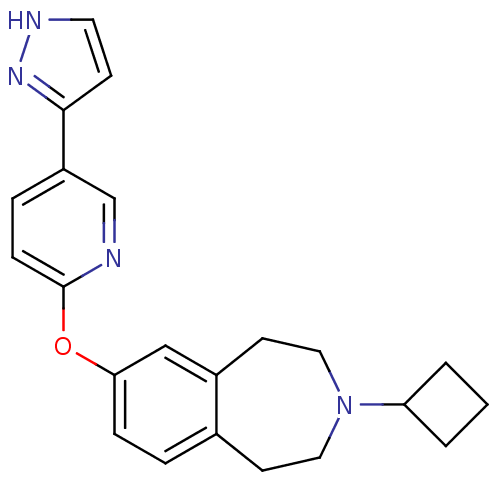 (CHEMBL3092649) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444499 (CHEMBL3092835) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50247054 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444498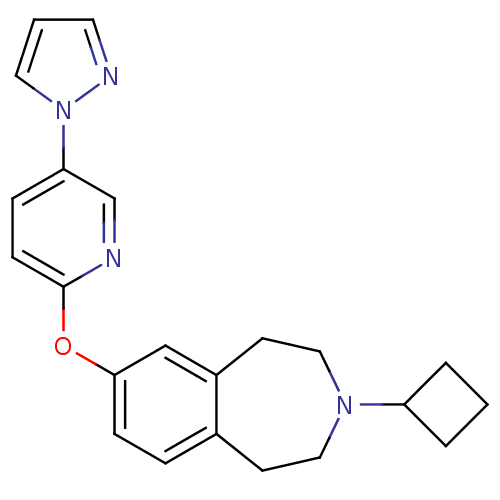 (CHEMBL3092648) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010294 ((R)6-({2-[4-(7-Nitro-benzo[1,2,5]oxadiazol-4-ylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7840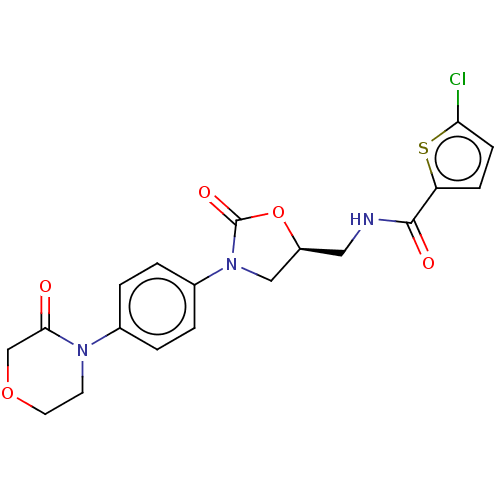 (RIVAROXABAN | US8822458, 44 | US8822458, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human activated Factor X using S-2765 as substrate incubated for 15 mins followed by substrate addition and measured after 1 hr by chro... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111722 BindingDB Entry DOI: 10.7270/Q2891943 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H](+)-PN200-110 binding to L-type calcium channel 1,4-DHP binding site of rat ventricular myocytes | J Med Chem 36: 3743-5 (1994) BindingDB Entry DOI: 10.7270/Q2C24X29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444511 (CHEMBL3092838) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444502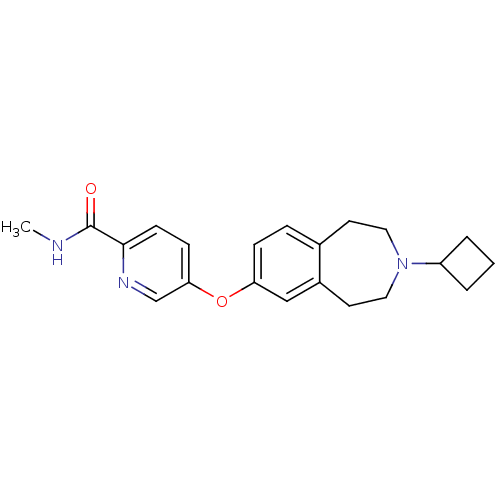 (CHEMBL3092831) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444501 (CHEMBL3092832) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004797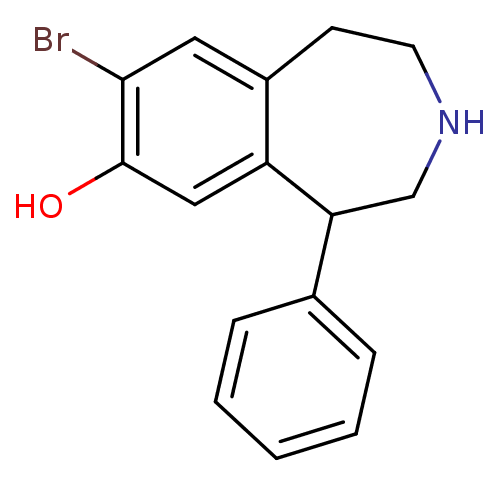 (8-Bromo-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]aze...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum | J Med Chem 35: 67-72 (1992) BindingDB Entry DOI: 10.7270/Q2P84CHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010296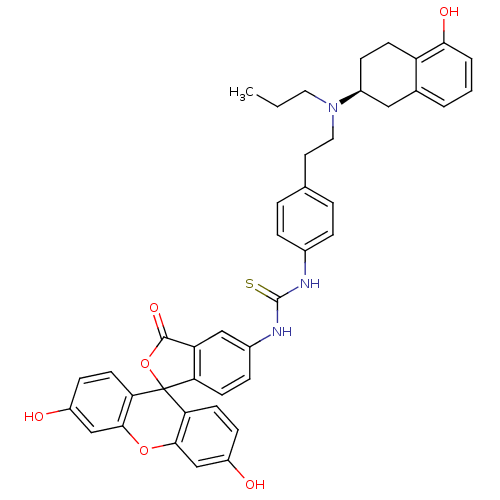 ((S)3',6'-dihydroxy-5-[4-{2-[5-hydroxy-1,2,3,4-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 723 total ) | Next | Last >> |