

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50401763 (CHEMBL2207565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401764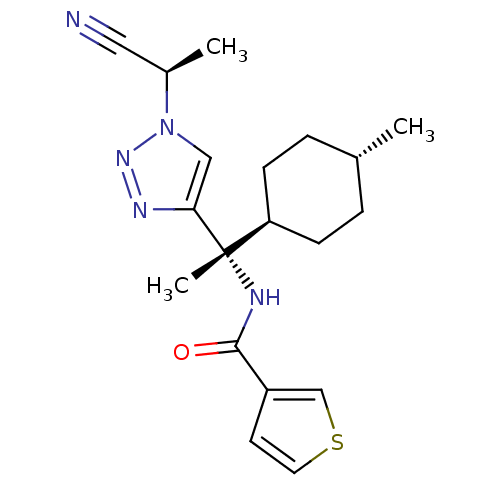 (CHEMBL2207564) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249132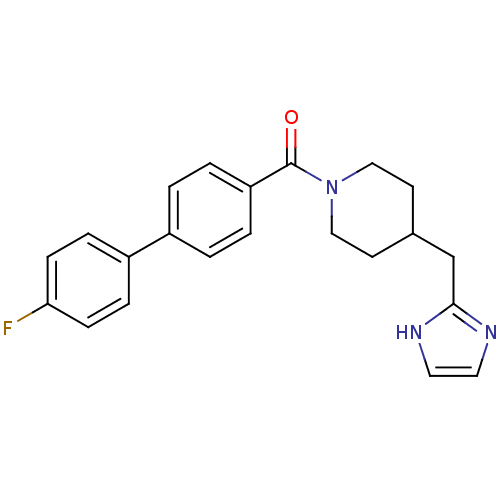 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(4'-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Src protein tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474951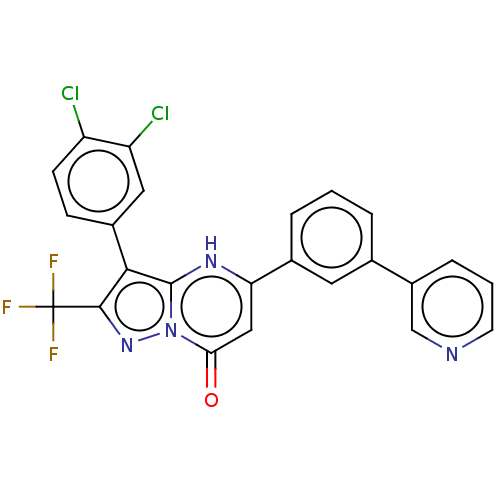 (US10849901, Compound 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401766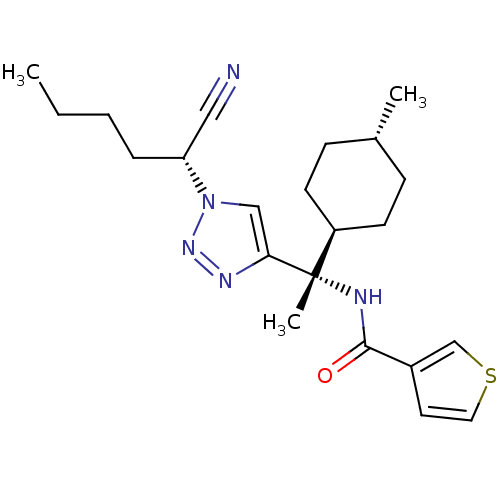 (CHEMBL2207562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249483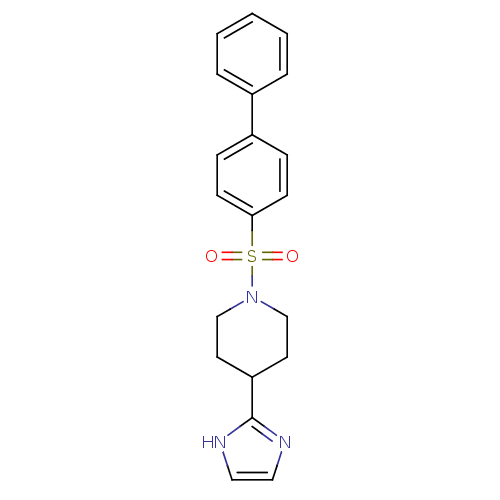 (1-(biphenyl-4-ylsulfonyl)-4-(1H-imidazol-2-yl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474952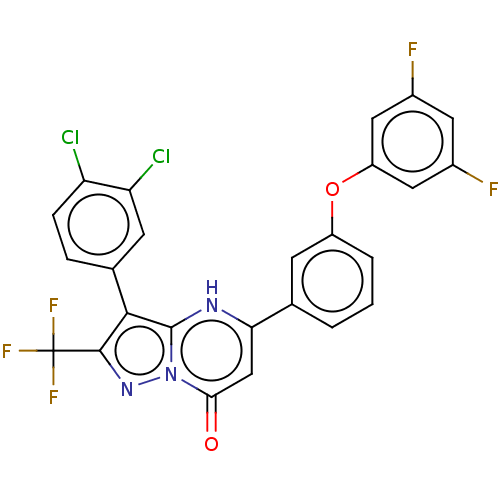 (US10849901, Compound 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921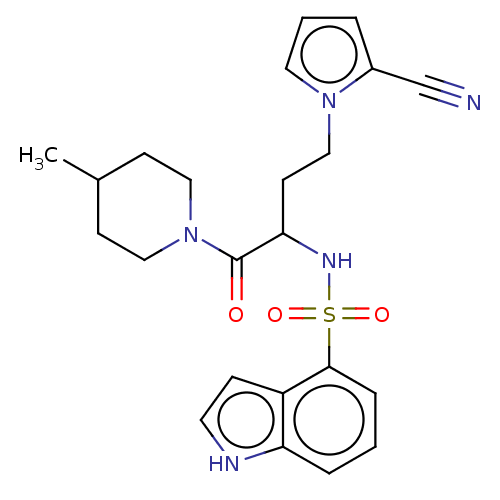 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in CHOK1 cells coexpressing aequorin/Galphaq assessed as inhibition of CCL27-dependent calcium flux in p... | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249464 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Brutons tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19502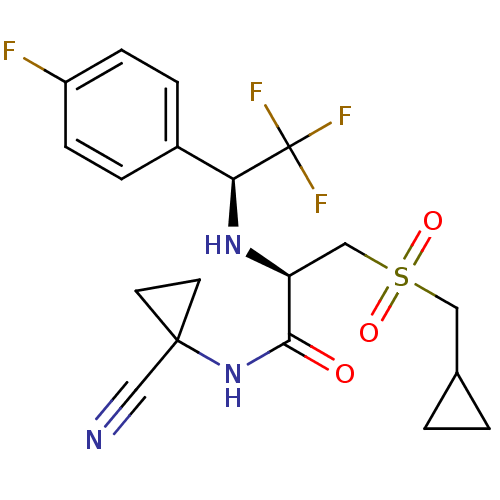 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474954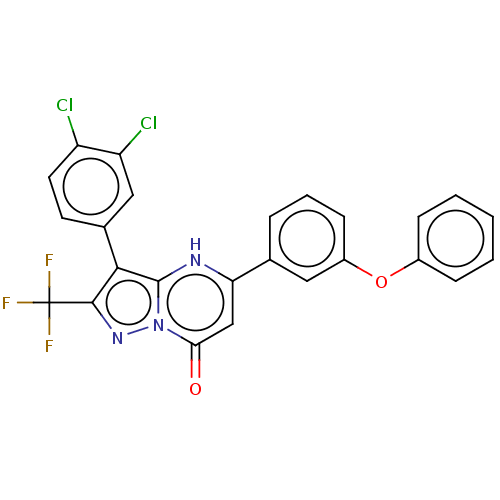 (US10849901, Compound 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474953 (US10849901, Compound 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401814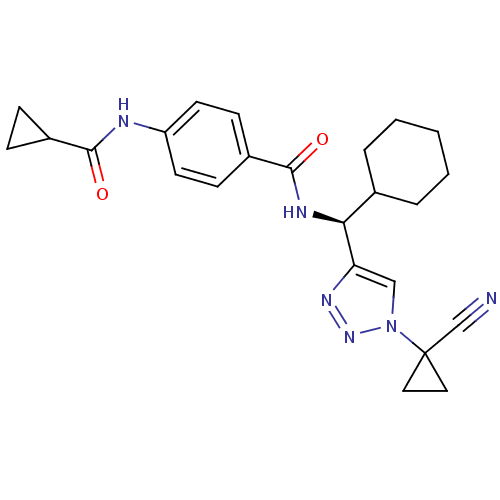 (CHEMBL2207591) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401765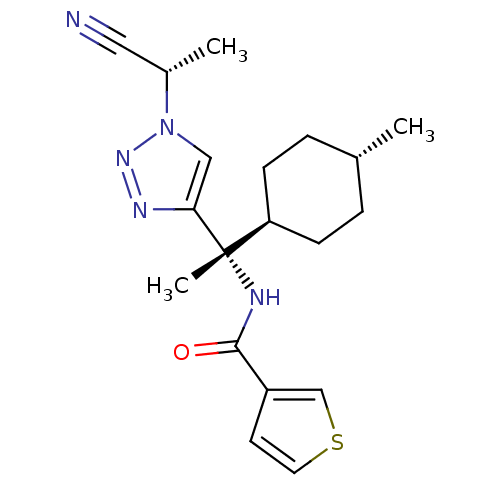 (CHEMBL2207563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474954 (US10849901, Compound 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM475063 (US10849901, Compound 113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474955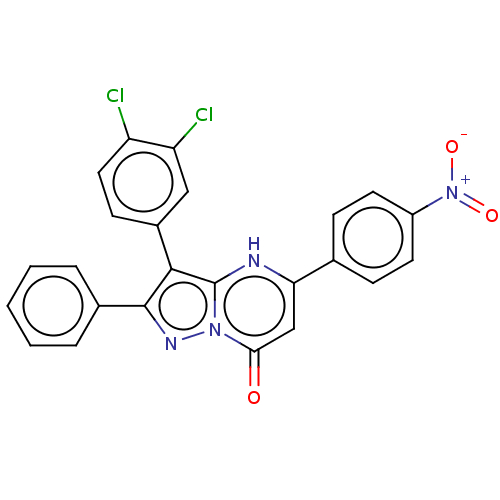 (US10849901, Compound 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401834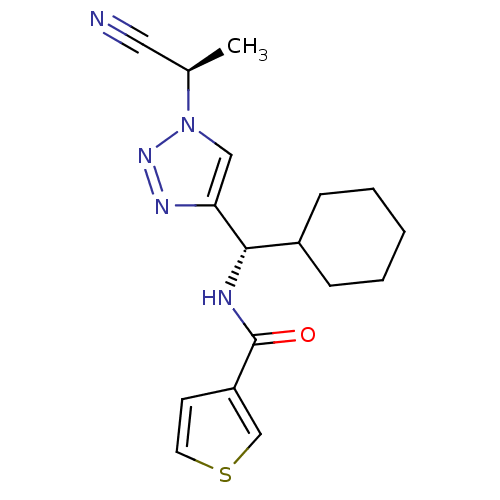 (CHEMBL2207571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249131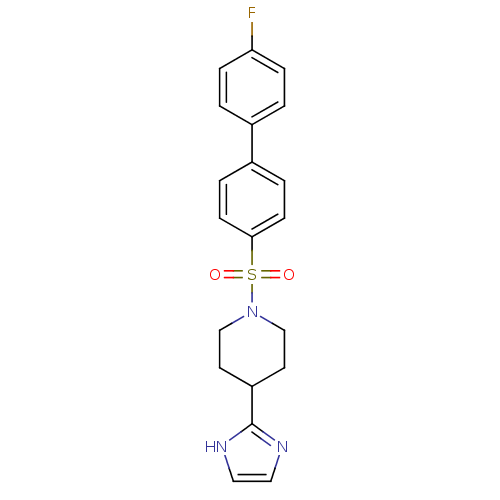 (1-(4'-fluorobiphenyl-4-ylsulfonyl)-4-(1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053967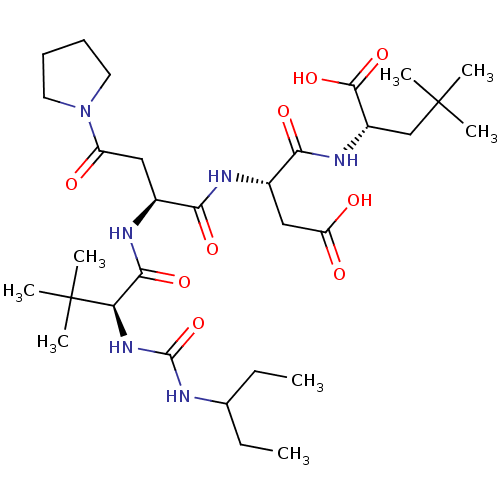 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401835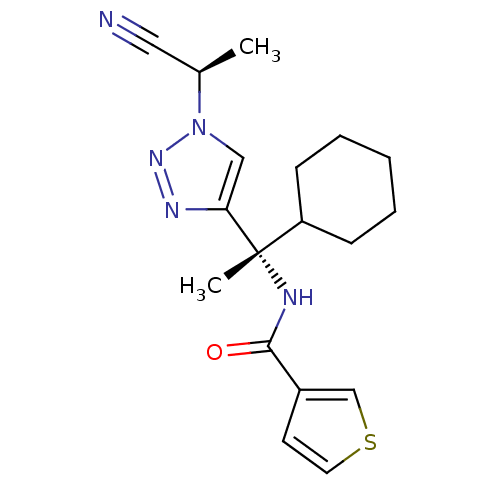 (CHEMBL2207570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474956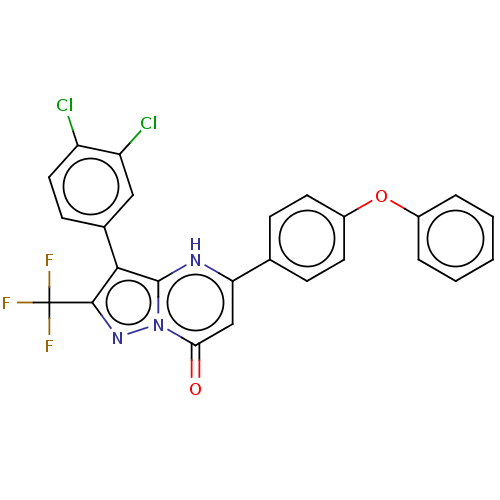 (US10849901, Compound 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474957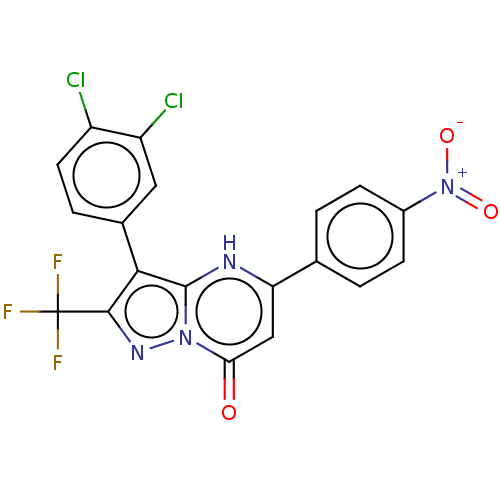 (US10849901, Compound 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474958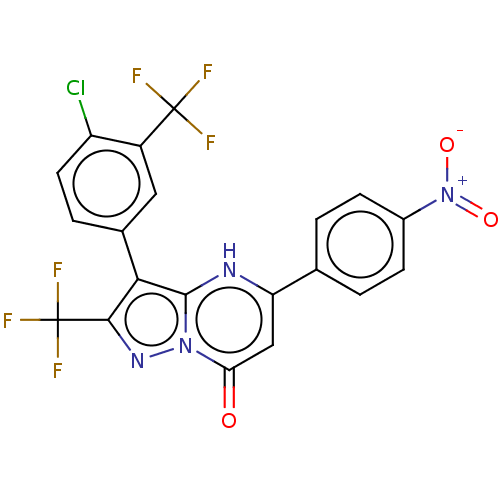 (US10849901, Compound 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033458 ((S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Benzyl-3-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249134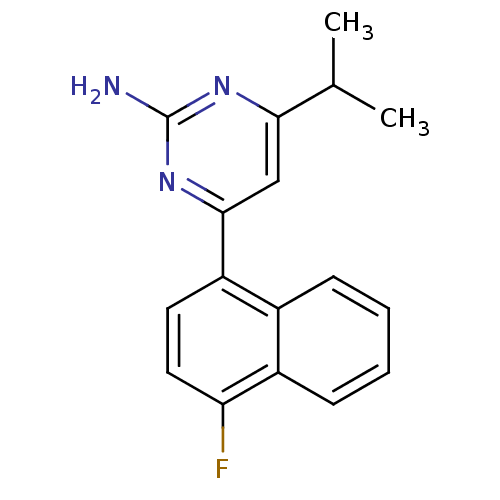 (4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401761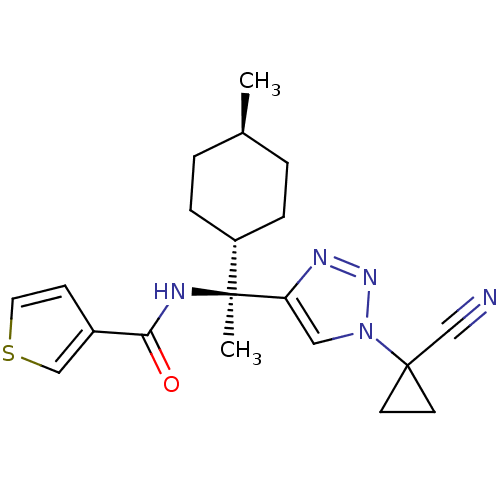 (CHEMBL2207567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474959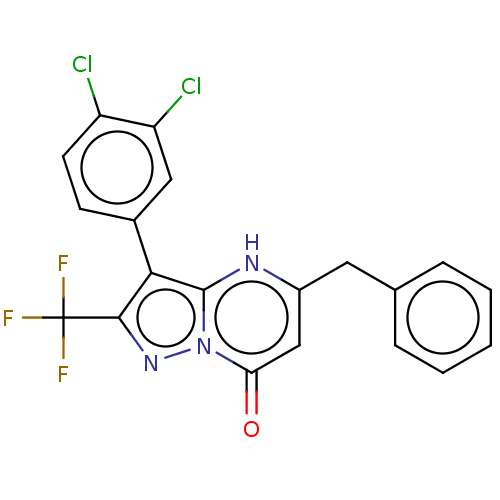 (US10849901, Compound 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474960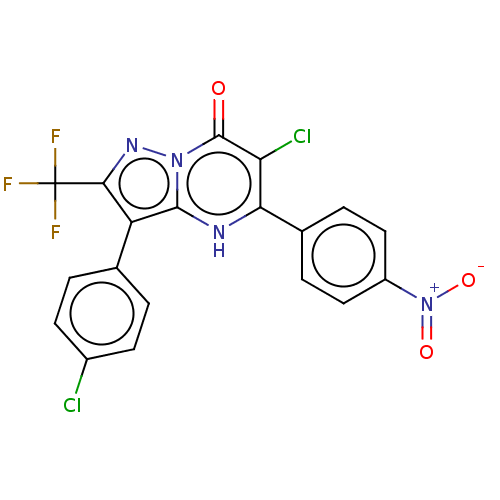 (US10849901, Compound 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401770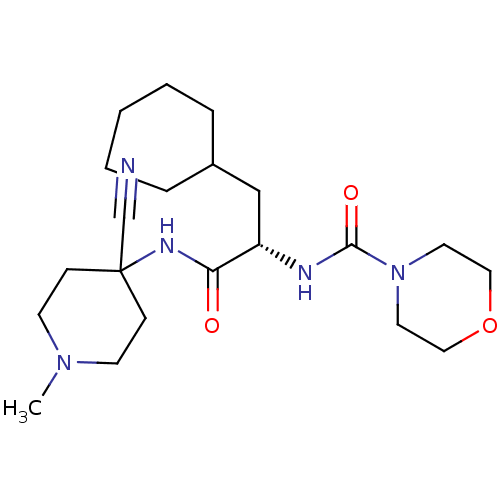 (CHEMBL1236882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S-mediated antigen presentation in B/T hybridoma cells assessed as IL-2 level | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474961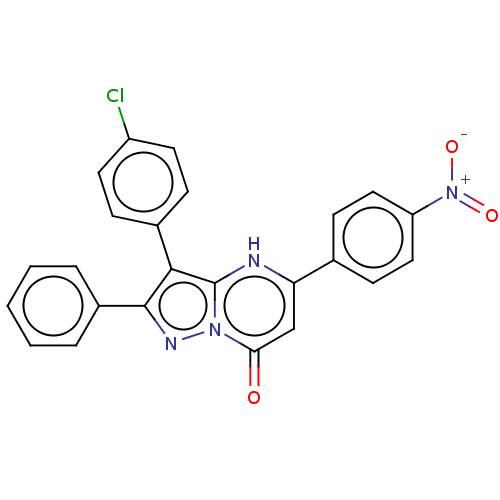 (US10849901, Compound 