 Found 1283 hits with Last Name = 'george' and Initial = 'n'
Found 1283 hits with Last Name = 'george' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198694
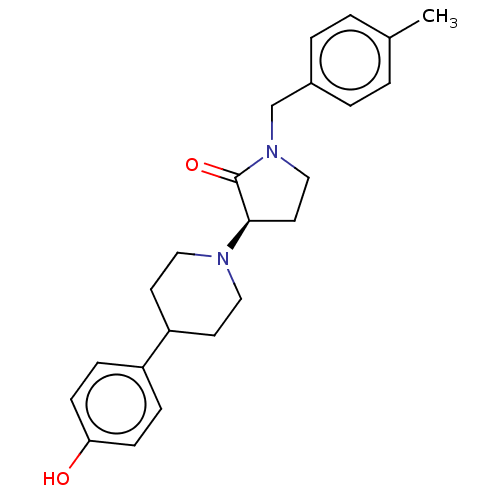
(US9221796, 23b)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(CC3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c1-17-2-4-18(5-3-17)16-25-15-12-22(23(25)27)24-13-10-20(11-14-24)19-6-8-21(26)9-7-19/h2-9,20,22,26H,10-16H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198665
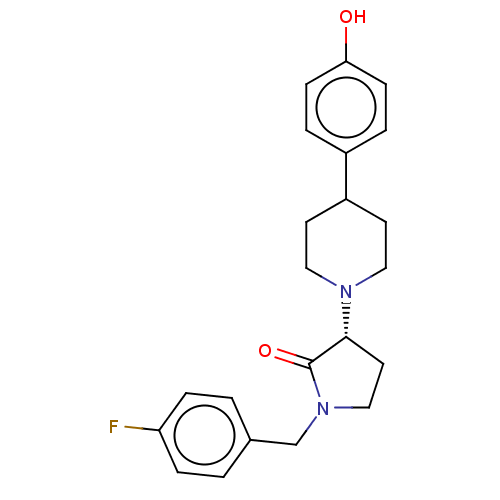
(US9221796, 2b)Show SMILES Oc1ccc(cc1)C1CCN(CC1)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H25FN2O2/c23-19-5-1-16(2-6-19)15-25-14-11-21(22(25)27)24-12-9-18(10-13-24)17-3-7-20(26)8-4-17/h1-8,18,21,26H,9-15H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198726
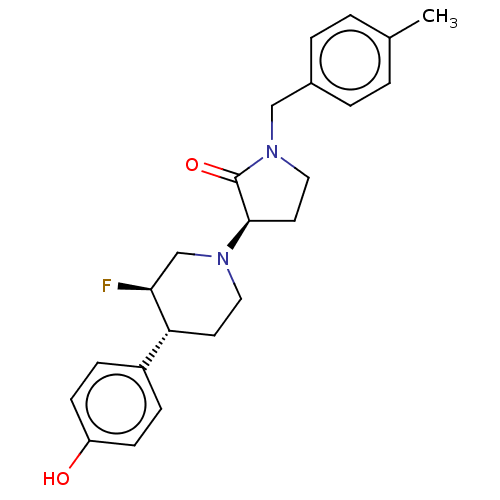
(US9221796, 46, P-2)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330324

(CHEMBL4170867)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to GluN2B receptor in human cortex |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330409

(CHEMBL4168402)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(c4ccc(O)cc4)C(F)(F)C3)C2=O)cc1 |r| Show InChI InChI=1S/C23H26F2N2O2/c1-16-2-4-17(5-3-16)14-26-13-11-21(22(26)29)27-12-10-20(23(24,25)15-27)18-6-8-19(28)9-7-18/h2-9,20-21,28H,10-15H2,1H3/t20?,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330410

