 Found 115 hits with Last Name = 'roux' and Initial = 'p'
Found 115 hits with Last Name = 'roux' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 5
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM82253
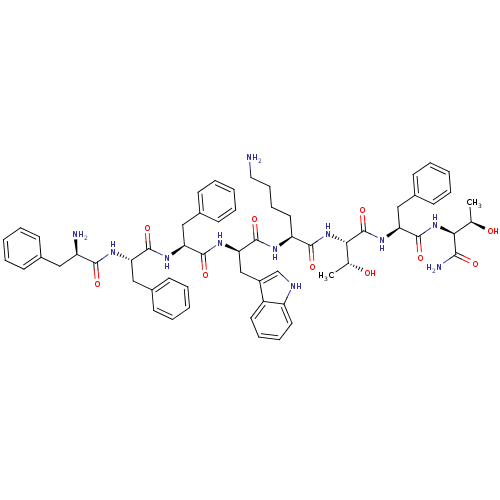
(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM84629
(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(RAT) | BDBM85080
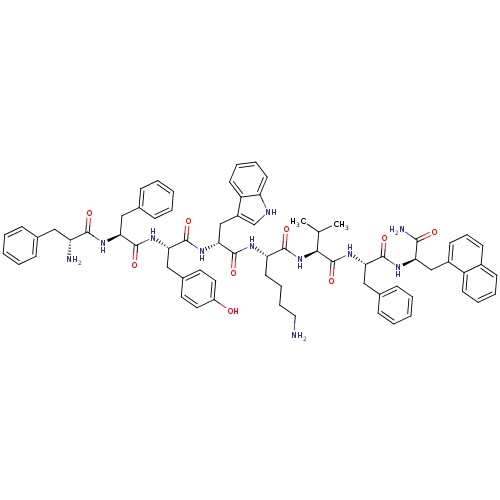
(CH-275 | CH275 | L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-...)Show SMILES CC(C)NCc1ccc(C[C@H]2NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)C(Cc3ccccc3)NC(=O)C(Cc3ccccc3)NC(=O)C(CCCCN)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC(=O)C(NC2=O)C(C)O)C(C)O)C(O)=O)cc1 Show InChI InChI=1S/C74H96N14O15S2/c1-42(2)77-37-49-29-27-48(28-30-49)35-57-69(97)87-62(43(3)90)72(100)84-58(34-47-22-12-7-13-23-47)70(98)88-63(44(4)91)73(101)85-60(39-89)71(99)86-61(74(102)103)41-105-104-40-52(76)64(92)79-54(26-16-17-31-75)65(93)80-55(32-45-18-8-5-9-19-45)66(94)81-56(33-46-20-10-6-11-21-46)67(95)83-59(68(96)82-57)36-50-38-78-53-25-15-14-24-51(50)53/h5-15,18-25,27-30,38,42-44,52,54-63,77-78,89-91H,16-17,26,31-37,39-41,75-76H2,1-4H3,(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,95)(H,84,100)(H,85,101)(H,86,99)(H,87,97)(H,88,98)(H,102,103)/t43?,44?,52-,54?,55?,56?,57-,58-,59+,60-,61-,62?,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM84629

(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM85080

