 Found 83 hits with Last Name = 'prasher' and Initial = 'p'
Found 83 hits with Last Name = 'prasher' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089653

(CHEMBL3576974)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446681
(CHEMBL3113617)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(O)=O)c2ccccc12 Show InChI InChI=1S/C19H16N2O6S/c1-12-6-8-13(9-7-12)28(26,27)21-11-15(14-4-2-3-5-16(14)21)18(24)19(25)20-10-17(22)23/h2-9,11H,10H2,1H3,(H,20,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446686
(CHEMBL3113612)Show SMILES COC(=O)CNC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C20H18N2O6S/c1-13-7-9-14(10-8-13)29(26,27)22-12-16(15-5-3-4-6-17(15)22)19(24)20(25)21-11-18(23)28-2/h3-10,12H,11H2,1-2H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446680
(CHEMBL3113618)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C28H23N3O6S/c1-17-10-12-19(13-11-17)38(36,37)31-16-22(21-7-3-5-9-25(21)31)26(32)27(33)30-24(28(34)35)14-18-15-29-23-8-4-2-6-20(18)23/h2-13,15-16,24,29H,14H2,1H3,(H,30,33)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089655
(CHEMBL3576976)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446685
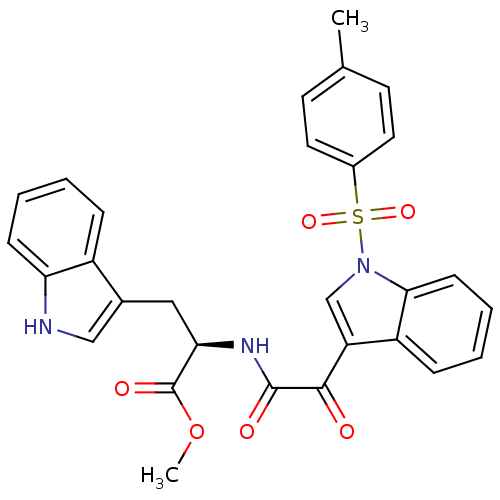
(CHEMBL3113613)Show SMILES COC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C29H25N3O6S/c1-18-11-13-20(14-12-18)39(36,37)32-17-23(22-8-4-6-10-26(22)32)27(33)28(34)31-25(29(35)38-2)15-19-16-30-24-9-5-3-7-21(19)24/h3-14,16-17,25,30H,15H2,1-2H3,(H,31,34)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446678
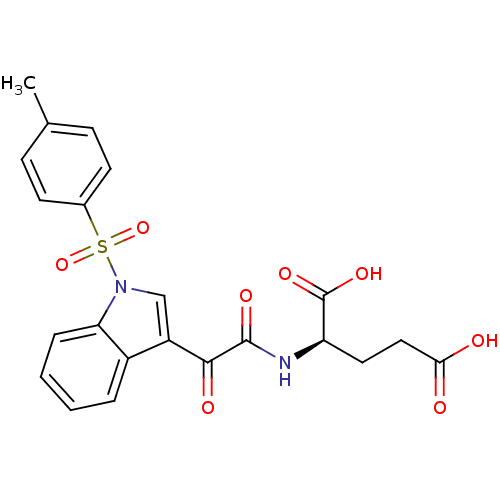