(CHEMBL4161899)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198735
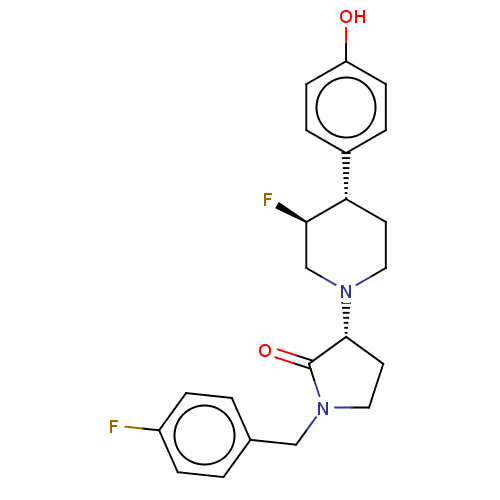
(US9221796, 48, P-3)Show SMILES Oc1ccc(cc1)[C@@H]1CCN(C[C@H]1F)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-1-15(2-6-17)13-26-12-10-21(22(26)28)25-11-9-19(20(24)14-25)16-3-7-18(27)8-4-16/h1-8,19-21,27H,9-14H2/t19-,20+,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513015
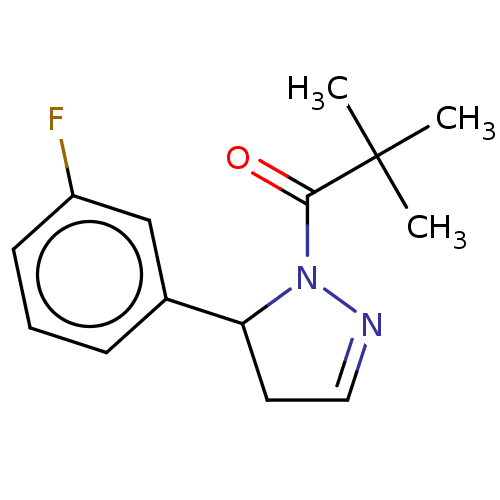
(CHEMBL4537171)Show InChI InChI=1S/C14H17FN2O/c1-14(2,3)13(18)17-12(7-8-16-17)10-5-4-6-11(15)9-10/h4-6,8-9,12H,7H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50613087

(CHEMBL5091238)Show SMILES O=C1NC(NC23CC4CC(CC(C4)C2)C3)=N\C1=C/c1ccc2ncsc2c1 |c:17,TLB:8:9:13:7.12.6,THB:8:7:14.9.10:13,10:9:12.11.13:6,10:11:14.9.8:6| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50507336
