 Found 2449 hits with Last Name = 'orth' and Initial = 'p'
Found 2449 hits with Last Name = 'orth' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548
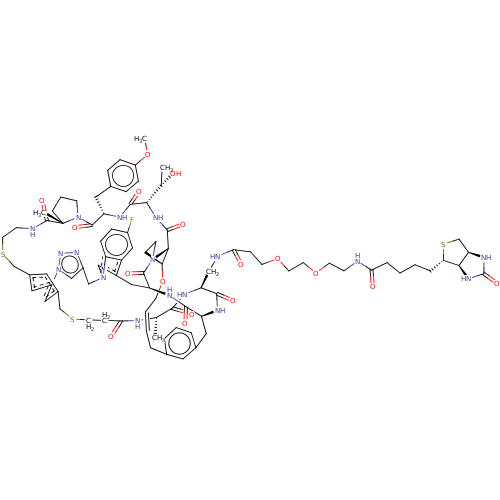
(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547
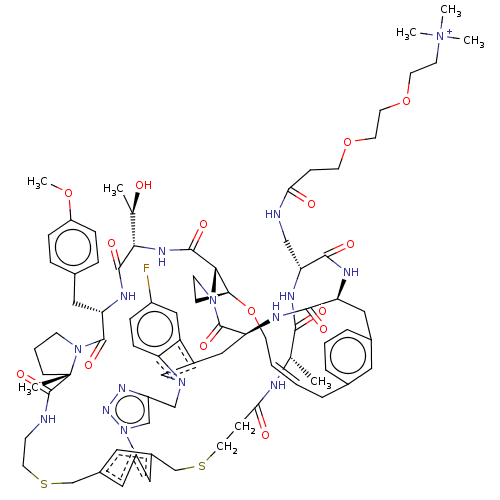
(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546
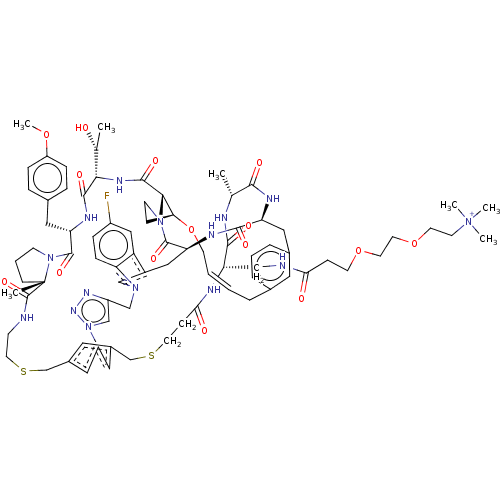
(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545
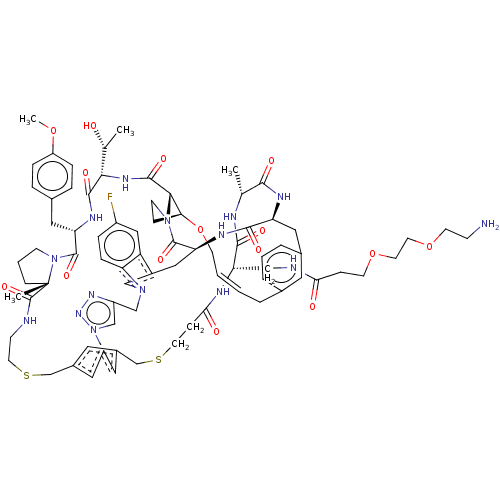
(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544
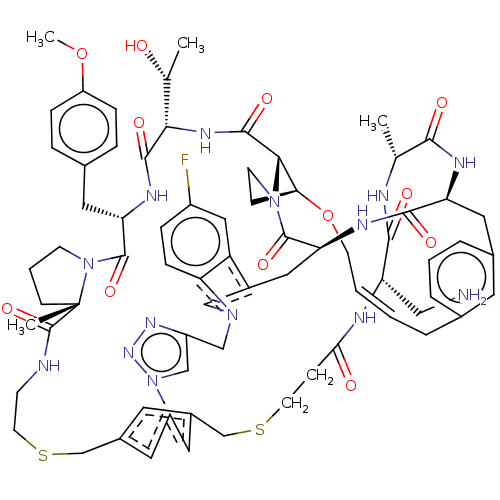
(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50035179
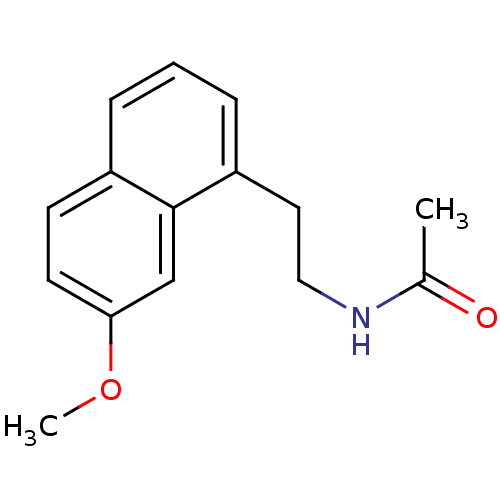
(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581549
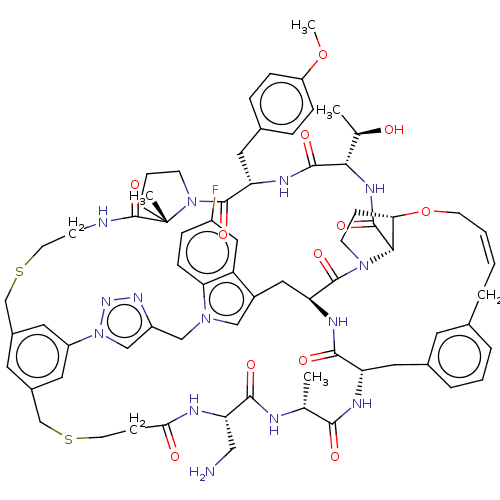
(CHEMBL5082483)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C/CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,c:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332270
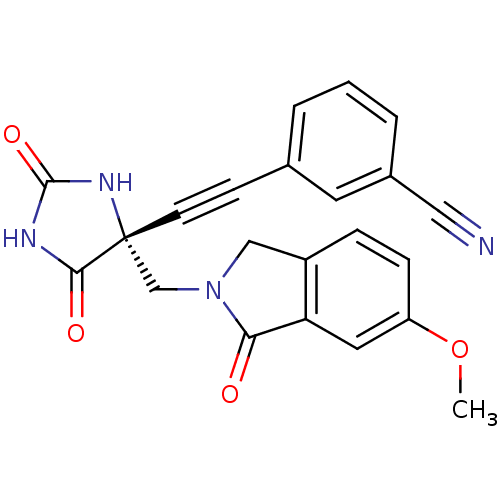
((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(c3)C#N)C(=O)c2c1 |r| Show InChI InChI=1S/C22H16N4O4/c1-30-17-6-5-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)8-7-14-3-2-4-15(9-14)11-23/h2-6,9-10H,12-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26526