(CHEMBL3113620)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](CCC(O)=O)C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C22H20N2O8S/c1-13-6-8-14(9-7-13)33(31,32)24-12-16(15-4-2-3-5-18(15)24)20(27)21(28)23-17(22(29)30)10-11-19(25)26/h2-9,12,17H,10-11H2,1H3,(H,23,28)(H,25,26)(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089651
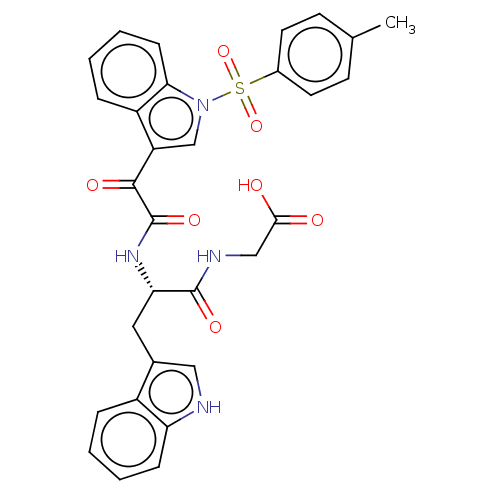
(CHEMBL3576979)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)NCC(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446682
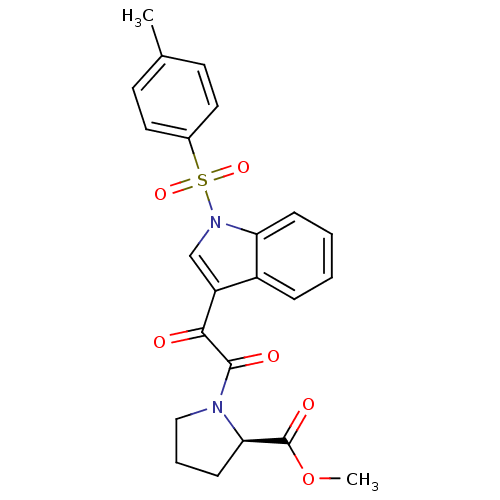
(CHEMBL3113616)Show SMILES COC(=O)[C@H]1CCCN1C(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C23H22N2O6S/c1-15-9-11-16(12-10-15)32(29,30)25-14-18(17-6-3-4-7-19(17)25)21(26)22(27)24-13-5-8-20(24)23(28)31-2/h3-4,6-7,9-12,14,20H,5,8,13H2,1-2H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446677
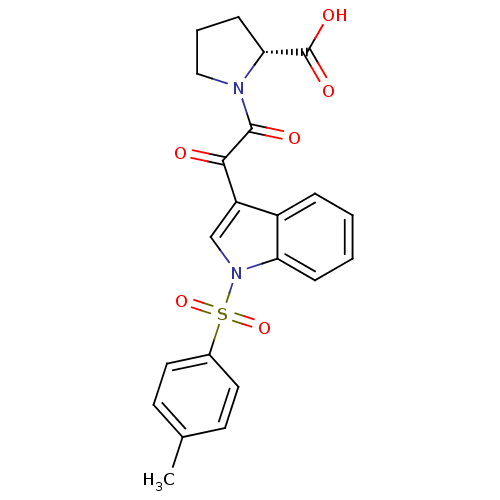
(CHEMBL3113621)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N2CCC[C@@H]2C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C22H20N2O6S/c1-14-8-10-15(11-9-14)31(29,30)24-13-17(16-5-2-3-6-18(16)24)20(25)21(26)23-12-4-7-19(23)22(27)28/h2-3,5-6,8-11,13,19H,4,7,12H2,1H3,(H,27,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446683
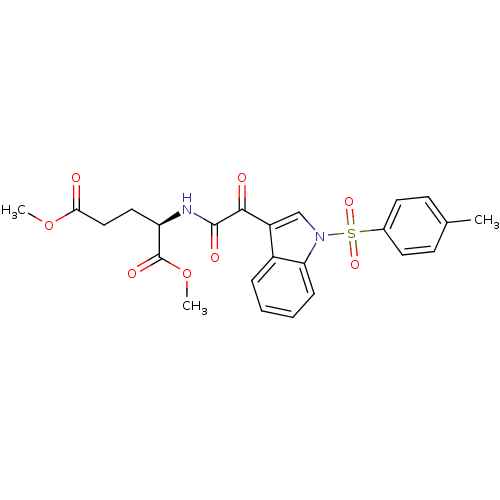
(CHEMBL3113615)Show SMILES COC(=O)CC[C@@H](NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1)C(=O)OC |r| Show InChI InChI=1S/C24H24N2O8S/c1-15-8-10-16(11-9-15)35(31,32)26-14-18(17-6-4-5-7-20(17)26)22(28)23(29)25-19(24(30)34-3)12-13-21(27)33-2/h4-11,14,19H,12-13H2,1-3H3,(H,25,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089648
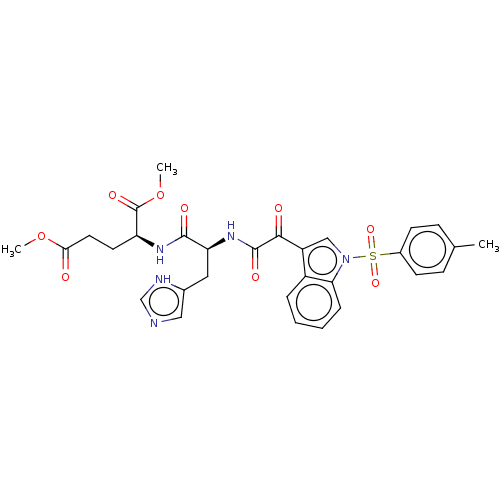
(CHEMBL3576983)Show SMILES COC(=O)CC[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 983 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089646