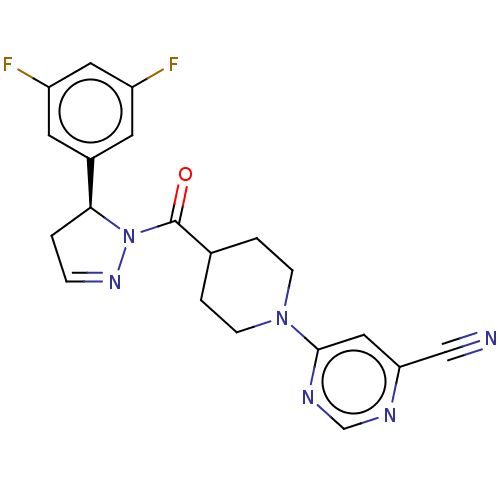
(CHEMBL4514271)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1cc(ncn1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-7-14(8-16(22)9-15)18-1-4-26-28(18)20(29)13-2-5-27(6-3-13)19-10-17(11-23)24-12-25-19/h4,7-10,12-13,18H,1-3,5-6H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM25036
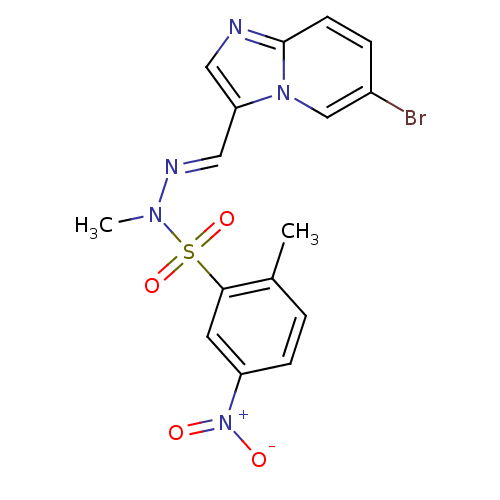
(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50583755
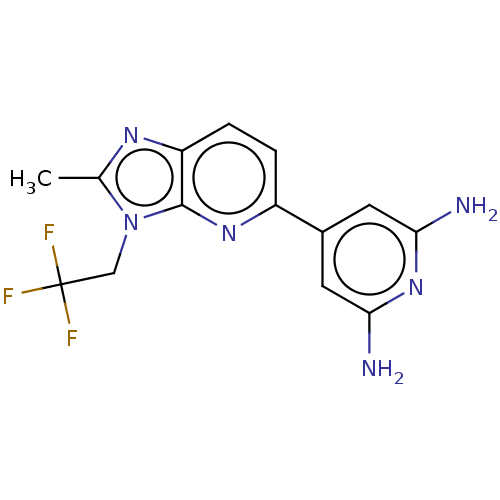
(CHEMBL5070553) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50530417
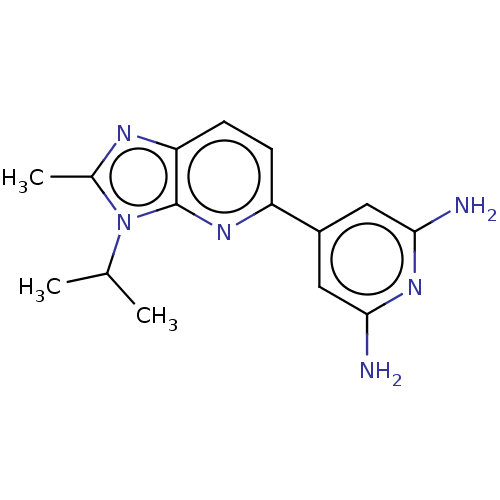
(CHEMBL4441878)Show InChI InChI=1S/C15H18N6/c1-8(2)21-9(3)18-12-5-4-11(19-15(12)21)10-6-13(16)20-14(17)7-10/h4-8H,1-3H3,(H4,16,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50530417