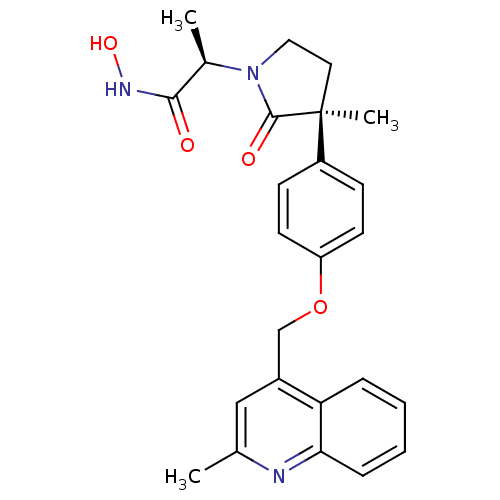
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332292
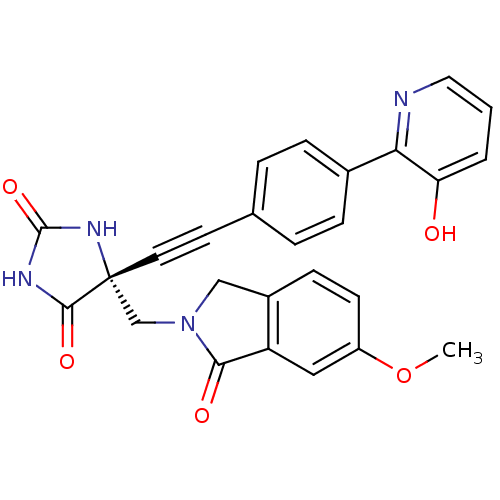
((R)-5-((4-(3-hydroxypyridin-2-yl)phenyl)ethynyl)-5...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H20N4O5/c1-35-19-9-8-18-14-30(23(32)20(18)13-19)15-26(24(33)28-25(34)29-26)11-10-16-4-6-17(7-5-16)22-21(31)3-2-12-27-22/h2-9,12-13,31H,14-15H2,1H3,(H2,28,29,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM102669
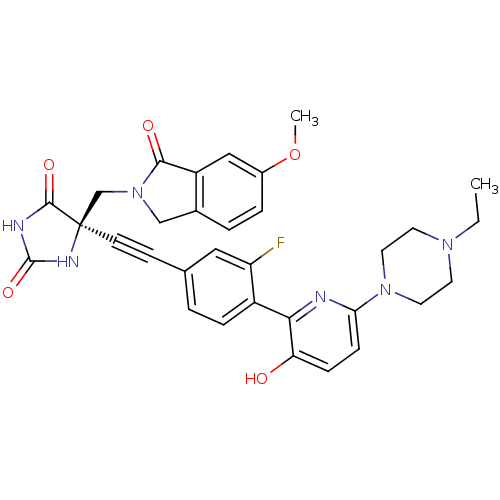
(CHEMBL1288726 | US8541572, 976)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1F)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H31FN6O5/c1-3-37-12-14-38(15-13-37)27-9-8-26(40)28(34-27)23-7-4-20(16-25(23)33)10-11-32(30(42)35-31(43)36-32)19-39-18-21-5-6-22(44-2)17-24(21)29(39)41/h4-9,16-17,40H,3,12-15,18-19H2,1-2H3,(H2,35,36,42,43)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325003
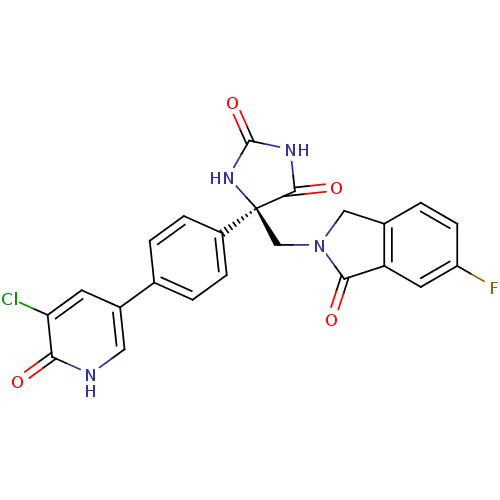
((R)-5-(4-(5-chloro-6-oxo-1,6-dihydropyridin-3-yl)p...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3c[nH]c(=O)c(Cl)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H16ClFN4O4/c24-18-7-14(9-26-19(18)30)12-1-4-15(5-2-12)23(21(32)27-22(33)28-23)11-29-10-13-3-6-16(25)8-17(13)20(29)31/h1-9H,10-11H2,(H,26,30)(H2,27,28,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545