(CHEMBL3576981)Show SMILES COC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446679
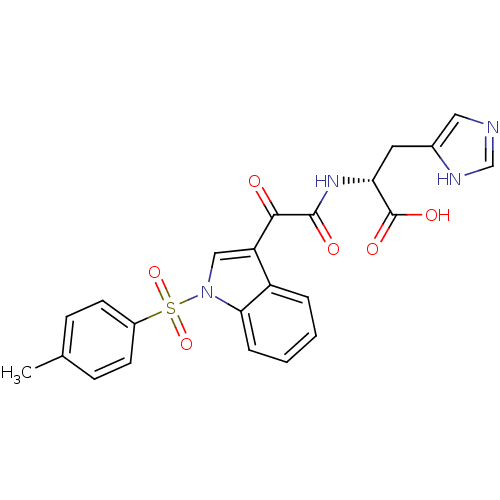
(CHEMBL3113619)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](Cc2cnc[nH]2)C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C23H20N4O6S/c1-14-6-8-16(9-7-14)34(32,33)27-12-18(17-4-2-3-5-20(17)27)21(28)22(29)26-19(23(30)31)10-15-11-24-13-25-15/h2-9,11-13,19H,10H2,1H3,(H,24,25)(H,26,29)(H,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089644
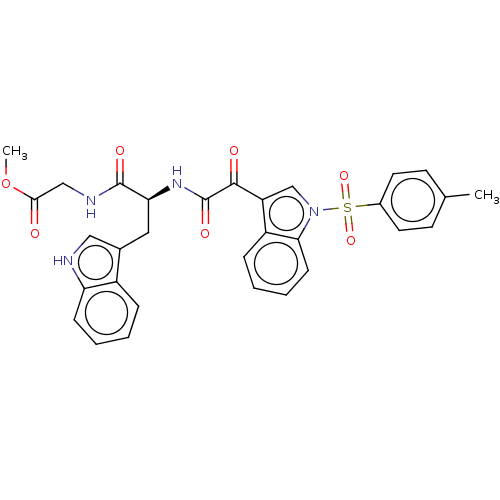
(CHEMBL3576986)Show SMILES COC(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089656
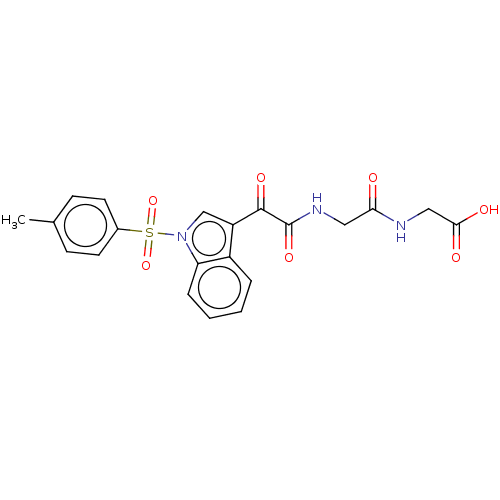
(CHEMBL3576973)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(=O)NCC(O)=O)c2ccccc12 Show InChI InChI=1S/C21H19N3O7S/c1-13-6-8-14(9-7-13)32(30,31)24-12-16(15-4-2-3-5-17(15)24)20(28)21(29)23-10-18(25)22-11-19(26)27/h2-9,12H,10-11H2,1H3,(H,22,25)(H,23,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446684
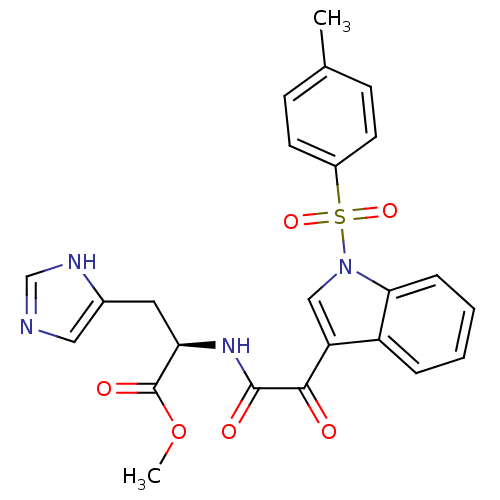
(CHEMBL3113614)Show SMILES COC(=O)[C@@H](Cc1cnc[nH]1)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C24H22N4O6S/c1-15-7-9-17(10-8-15)35(32,33)28-13-19(18-5-3-4-6-21(18)28)22(29)23(30)27-20(24(31)34-2)11-16-12-25-14-26-16/h3-10,12-14,20H,11H2,1-2H3,(H,25,26)(H,27,30)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) by UV-Vis spectrophotometry |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089649