(CHEMBL4441878)Show InChI InChI=1S/C15H18N6/c1-8(2)21-9(3)18-12-5-4-11(19-15(12)21)10-6-13(16)20-14(17)7-10/h4-8H,1-3H3,(H4,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538084

(CHEMBL4647659)Show SMILES CC(C)Oc1ccc(F)c(c1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1N1CCC[C@H](C1)C(O)=O |r| Show InChI InChI=1S/C25H27FN6O4/c1-14(2)36-16-5-6-18(26)17(10-16)19-12-29-23(27)22(30-19)24(33)31-20-11-28-8-7-21(20)32-9-3-4-15(13-32)25(34)35/h5-8,10-12,14-15H,3-4,9,13H2,1-2H3,(H2,27,29)(H,31,33)(H,34,35)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50613087

(CHEMBL5091238)Show SMILES O=C1NC(NC23CC4CC(CC(C4)C2)C3)=N\C1=C/c1ccc2ncsc2c1 |c:17,TLB:8:9:13:7.12.6,THB:8:7:14.9.10:13,10:9:12.11.13:6,10:11:14.9.8:6| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513040

(CHEMBL4593226)Show InChI InChI=1S/C14H16F2N2O/c1-14(2,3)13(19)18-12(4-5-17-18)9-6-10(15)8-11(16)7-9/h5-8,12H,4H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM293424
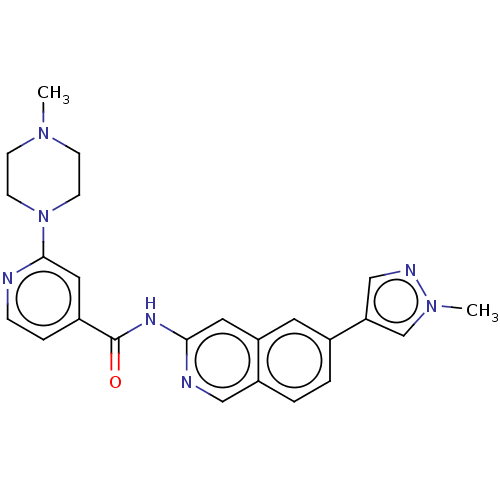
(US10106527, Compound 10 | US10106527, Compound 142...)Show SMILES CN1CCN(CC1)c1cc(ccn1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C24H25N7O/c1-29-7-9-31(10-8-29)23-13-18(5-6-25-23)24(32)28-22-12-20-11-17(3-4-19(20)14-26-22)21-15-27-30(2)16-21/h3-6,11-16H,7-10H2,1-2H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513011