(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332265

((R)-5-((2-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3F)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-15-7-6-14-11-25(18(26)16(14)10-15)12-21(19(27)23-20(28)24-21)9-8-13-4-2-3-5-17(13)22/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50043289
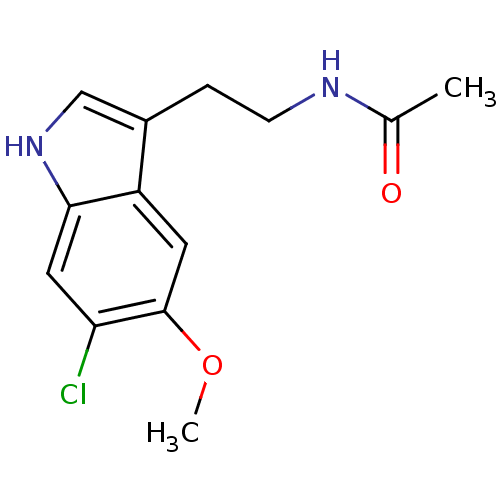
(CHEMBL34730 | N-[2-(6-Chloro-5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H15ClN2O2/c1-8(17)15-4-3-9-7-16-12-6-11(14)13(18-2)5-10(9)12/h5-7,16H,3-4H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332262
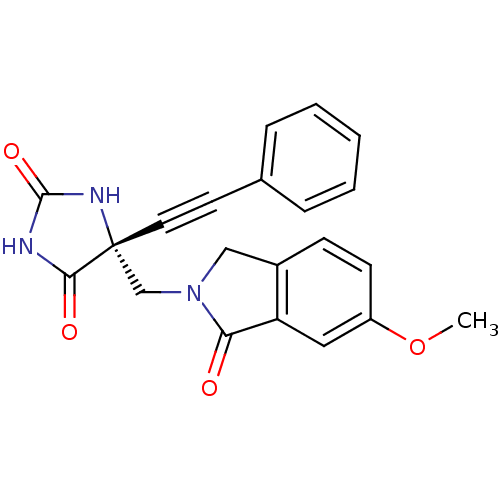
((R)-5-((6-methoxy-1-oxoisoindolin-2-yl)methyl)-5-(...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H17N3O4/c1-28-16-8-7-15-12-24(18(25)17(15)11-16)13-21(19(26)22-20(27)23-21)10-9-14-5-3-2-4-6-14/h2-8,11H,12-13H2,1H3,(H2,22,23,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM85064
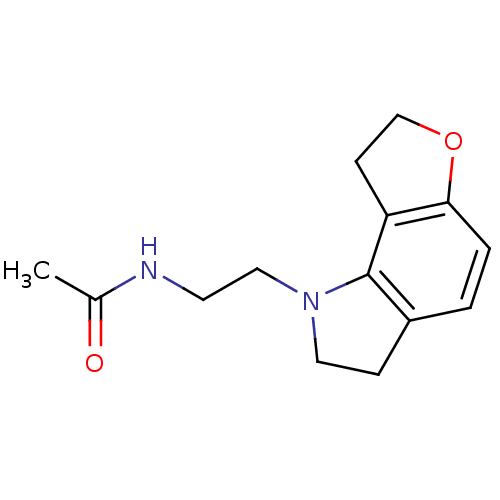
(CAS_5311134 | GR 196429 | NSC_5311134)Show InChI InChI=1S/C14H18N2O2/c1-10(17)15-6-8-16-7-4-11-2-3-13-12(14(11)16)5-9-18-13/h2-3H,4-9H2,1H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292694
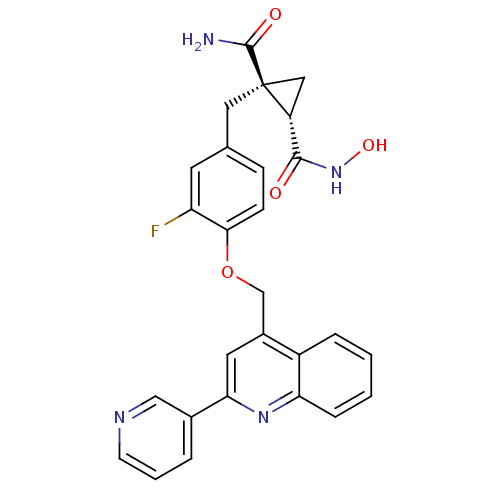