(CH-275 | CH275 | L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-...)Show SMILES CC(C)NCc1ccc(C[C@H]2NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)C(Cc3ccccc3)NC(=O)C(Cc3ccccc3)NC(=O)C(CCCCN)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC(=O)C(NC2=O)C(C)O)C(C)O)C(O)=O)cc1 Show InChI InChI=1S/C74H96N14O15S2/c1-42(2)77-37-49-29-27-48(28-30-49)35-57-69(97)87-62(43(3)90)72(100)84-58(34-47-22-12-7-13-23-47)70(98)88-63(44(4)91)73(101)85-60(39-89)71(99)86-61(74(102)103)41-105-104-40-52(76)64(92)79-54(26-16-17-31-75)65(93)80-55(32-45-18-8-5-9-19-45)66(94)81-56(33-46-20-10-6-11-21-46)67(95)83-59(68(96)82-57)36-50-38-78-53-25-15-14-24-51(50)53/h5-15,18-25,27-30,38,42-44,52,54-63,77-78,89-91H,16-17,26,31-37,39-41,75-76H2,1-4H3,(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,95)(H,84,100)(H,85,101)(H,86,99)(H,87,97)(H,88,98)(H,102,103)/t43?,44?,52-,54?,55?,56?,57-,58-,59+,60-,61-,62?,63+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(RAT) | BDBM84629

(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM84629

(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM85080

(CH-275 | CH275 | L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-...)Show SMILES CC(C)NCc1ccc(C[C@H]2NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)C(Cc3ccccc3)NC(=O)C(Cc3ccccc3)NC(=O)C(CCCCN)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC(=O)C(NC2=O)C(C)O)C(C)O)C(O)=O)cc1 Show InChI InChI=1S/C74H96N14O15S2/c1-42(2)77-37-49-29-27-48(28-30-49)35-57-69(97)87-62(43(3)90)72(100)84-58(34-47-22-12-7-13-23-47)70(98)88-63(44(4)91)73(101)85-60(39-89)71(99)86-61(74(102)103)41-105-104-40-52(76)64(92)79-54(26-16-17-31-75)65(93)80-55(32-45-18-8-5-9-19-45)66(94)81-56(33-46-20-10-6-11-21-46)67(95)83-59(68(96)82-57)36-50-38-78-53-25-15-14-24-51(50)53/h5-15,18-25,27-30,38,42-44,52,54-63,77-78,89-91H,16-17,26,31-37,39-41,75-76H2,1-4H3,(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,95)(H,84,100)(H,85,101)(H,86,99)(H,87,97)(H,88,98)(H,102,103)/t43?,44?,52-,54?,55?,56?,57-,58-,59+,60-,61-,62?,63+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM85080