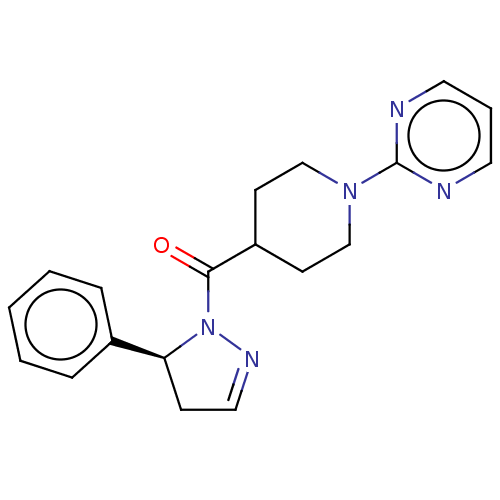
(CHEMBL4455042)Show SMILES O=C(C1CCN(CC1)c1ncccn1)N1N=CC[C@H]1c1ccccc1 |r,c:17| Show InChI InChI=1S/C19H21N5O/c25-18(24-17(7-12-22-24)15-5-2-1-3-6-15)16-8-13-23(14-9-16)19-20-10-4-11-21-19/h1-6,10-12,16-17H,7-9,13-14H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513004
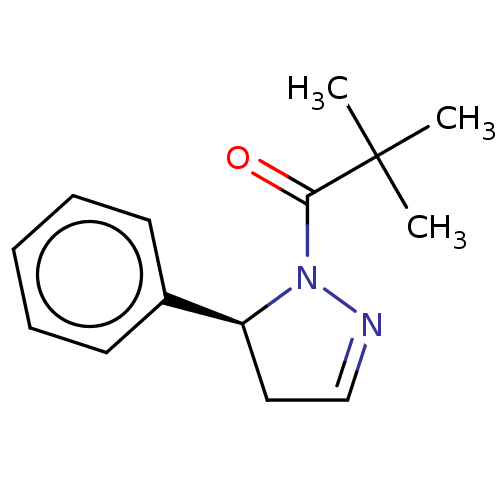
(CHEMBL4521353)Show InChI InChI=1S/C14H18N2O/c1-14(2,3)13(17)16-12(9-10-15-16)11-7-5-4-6-8-11/h4-8,10,12H,9H2,1-3H3/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513024
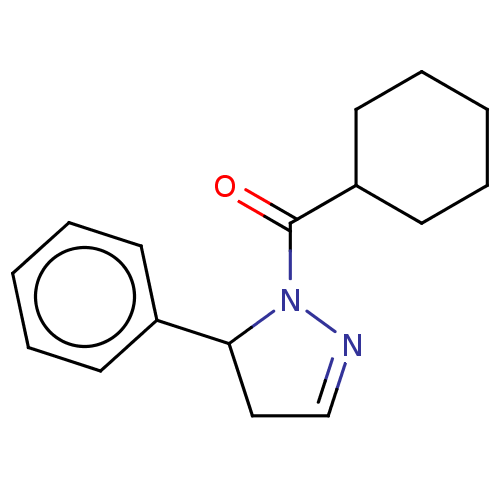
(CHEMBL4471642)Show InChI InChI=1S/C16H20N2O/c19-16(14-9-5-2-6-10-14)18-15(11-12-17-18)13-7-3-1-4-8-13/h1,3-4,7-8,12,14-15H,2,5-6,9-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM385170
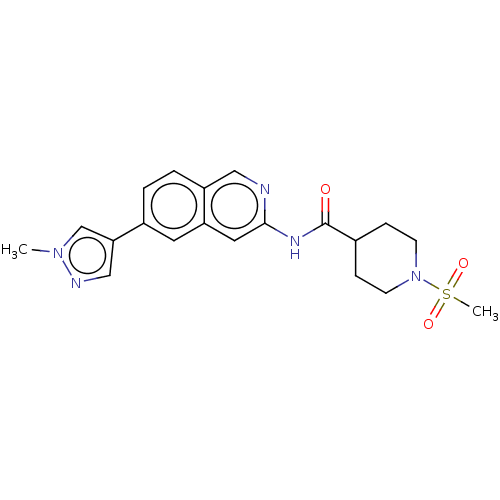
(US10287267, Compound 330 | US10508099, Compound 33...)Show SMILES Cn1cc(cn1)-c1ccc2cnc(NC(=O)C3CCN(CC3)S(C)(=O)=O)cc2c1 Show InChI InChI=1S/C20H23N5O3S/c1-24-13-18(12-22-24)15-3-4-16-11-21-19(10-17(16)9-15)23-20(26)14-5-7-25(8-6-14)29(2,27)28/h3-4,9-14H,5-8H2,1-2H3,(H,21,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50613087