(CHEMBL3576977)Show SMILES COC(=O)CNC(=O)CNC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C22H21N3O7S/c1-14-7-9-15(10-8-14)33(30,31)25-13-17(16-5-3-4-6-18(16)25)21(28)22(29)24-11-19(26)23-12-20(27)32-2/h3-10,13H,11-12H2,1-2H3,(H,23,26)(H,24,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50029559
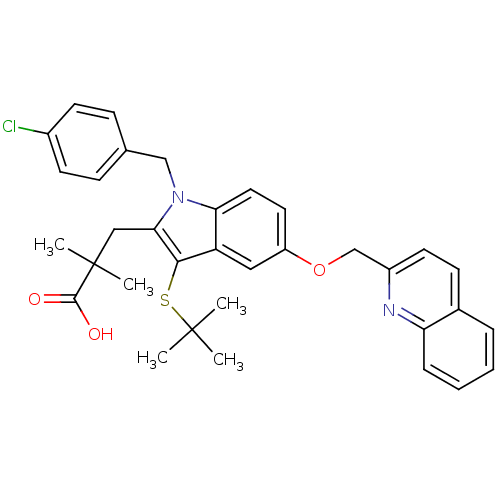
(2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089651

(CHEMBL3576979)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)NCC(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
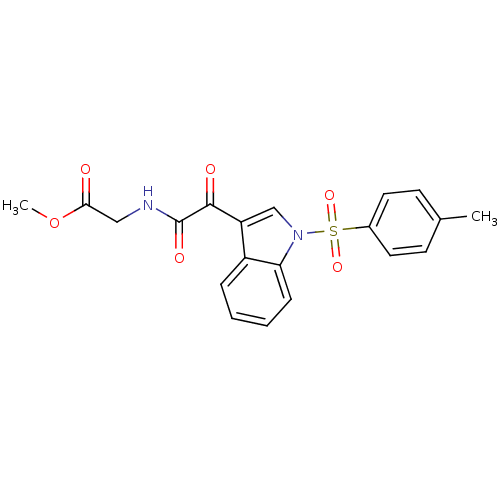
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446686