((1R,2S)-1-(3-fluoro-4-((2-(pyridin-3-yl)quinolin-4...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3cccnc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C27H23FN4O4/c28-21-10-16(12-27(26(29)34)13-20(27)25(33)32-35)7-8-24(21)36-15-18-11-23(17-4-3-9-30-14-17)31-22-6-2-1-5-19(18)22/h1-11,14,20,35H,12-13,15H2,(H2,29,34)(H,32,33)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292693
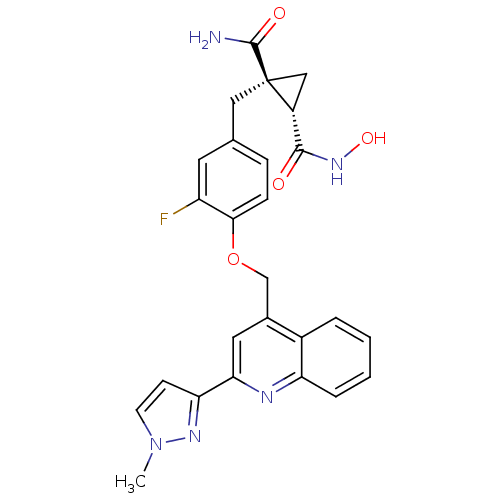
((1R,2S)-1-(3-fluoro-4-((2-(1-methyl-1H-pyrazol-3-y...)Show SMILES Cn1ccc(n1)-c1cc(COc2ccc(C[C@@]3(C[C@@H]3C(=O)NO)C(N)=O)cc2F)c2ccccc2n1 |r| Show InChI InChI=1S/C26H24FN5O4/c1-32-9-8-21(30-32)22-11-16(17-4-2-3-5-20(17)29-22)14-36-23-7-6-15(10-19(23)27)12-26(25(28)34)13-18(26)24(33)31-35/h2-11,18,35H,12-14H2,1H3,(H2,28,34)(H,31,33)/t18-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292691

((1R,2S)-1-(3-fluoro-4-((2-(pyrrolidin-1-yl)quinoli...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)N3CCCC3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C26H27FN4O4/c27-20-11-16(13-26(25(28)33)14-19(26)24(32)30-34)7-8-22(20)35-15-17-12-23(31-9-3-4-10-31)29-21-6-2-1-5-18(17)21/h1-2,5-8,11-12,19,34H,3-4,9-10,13-15H2,(H2,28,33)(H,30,32)/t19-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM85064