(CHEMBL5091238)Show SMILES O=C1NC(NC23CC4CC(CC(C4)C2)C3)=N\C1=C/c1ccc2ncsc2c1 |c:17,TLB:8:9:13:7.12.6,THB:8:7:14.9.10:13,10:9:12.11.13:6,10:11:14.9.8:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513013
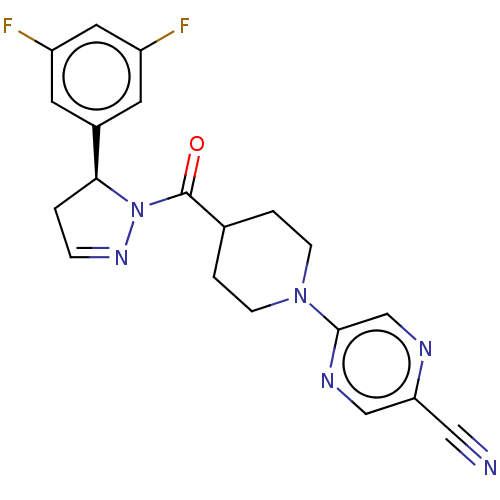
(CHEMBL4450890)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1cnc(cn1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-7-14(8-16(22)9-15)18-1-4-26-28(18)20(29)13-2-5-27(6-3-13)19-12-24-17(10-23)11-25-19/h4,7-9,11-13,18H,1-3,5-6H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513034
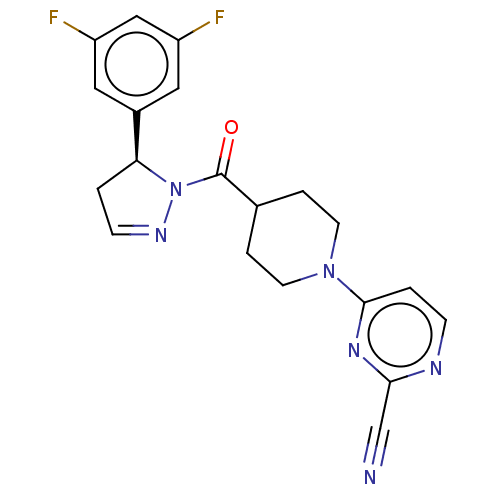
(CHEMBL4541955)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1ccnc(n1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-9-14(10-16(22)11-15)17-1-6-25-28(17)20(29)13-3-7-27(8-4-13)19-2-5-24-18(12-23)26-19/h2,5-6,9-11,13,17H,1,3-4,7-8H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513016
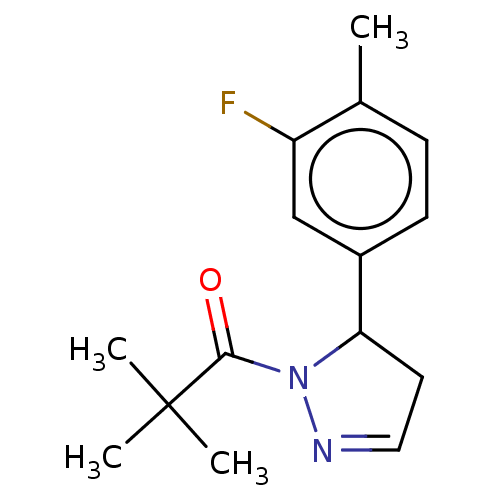
(CHEMBL4514028)Show InChI InChI=1S/C15H19FN2O/c1-10-5-6-11(9-12(10)16)13-7-8-17-18(13)14(19)15(2,3)4/h5-6,8-9,13H,7H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50097869
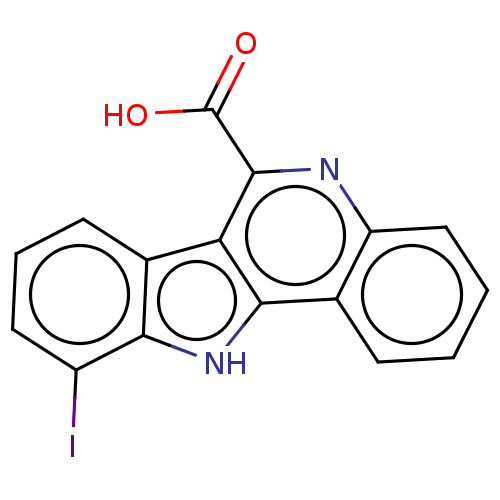
(CHEMBL3589662)Show InChI InChI=1S/C16H9IN2O2/c17-10-6-3-5-9-12-14(19-13(9)10)8-4-1-2-7-11(8)18-15(12)16(20)21/h1-7,19H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50512998
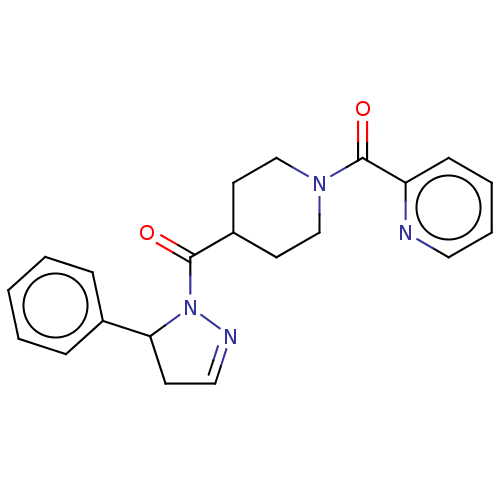
(CHEMBL4575848)Show SMILES O=C(C1CCN(CC1)C(=O)c1ccccn1)N1N=CCC1c1ccccc1 |c:19| Show InChI InChI=1S/C21H22N4O2/c26-20(25-19(9-13-23-25)16-6-2-1-3-7-16)17-10-14-24(15-11-17)21(27)18-8-4-5-12-22-18/h1-8,12-13,17,19H,9-11,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513034