(CHEMBL3113612)Show SMILES COC(=O)CNC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C20H18N2O6S/c1-13-7-9-14(10-8-13)29(26,27)22-12-16(15-5-3-4-6-17(15)22)19(24)20(25)21-11-18(23)28-2/h3-10,12H,11H2,1-2H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA in presence of pig liver esterase |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
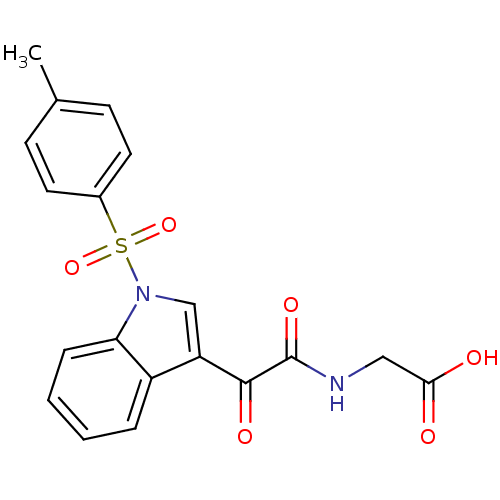
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446681

(CHEMBL3113617)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(O)=O)c2ccccc12 Show InChI InChI=1S/C19H16N2O6S/c1-12-6-8-13(9-7-12)28(26,27)21-11-15(14-4-2-3-5-16(14)21)18(24)19(25)20-10-17(22)23/h2-9,11H,10H2,1H3,(H,20,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446686

(CHEMBL3113612)Show SMILES COC(=O)CNC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C20H18N2O6S/c1-13-7-9-14(10-8-13)29(26,27)22-12-16(15-5-3-4-6-17(15)22)19(24)20(25)21-11-18(23)28-2/h3-10,12H,11H2,1-2H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
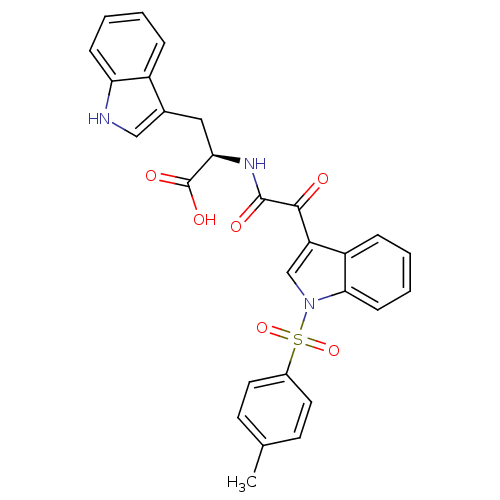
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446680

(CHEMBL3113618)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C28H23N3O6S/c1-17-10-12-19(13-11-17)38(36,37)31-16-22(21-7-3-5-9-25(21)31)26(32)27(33)30-24(28(34)35)14-18-15-29-23-8-4-2-6-20(18)23/h2-13,15-16,24,29H,14H2,1H3,(H,30,33)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446685

(CHEMBL3113613)Show SMILES COC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C29H25N3O6S/c1-18-11-13-20(14-12-18)39(36,37)32-17-23(22-8-4-6-10-26(22)32)27(33)28(34)31-25(29(35)38-2)15-19-16-30-24-9-5-3-7-21(19)24/h3-14,16-17,25,30H,15H2,1-2H3,(H,31,34)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA in presence of pig liver esterase |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089644