(CH-275 | CH275 | L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-...)Show SMILES CC(C)NCc1ccc(C[C@H]2NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)C(Cc3ccccc3)NC(=O)C(Cc3ccccc3)NC(=O)C(CCCCN)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC(=O)C(NC2=O)C(C)O)C(C)O)C(O)=O)cc1 Show InChI InChI=1S/C74H96N14O15S2/c1-42(2)77-37-49-29-27-48(28-30-49)35-57-69(97)87-62(43(3)90)72(100)84-58(34-47-22-12-7-13-23-47)70(98)88-63(44(4)91)73(101)85-60(39-89)71(99)86-61(74(102)103)41-105-104-40-52(76)64(92)79-54(26-16-17-31-75)65(93)80-55(32-45-18-8-5-9-19-45)66(94)81-56(33-46-20-10-6-11-21-46)67(95)83-59(68(96)82-57)36-50-38-78-53-25-15-14-24-51(50)53/h5-15,18-25,27-30,38,42-44,52,54-63,77-78,89-91H,16-17,26,31-37,39-41,75-76H2,1-4H3,(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,95)(H,84,100)(H,85,101)(H,86,99)(H,87,97)(H,88,98)(H,102,103)/t43?,44?,52-,54?,55?,56?,57-,58-,59+,60-,61-,62?,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 577 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM84629

(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 835 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM85080

(CH-275 | CH275 | L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-...)Show SMILES CC(C)NCc1ccc(C[C@H]2NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)C(Cc3ccccc3)NC(=O)C(Cc3ccccc3)NC(=O)C(CCCCN)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC(=O)C(NC2=O)C(C)O)C(C)O)C(O)=O)cc1 Show InChI