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474962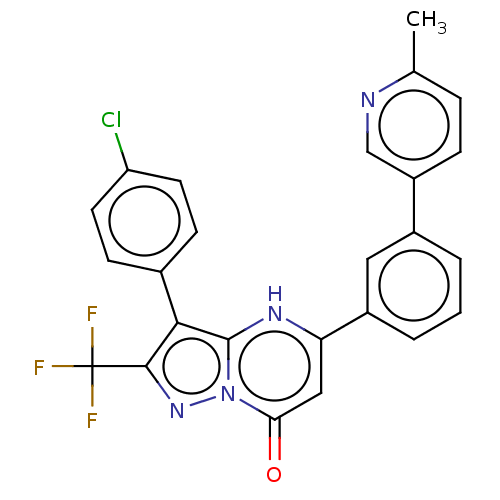 (US10849901, Compound 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474963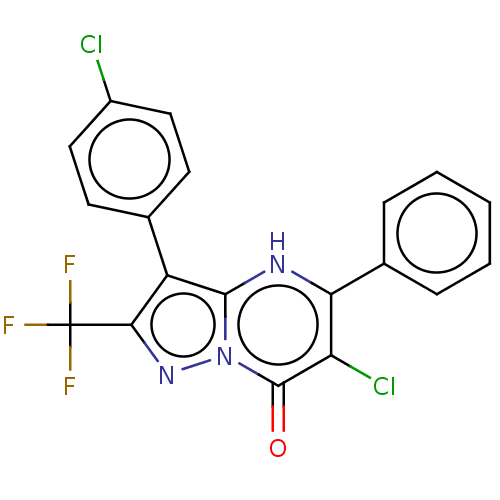 (US10849901, Compound 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in CHOK1 cells coexpressing aequorin/Galphaq assessed as inhibition of human CCL28-dependent calcium flu... | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in CHOK1 cells coexpressing aequorin/Galphaq assessed as inhibition of human CCL27-dependent calcium flu... | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033457 (1-[(S)-((S)-1-Carboxy-3-methyl-butylcarbamoyl)-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in mouse BA/F3 cells assessed as inhibition of human