(CHEMBL3576986)Show SMILES COC(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50096276
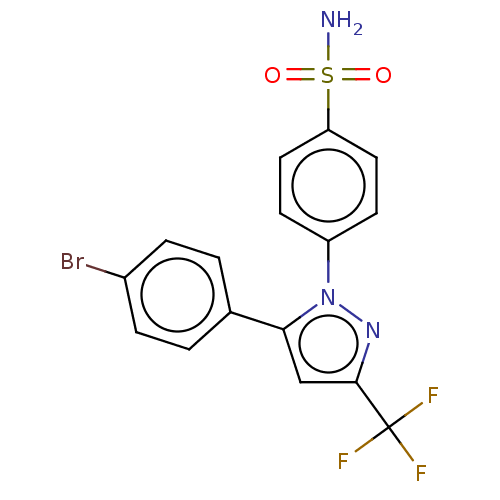
(CHEMBL1235806)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Br)cc1)C(F)(F)F Show InChI InChI=1S/C12H6N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H2,13,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446685

(CHEMBL3113613)Show SMILES COC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C29H25N3O6S/c1-18-11-13-20(14-12-18)39(36,37)32-17-23(22-8-4-6-10-26(22)32)27(33)28(34)31-25(29(35)38-2)15-19-16-30-24-9-5-3-7-21(19)24/h3-14,16-17,25,30H,15H2,1-2H3,(H,31,34)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446678

(CHEMBL3113620)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](CCC(O)=O)C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C22H20N2O8S/c1-13-6-8-14(9-7-13)33(31,32)24-12-16(15-4-2-3-5-18(15)24)20(27)21(28)23-17(22(29)30)10-11-19(25)26/h2-9,12,17H,10-11H2,1H3,(H,23,28)(H,25,26)(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089653

(CHEMBL3576974)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446683

(CHEMBL3113615)Show SMILES COC(=O)CC[C@@H](NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1)C(=O)OC |r| Show InChI InChI=1S/C24H24N2O8S/c1-15-8-10-16(11-9-15)35(31,32)26-14-18(17-6-4-5-7-20(17)26)22(28)23(29)25-19(24(30)34-3)12-13-21(27)33-2/h4-11,14,19H,12-13H2,1-3H3,(H,25,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA in presence of pig liver esterase |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446682

(CHEMBL3113616)Show SMILES COC(=O)[C@H]1CCCN1C(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C23H22N2O6S/c1-15-9-11-16(12-10-15)32(29,30)25-14-18(17-6-3-4-7-19(17)25)21(26)22(27)24-13-5-8-20(24)23(28)31-2/h3-4,6-7,9-12,14,20H,5,8,13H2,1-2H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
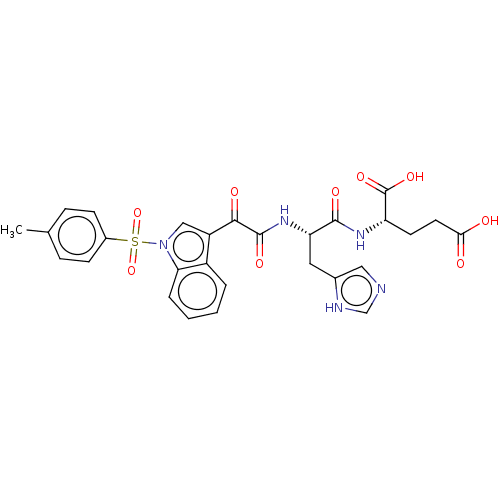
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089655

(CHEMBL3576976)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446677

(CHEMBL3113621)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N2CCC[C@@H]2C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C22H20N2O6S/c1-14-8-10-15(11-9-14)31(29,30)24-13-17(16-5-2-3-6-18(16)24)20(25)21(26)23-12-4-7-19(23)22(27)28/h2-3,5-6,8-11,13,19H,4,7,12H2,1H3,(H,27,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM13066
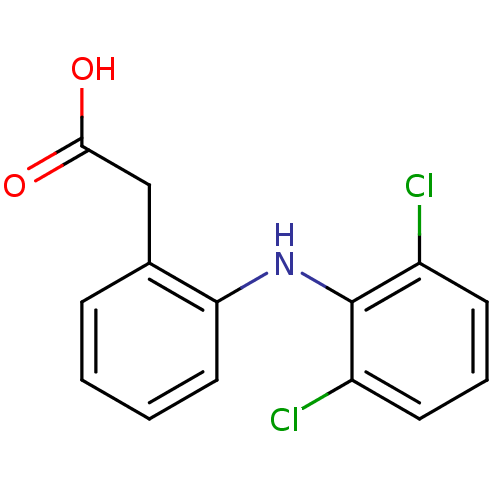
(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13066