(CHEMBL4541955)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1ccnc(n1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-9-14(10-16(22)11-15)17-1-6-25-28(17)20(29)13-3-7-27(8-4-13)19-2-5-24-18(12-23)26-19/h2,5-6,9-11,13,17H,1,3-4,7-8H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513016

(CHEMBL4514028)Show InChI InChI=1S/C15H19FN2O/c1-10-5-6-11(9-12(10)16)13-7-8-17-18(13)14(19)15(2,3)4/h5-6,8-9,13H,7H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50512996
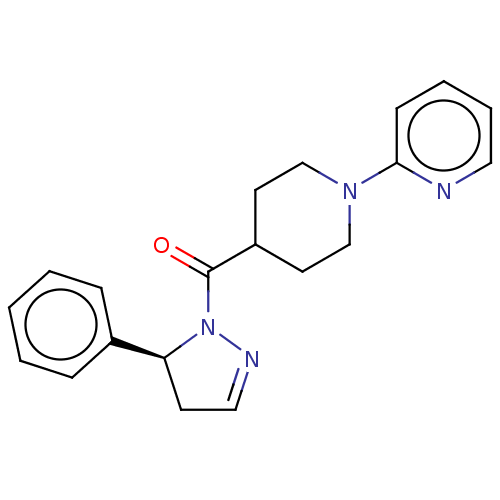
(CHEMBL4454462)Show SMILES O=C(C1CCN(CC1)c1ccccn1)N1N=CC[C@H]1c1ccccc1 |r,c:17| Show InChI InChI=1S/C20H22N4O/c25-20(24-18(9-13-22-24)16-6-2-1-3-7-16)17-10-14-23(15-11-17)19-8-4-5-12-21-19/h1-8,12-13,17-18H,9-11,14-15H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513030
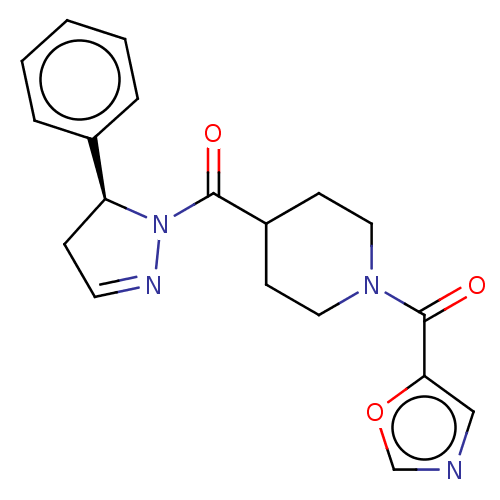
(CHEMBL4548784)Show SMILES O=C(C1CCN(CC1)C(=O)c1cnco1)N1N=CC[C@H]1c1ccccc1 |r,c:18| Show InChI InChI=1S/C19H20N4O3/c24-18(23-16(6-9-21-23)14-4-2-1-3-5-14)15-7-10-22(11-8-15)19(25)17-12-20-13-26-17/h1-5,9,12-13,15-16H,6-8,10-11H2/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK4
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50613085

(CHEMBL5081787)Show SMILES Clc1cccc(Cl)c1NC1=NC(=O)\C(S1)=C\c1ccc2nccnc2c1 |t:10| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50583755

(CHEMBL5070553) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50512997
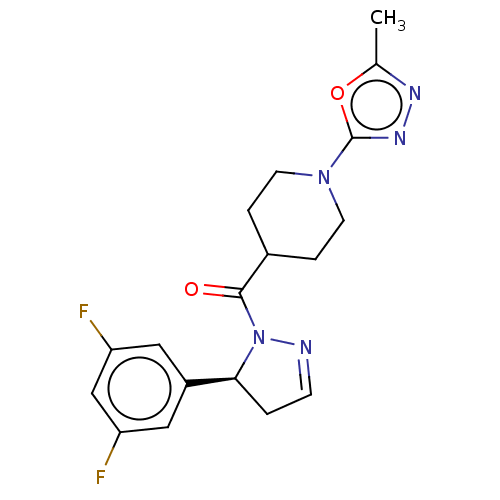
(CHEMBL4545939)Show SMILES Cc1nnc(o1)N1CCC(CC1)C(=O)N1N=CC[C@H]1c1cc(F)cc(F)c1 |r,c:17| Show InChI InChI=1S/C18H19F2N5O2/c1-11-22-23-18(27-11)24-6-3-12(4-7-24)17(26)25-16(2-5-21-25)13-8-14(19)10-15(20)9-13/h5,8-10,12,16H,2-4,6-7H2,1H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in mouse L929 cells assessed as reduction in TNF/zVAD.fmk-induced necrotic death measured after 24 hrs by cell titer-glo luminesce... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM335593