CCL27-dependent chemotaxis | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474966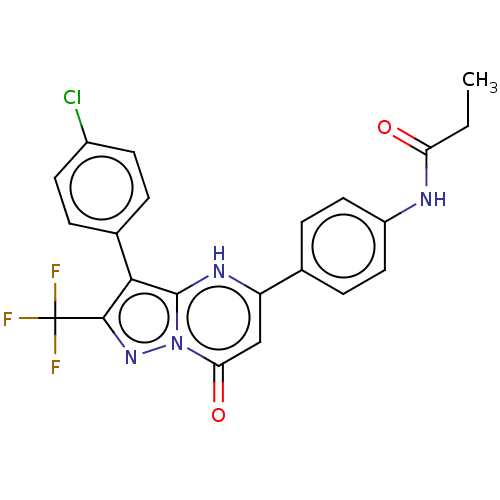 (US10849901, Compound 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401762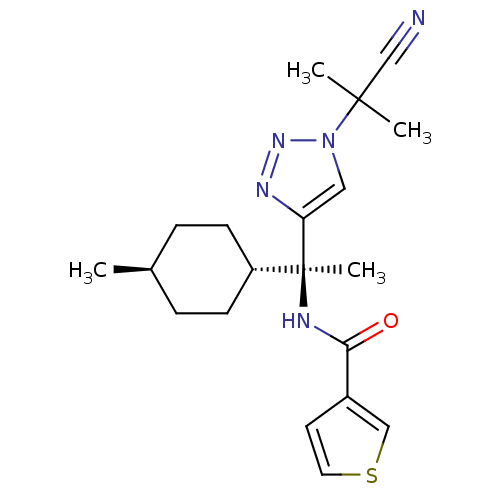 (CHEMBL2207566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474964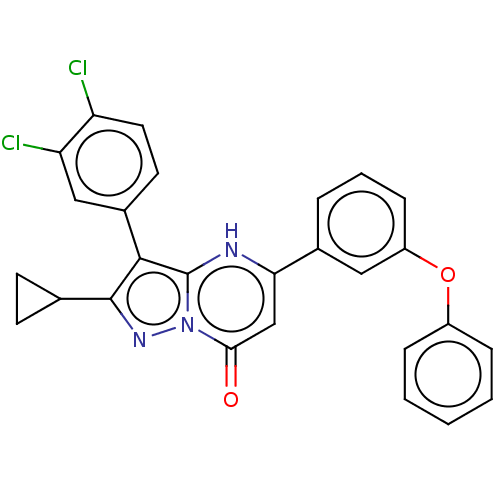 (US10849901, Compound 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401818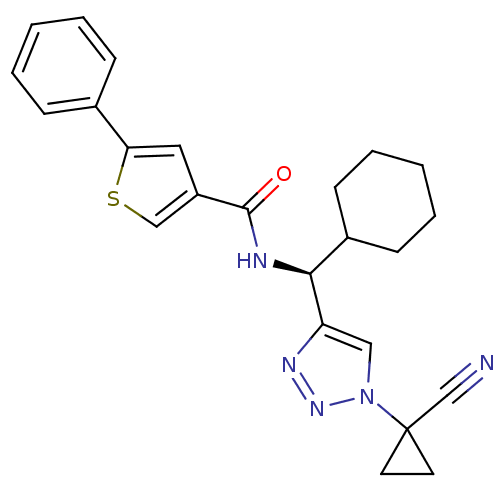 (CHEMBL2207587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474970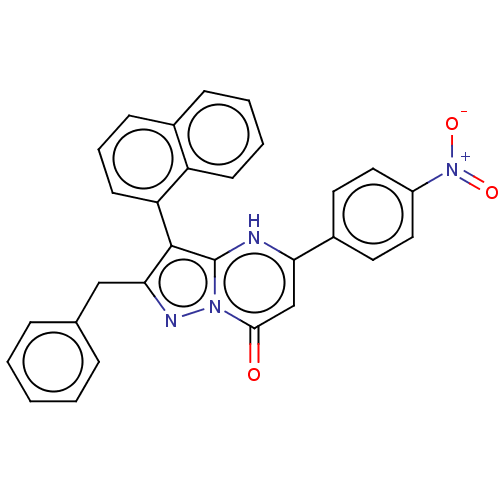 (US10849901, Compound 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474969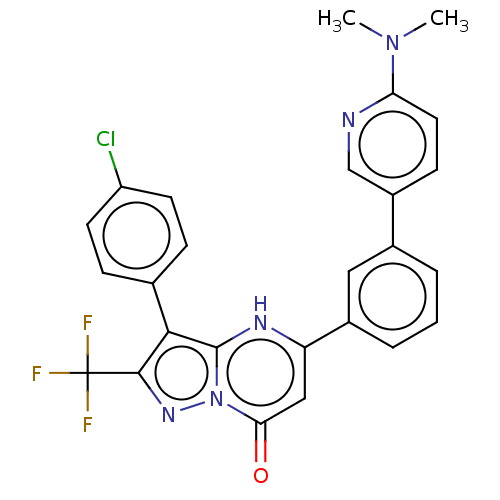 (US10849901, Compound 