InChI=1S/C74H96N14O15S2/c1-42(2)77-37-49-29-27-48(28-30-49)35-57-69(97)87-62(43(3)90)72(100)84-58(34-47-22-12-7-13-23-47)70(98)88-63(44(4)91)73(101)85-60(39-89)71(99)86-61(74(102)103)41-105-104-40-52(76)64(92)79-54(26-16-17-31-75)65(93)80-55(32-45-18-8-5-9-19-45)66(94)81-56(33-46-20-10-6-11-21-46)67(95)83-59(68(96)82-57)36-50-38-78-53-25-15-14-24-51(50)53/h5-15,18-25,27-30,38,42-44,52,54-63,77-78,89-91H,16-17,26,31-37,39-41,75-76H2,1-4H3,(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,95)(H,84,100)(H,85,101)(H,86,99)(H,87,97)(H,88,98)(H,102,103)/t43?,44?,52-,54?,55?,56?,57-,58-,59+,60-,61-,62?,63+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176622
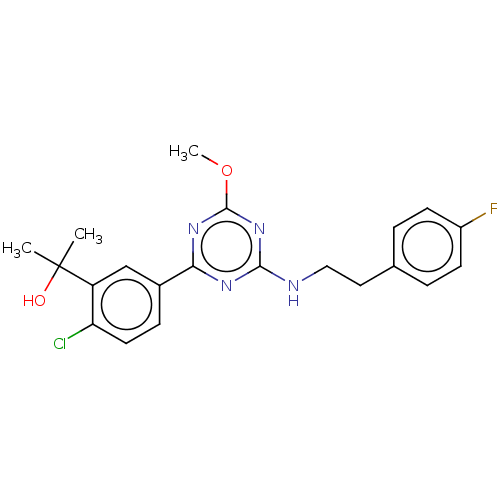
(US9115121, 13)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(Cl)c(c1)C(C)(C)O Show InChI InChI=1S/C21H22ClFN4O2/c1-21(2,28)16-12-14(6-9-17(16)22)18-25-19(27-20(26-18)29-3)24-11-10-13-4-7-15(23)8-5-13/h4-9,12,28H,10-11H2,1-3H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176622

(US9115121, 13)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(Cl)c(c1)C(C)(C)O Show InChI InChI=1S/C21H22ClFN4O2/c1-21(2,28)16-12-14(6-9-17(16)22)18-25-19(27-20(26-18)29-3)24-11-10-13-4-7-15(23)8-5-13/h4-9,12,28H,10-11H2,1-3H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 24: 283-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.023
BindingDB Entry DOI: 10.7270/Q2ZC85VD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176668
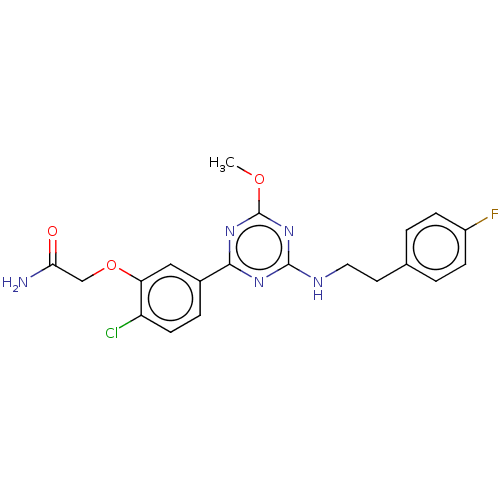
(US9115121, 65)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(Cl)c(OCC(N)=O)c1 Show InChI InChI=1S/C20H19ClFN5O3/c1-29-20-26-18(13-4-7-15(21)16(10-13)30-11-17(23)28)25-19(27-20)24-9-8-12-2-5-14(22)6-3-12/h2-7,10H,8-9,11H2,1H3,(H2,23,28)(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176606