(3-((5-((4-aminopiperidin-1-yl)methyl)pyrrolo[2,1-f...)Show SMILES CC(C)n1nnc(n1)-c1cc(O)cc(Nc2ncnn3ccc(CN4CCC(N)CC4)c23)c1 Show InChI InChI=1S/C22H28N10O/c1-14(2)32-28-21(27-29-32)16-9-18(11-19(33)10-16)26-22-20-15(3-8-31(20)25-13-24-22)12-30-6-4-17(23)5-7-30/h3,8-11,13-14,17,33H,4-7,12,23H2,1-2H3,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and substra... |
US Patent US9737542 (2017)
BindingDB Entry DOI: 10.7270/Q2N018NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50110183
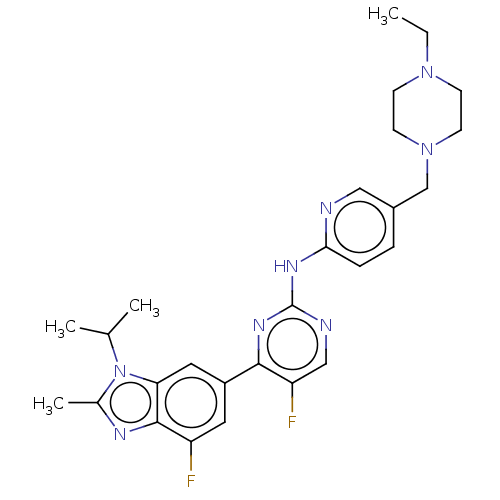
(Abemaciclib | LY-2835219 | US10626107, Example LY2...)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1 Show InChI InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM293424

(US10106527, Compound 10 | US10106527, Compound 142...)Show SMILES CN1CCN(CC1)c1cc(ccn1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C24H25N7O/c1-29-7-9-31(10-8-29)23-13-18(5-6-25-23)24(32)28-22-12-20-11-17(3-4-19(20)14-26-22)21-15-27-30(2)16-21/h3-6,11-16H,7-10H2,1-2H3,(H,26,28,32) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM293424

(US10106527, Compound 10 | US10106527, Compound 142...)Show SMILES CN1CCN(CC1)c1cc(ccn1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C24H25N7O/c1-29-7-9-31(10-8-29)23-13-18(5-6-25-23)24(32)28-22-12-20-11-17(3-4-19(20)14-26-22)21-15-27-30(2)16-21/h3-6,11-16H,7-10H2,1-2H3,(H,26,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538084

(CHEMBL4647659)Show SMILES CC(C)Oc1ccc(F)c(c1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1N1CCC[C@H](C1)C(O)=O |r| Show InChI InChI=1S/C25H27FN6O4/c1-14(2)36-16-5-6-18(26)17(10-16)19-12-29-23(27)22(30-19)24(33)31-20-11-28-8-7-21(20)32-9-3-4-15(13-32)25(34)35/h5-8,10-12,14-15H,3-4,9,13H2,1-2H3,(H2,27,29)(H,31,33)(H,34,35)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513004

(CHEMBL4521353)Show InChI InChI=1S/C14H18N2O/c1-14(2,3)13(17)16-12(9-10-15-16)11-7-5-4-6-8-11/h4-8,10,12H,9H2,1-3H3/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513007
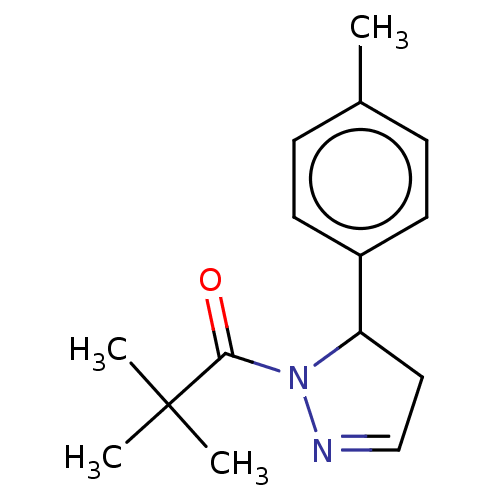
(CHEMBL4436215)Show InChI InChI=1S/C15H20N2O/c1-11-5-7-12(8-6-11)13-9-10-16-17(13)14(18)15(2,3)4/h5-8,10,13H,9H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50507336

(CHEMBL4514271)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1cc(ncn1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-7-14(8-16(22)9-15)18-1-4-26-28(18)20(29)13-2-5-27(6-3-13)19-10-17(11-23)24-12-25-19/h4,7-10,12-13,18H,1-3,5-6H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data