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474968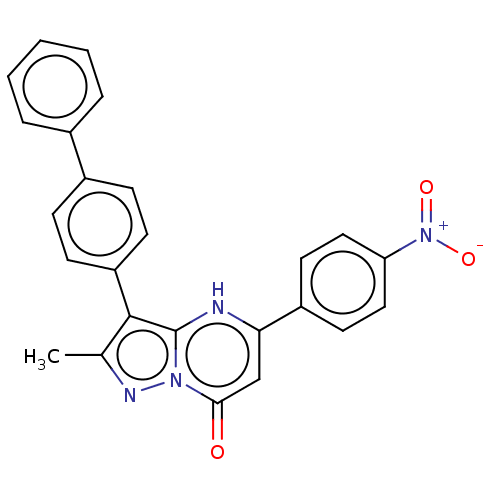 (US10849901, Compound 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474967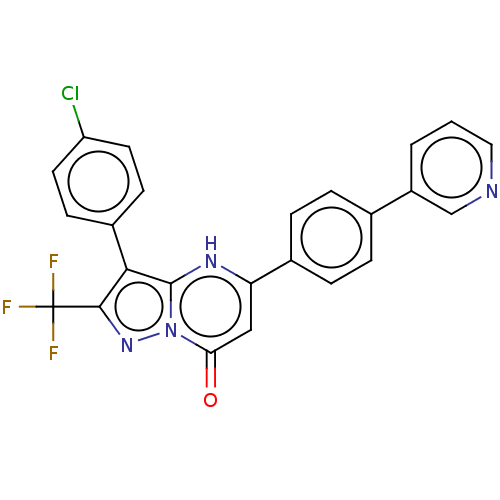 (US10849901, Compound 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 6 (Human) | BDBM474965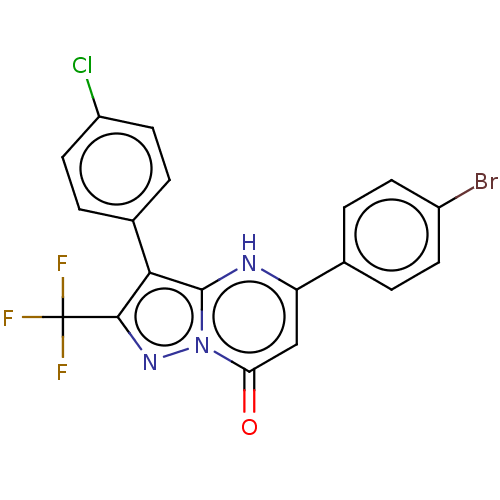 (US10849901, Compound 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Navigen, Inc.; The University of Utah Research Foundation US Patent | Assay Description The primary biochemical assay uses a fluorometric assay for monitoring the replacement of bound nucleotide with a fluorogenic GTP derivative bearing ... | US Patent US10849901 (2020) BindingDB Entry DOI: 10.7270/Q2T156Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 996 total ) | Next | Last >> |