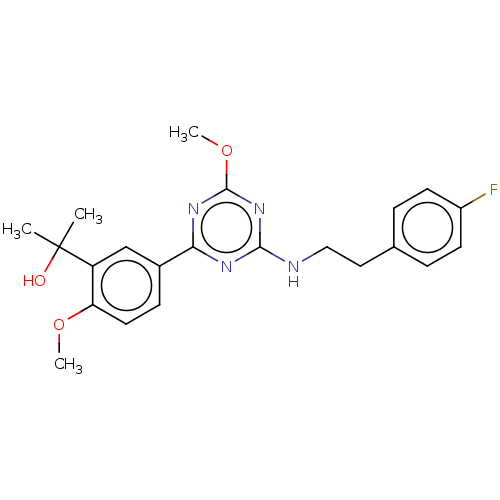
(US9115121, 58)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(OC)c(c1)C(C)(C)O Show InChI InChI=1S/C22H25FN4O3/c1-22(2,28)17-13-15(7-10-18(17)29-3)19-25-20(27-21(26-19)30-4)24-12-11-14-5-8-16(23)9-6-14/h5-10,13,28H,11-12H2,1-4H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176645
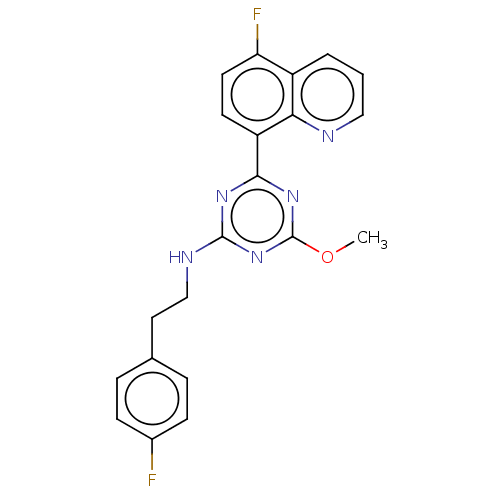
(US9115121, 37)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(F)c2cccnc12 Show InChI InChI=1S/C21H17F2N5O/c1-29-21-27-19(16-8-9-17(23)15-3-2-11-24-18(15)16)26-20(28-21)25-12-10-13-4-6-14(22)7-5-13/h2-9,11H,10,12H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176604
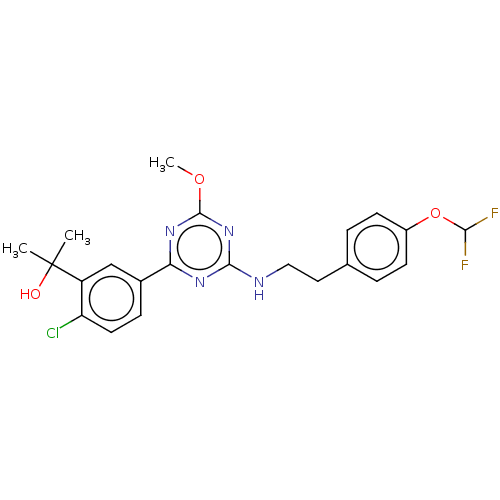
(US9115121, 46)Show SMILES COc1nc(NCCc2ccc(OC(F)F)cc2)nc(n1)-c1ccc(Cl)c(c1)C(C)(C)O Show InChI InChI=1S/C22H23ClF2N4O3/c1-22(2,30)16-12-14(6-9-17(16)23)18-27-20(29-21(28-18)31-3)26-11-10-13-4-7-15(8-5-13)32-19(24)25/h4-9,12,19,30H,10-11H2,1-3H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50494951
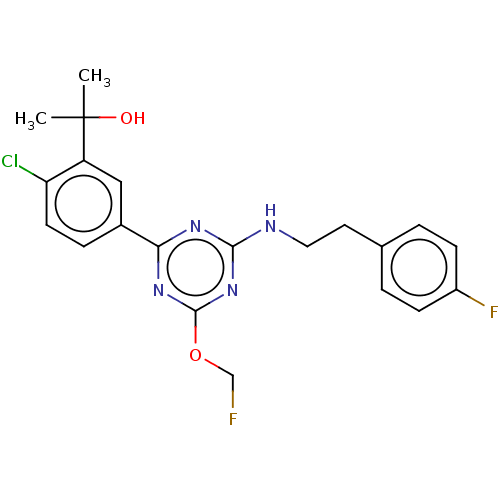
(CHEMBL3099054)Show SMILES CC(C)(O)c1cc(ccc1Cl)-c1nc(NCCc2ccc(F)cc2)nc(OCF)n1 Show InChI InChI=1S/C21H21ClF2N4O2/c1-21(2,29)16-11-14(5-8-17(16)22)18-26-19(28-20(27-18)30-12-23)25-10-9-13-3-6-15(24)7-4-13/h3-8,11,29H,9-10,12H2,1-2H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 24: 283-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.023
BindingDB Entry DOI: 10.7270/Q2ZC85VD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176620
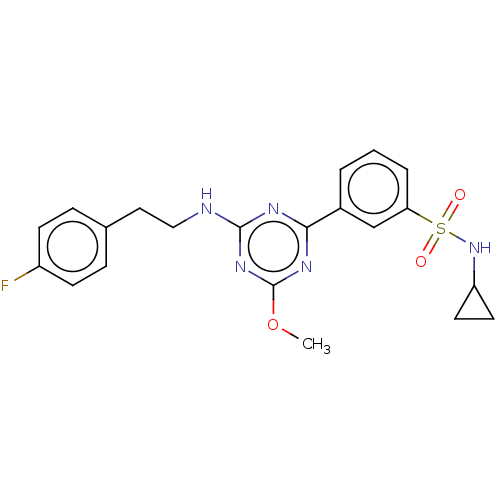