(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446682

(CHEMBL3113616)Show SMILES COC(=O)[C@H]1CCCN1C(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C23H22N2O6S/c1-15-9-11-16(12-10-15)32(29,30)25-14-18(17-6-3-4-7-19(17)25)21(26)22(27)24-13-5-8-20(24)23(28)31-2/h3-4,6-7,9-12,14,20H,5,8,13H2,1-2H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA in presence of pig liver esterase |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089656

(CHEMBL3576973)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(=O)NCC(O)=O)c2ccccc12 Show InChI InChI=1S/C21H19N3O7S/c1-13-6-8-14(9-7-13)32(30,31)24-12-16(15-4-2-3-5-17(15)24)20(28)21(29)23-10-18(25)22-11-19(26)27/h2-9,12H,10-11H2,1H3,(H,22,25)(H,23,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446683

(CHEMBL3113615)Show SMILES COC(=O)CC[C@@H](NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1)C(=O)OC |r| Show InChI InChI=1S/C24H24N2O8S/c1-15-8-10-16(11-9-15)35(31,32)26-14-18(17-6-4-5-7-20(17)26)22(28)23(29)25-19(24(30)34-3)12-13-21(27)33-2/h4-11,14,19H,12-13H2,1-3H3,(H,25,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089642

(CHEMBL3576984)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@@H](Cc2cnc[nH]2)C(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089650

(CHEMBL3576978)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)NCC(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50089651

(CHEMBL3576979)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)NCC(O)=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50089652

(CHEMBL3576980)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(=O)N[C@H](Cc2c[nH]c3ccccc23)C(O)=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... |
Eur J Med Chem 97: 104-23 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.044
BindingDB Entry DOI: 10.7270/Q2N87CJN |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446679

(CHEMBL3113619)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](Cc2cnc[nH]2)C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C23H20N4O6S/c1-14-6-8-16(9-7-14)34(32,33)27-12-18(17-4-2-3-5-20(17)27)21(28)22(29)26-19(23(30)31)10-15-11-24-13-25-15/h2-9,11-13,19H,10H2,1H3,(H,24,25)(H,26,29)(H,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446684

(CHEMBL3113614)Show SMILES COC(=O)[C@@H](Cc1cnc[nH]1)NC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C24H22N4O6S/c1-15-7-9-17(10-8-15)35(32,33)28-13-19(18-5-3-4-6-21(18)28)22(29)23(30)27-20(24(31)34-2)11-16-12-25-14-26-16/h3-10,12-14,20H,11H2,1-2H3,(H,25,26)(H,27,30)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA in presence of pig liver esterase |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446689
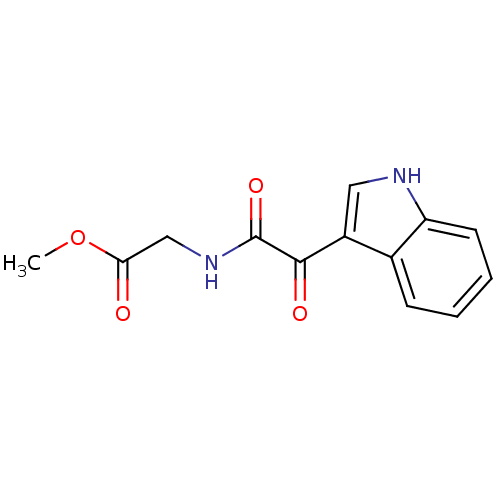
(CHEMBL3113607)Show InChI InChI=1S/C13H12N2O4/c1-19-11(16)7-15-13(18)12(17)9-6-14-10-5-3-2-4-8(9)10/h2-6,14H,7H2,1H3,(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data