(CAS_5311134 | GR 196429 | NSC_5311134)Show InChI InChI=1S/C14H18N2O2/c1-10(17)15-6-8-16-7-4-11-2-3-13-12(14(11)16)5-9-18-13/h2-3H,4-9H2,1H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581542
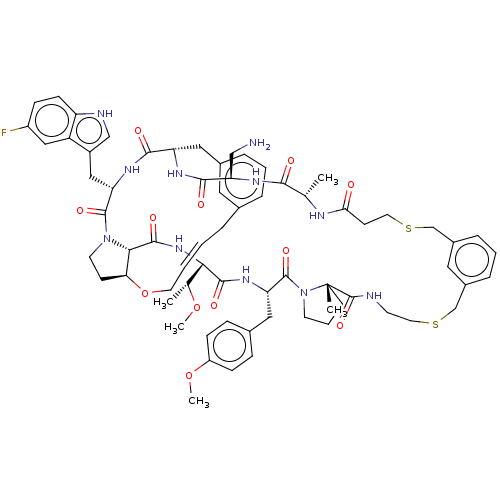
(CHEMBL5081587)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332289

((R)-5-((4-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C28H30N6O5/c1-3-32-12-14-33(15-13-32)24(31-38)20-6-4-19(5-7-20)10-11-28(26(36)29-27(37)30-28)18-34-17-21-8-9-22(39-2)16-23(21)25(34)35/h4-9,16,38H,3,12-15,17-18H2,1-2H3,(H2,29,30,36,37)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332288
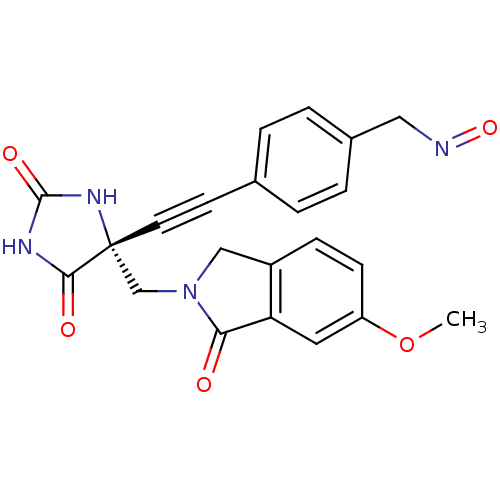
((R)-4-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(CN=O)cc3)C(=O)c2c1 |r| Show InChI InChI=1S/C22H18N4O5/c1-31-17-7-6-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)9-8-14-2-4-15(5-3-14)11-23-30/h2-7,10H,11-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332278
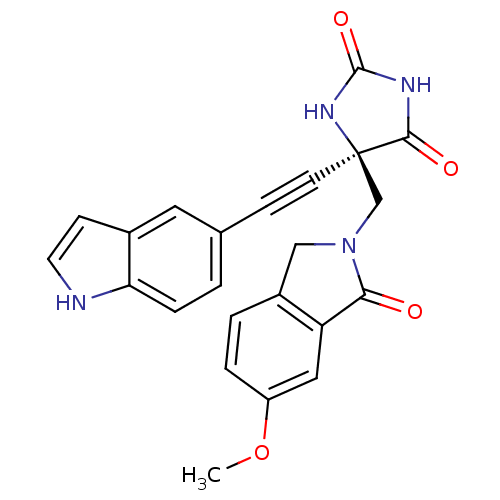
((R)-5-((1H-indol-5-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4[nH]ccc4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-4-3-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-5-19-15(10-14)7-9-24-19/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26525
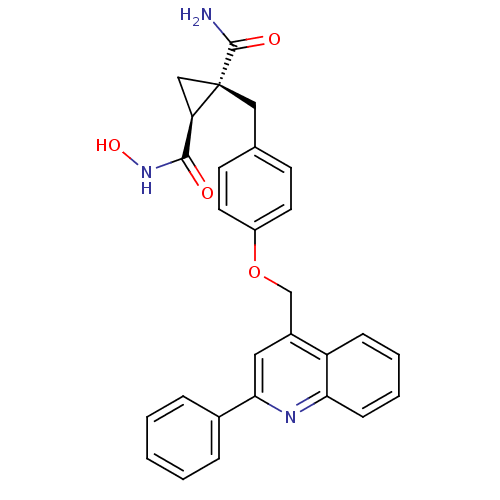