(US9115121, 11)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1cccc(c1)S(=O)(=O)NC1CC1 Show InChI InChI=1S/C21H22FN5O3S/c1-30-21-25-19(15-3-2-4-18(13-15)31(28,29)27-17-9-10-17)24-20(26-21)23-12-11-14-5-7-16(22)8-6-14/h2-8,13,17,27H,9-12H2,1H3,(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176660
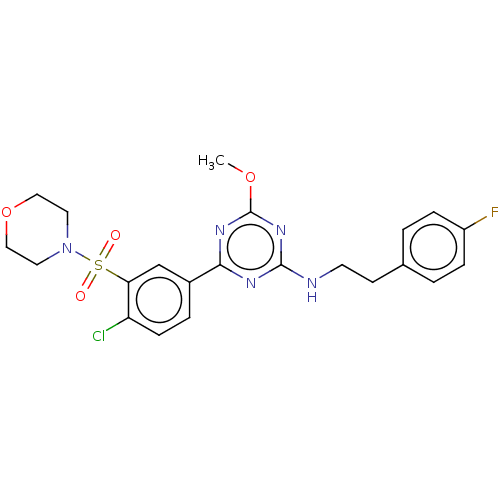
(US9115121, 53)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(Cl)c(c1)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C22H23ClFN5O4S/c1-32-22-27-20(26-21(28-22)25-9-8-15-2-5-17(24)6-3-15)16-4-7-18(23)19(14-16)34(30,31)29-10-12-33-13-11-29/h2-7,14H,8-13H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176610
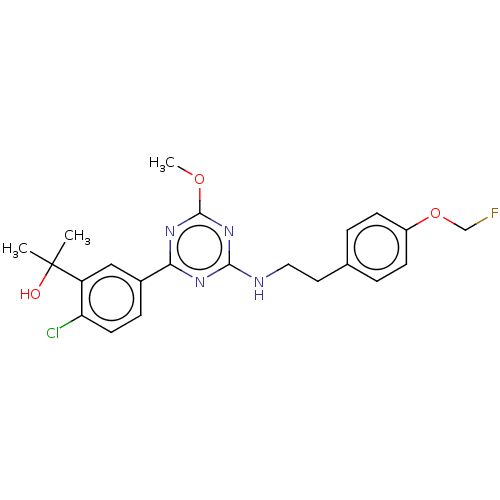
(US9115121, 69)Show SMILES COc1nc(NCCc2ccc(OCF)cc2)nc(n1)-c1ccc(Cl)c(c1)C(C)(C)O Show InChI InChI=1S/C22H24ClFN4O3/c1-22(2,29)17-12-15(6-9-18(17)23)19-26-20(28-21(27-19)30-3)25-11-10-14-4-7-16(8-5-14)31-13-24/h4-9,12,29H,10-11,13H2,1-3H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176654
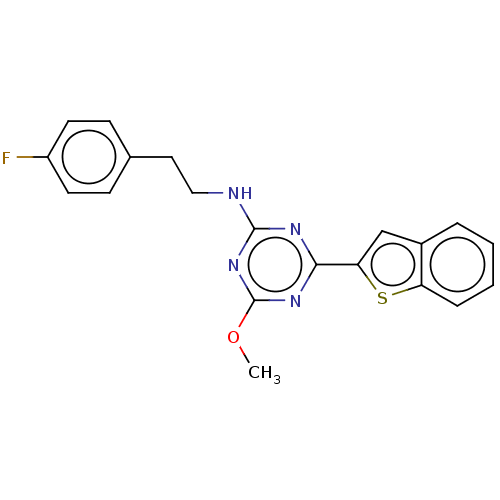
(US9115121, 47)Show InChI InChI=1S/C20H17FN4OS/c1-26-20-24-18(17-12-14-4-2-3-5-16(14)27-17)23-19(25-20)22-11-10-13-6-8-15(21)9-7-13/h2-9,12H,10-11H2,1H3,(H,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176656
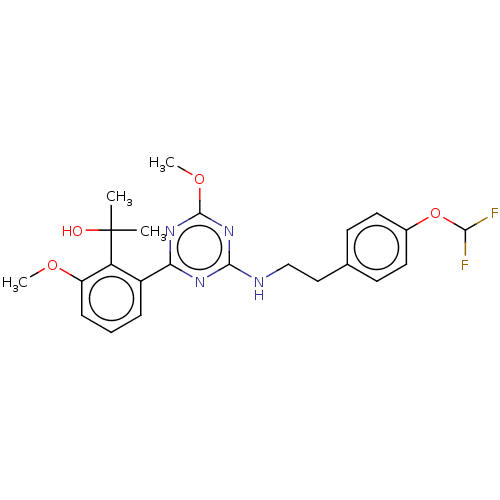
(US9115121, 49)Show SMILES COc1nc(NCCc2ccc(OC(F)F)cc2)nc(n1)-c1cccc(OC)c1C(C)(C)O Show InChI InChI=1S/C23H26F2N4O4/c1-23(2,30)18-16(6-5-7-17(18)31-3)19-27-21(29-22(28-19)32-4)26-13-12-14-8-10-15(11-9-14)33-20(24)25/h5-11,20,30H,12-13H2,1-4H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176666
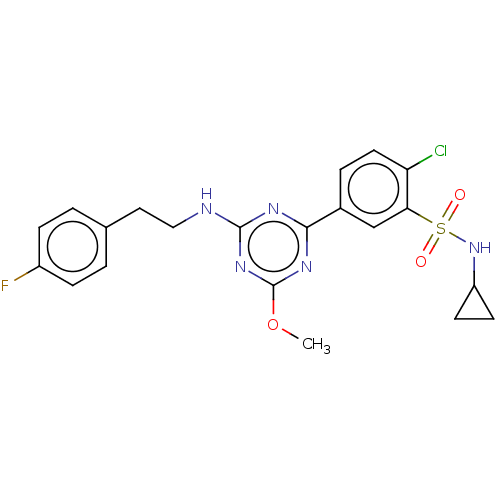
(US9115121, 62)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(Cl)c(c1)S(=O)(=O)NC1CC1 Show InChI InChI=1S/C21H21ClFN5O3S/c1-31-21-26-19(25-20(27-21)24-11-10-13-2-5-15(23)6-3-13)14-4-9-17(22)18(12-14)32(29,30)28-16-7-8-16/h2-6,9,12,16,28H,7-8,10-11H2,1H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176638
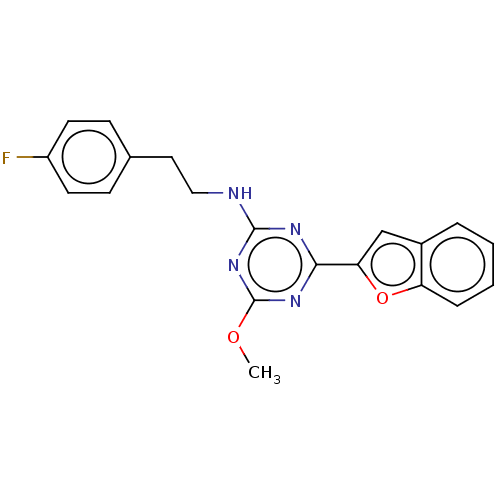
(US9115121, 30)Show InChI InChI=1S/C20H17FN4O2/c1-26-20-24-18(17-12-14-4-2-3-5-16(14)27-17)23-19(25-20)22-11-10-13-6-8-15(21)9-7-13/h2-9,12H,10-11H2,1H3,(H,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176602

(US9115121, 18)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1cc2c(Cl)cccc2[nH]1 Show InChI InChI=1S/C20H17ClFN5O/c1-28-20-26-18(17-11-14-15(21)3-2-4-16(14)24-17)25-19(27-20)23-10-9-12-5-7-13(22)8-6-12/h2-8,11,24H,9-10H2,1H3,(H,23,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176623

(US9115121, 14)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(F)c(c1)C(C)(C)O Show InChI InChI=1S/C21H22F2N4O2/c1-21(2,28)16-12-14(6-9-17(16)23)18-25-19(27-20(26-18)29-3)24-11-10-13-4-7-15(22)8-5-13/h4-9,12,28H,10-11H2,1-3H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176605
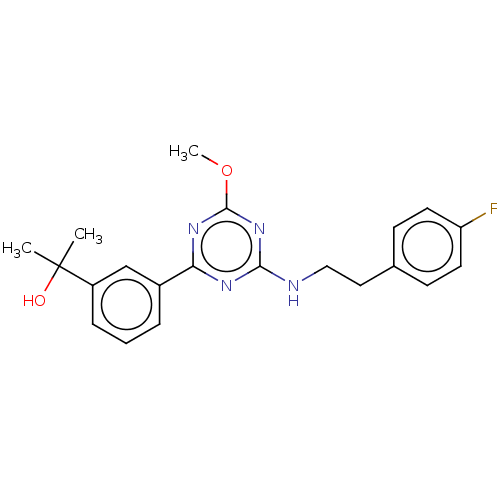
(US9115121, 56)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1cccc(c1)C(C)(C)O Show InChI InChI=1S/C21H23FN4O2/c1-21(2,27)16-6-4-5-15(13-16)18-24-19(26-20(25-18)28-3)23-12-11-14-7-9-17(22)10-8-14/h4-10,13,27H,11-12H2,1-3H3,(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176661
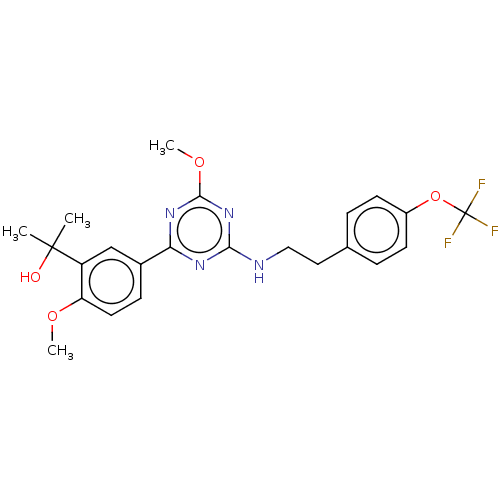
(US9115121, 54)Show SMILES COc1nc(NCCc2ccc(OC(F)(F)F)cc2)nc(n1)-c1ccc(OC)c(c1)C(C)(C)O Show InChI InChI=1S/C23H25F3N4O4/c1-22(2,31)17-13-15(7-10-18(17)32-3)19-28-20(30-21(29-19)33-4)27-12-11-14-5-8-16(9-6-14)34-23(24,25)26/h5-10,13,31H,11-12H2,1-4H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176671