((1S,2R)-1-N-hydroxy-2-({4-[(2-phenylquinolin-4-yl)...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H25N3O4/c29-27(33)28(16-23(28)26(32)31-34)15-18-10-12-21(13-11-18)35-17-20-14-25(19-6-2-1-3-7-19)30-24-9-5-4-8-22(20)24/h1-14,23,34H,15-17H2,(H2,29,33)(H,31,32)/t23-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26525

((1S,2R)-1-N-hydroxy-2-({4-[(2-phenylquinolin-4-yl)...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H25N3O4/c29-27(33)28(16-23(28)26(32)31-34)15-18-10-12-21(13-11-18)35-17-20-14-25(19-6-2-1-3-7-19)30-24-9-5-4-8-22(20)24/h1-14,23,34H,15-17H2,(H2,29,33)(H,31,32)/t23-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332290
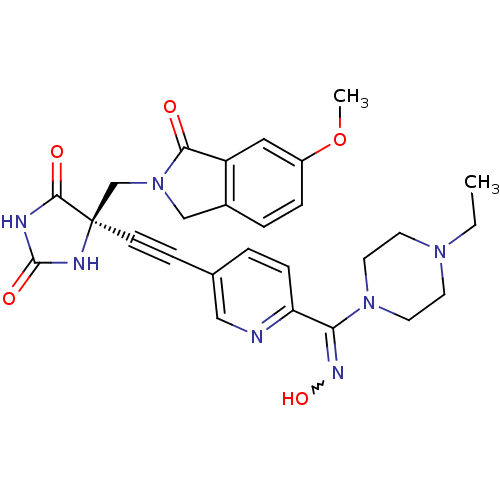
((R)-5-((6-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cn1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C27H29N7O5/c1-3-32-10-12-33(13-11-32)23(31-38)22-7-4-18(15-28-22)8-9-27(25(36)29-26(37)30-27)17-34-16-19-5-6-20(39-2)14-21(19)24(34)35/h4-7,14-15,38H,3,10-13,16-17H2,1-2H3,(H2,29,30,36,37)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332279
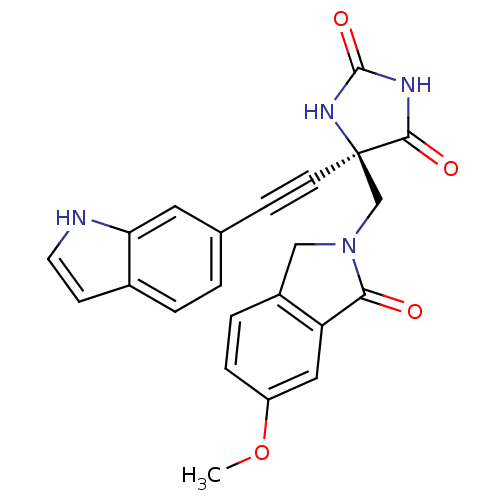
((R)-5-((1H-indol-6-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4cc[nH]c4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-5-4-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-3-15-7-9-24-19(15)10-14/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data