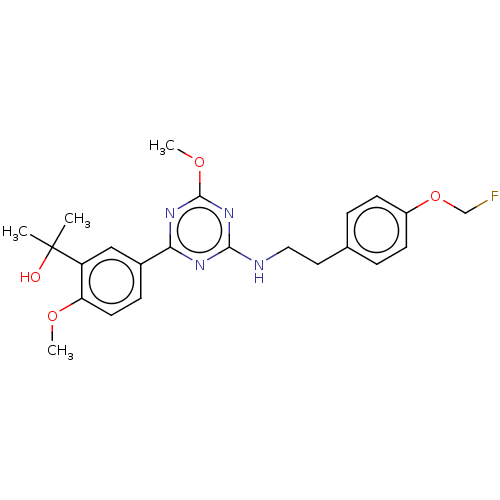
(US9115121, 70)Show SMILES COc1nc(NCCc2ccc(OCF)cc2)nc(n1)-c1ccc(OC)c(c1)C(C)(C)O Show InChI InChI=1S/C23H27FN4O4/c1-23(2,29)18-13-16(7-10-19(18)30-3)20-26-21(28-22(27-20)31-4)25-12-11-15-5-8-17(9-6-15)32-14-24/h5-10,13,29H,11-12,14H2,1-4H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176616
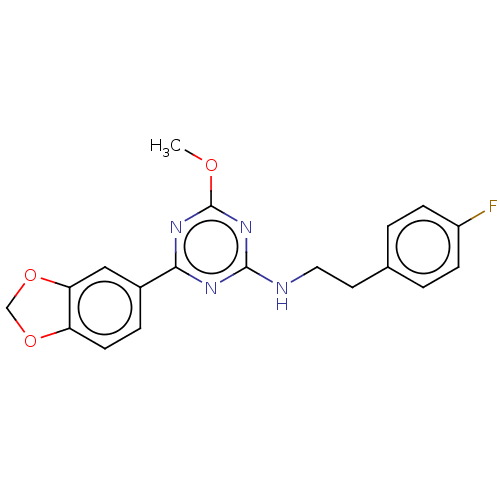
(US9115121, 7)Show InChI InChI=1S/C19H17FN4O3/c1-25-19-23-17(13-4-7-15-16(10-13)27-11-26-15)22-18(24-19)21-9-8-12-2-5-14(20)6-3-12/h2-7,10H,8-9,11H2,1H3,(H,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176608
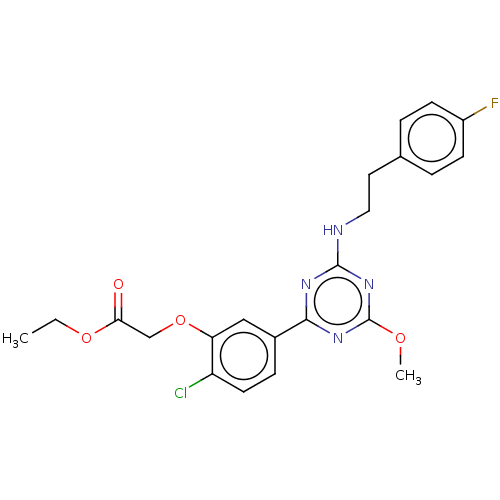
(US9115121, 63)Show SMILES CCOC(=O)COc1cc(ccc1Cl)-c1nc(NCCc2ccc(F)cc2)nc(OC)n1 Show InChI InChI=1S/C22H22ClFN4O4/c1-3-31-19(29)13-32-18-12-15(6-9-17(18)23)20-26-21(28-22(27-20)30-2)25-11-10-14-4-7-16(24)8-5-14/h4-9,12H,3,10-11,13H2,1-2H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176619
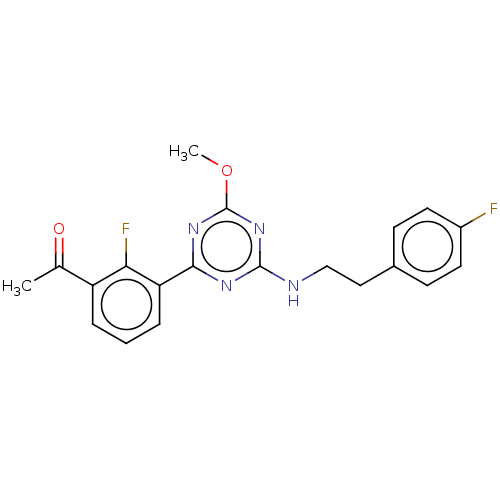
(US9115121, 10)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1cccc(C(C)=O)c1F Show InChI InChI=1S/C20H18F2N4O2/c1-12(27)15-4-3-5-16(17(15)22)18-24-19(26-20(25-18)28-2)23-11-10-13-6-8-14(21)9-7-13/h3-9H,10-11H2,1-2H3,(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176670
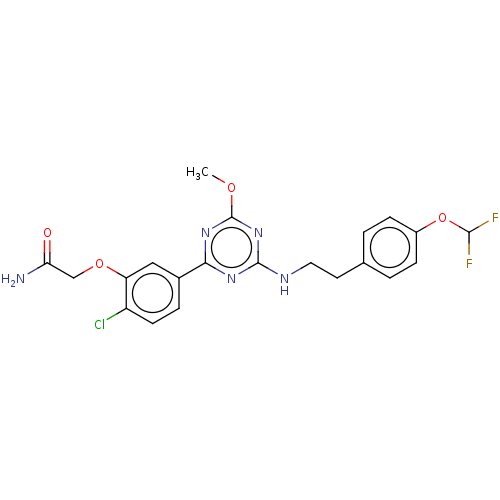
(US9115121, 68)Show SMILES COc1nc(NCCc2ccc(OC(F)F)cc2)nc(n1)-c1ccc(Cl)c(OCC(N)=O)c1 Show InChI InChI=1S/C21H20ClF2N5O4/c1-31-21-28-18(13-4-7-15(22)16(10-13)32-11-17(25)30)27-20(29-21)26-9-8-12-2-5-14(6-3-12)33-19(23)24/h2-7,10,19H,8-9,11H2,1H3,(H2,25,30)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176612
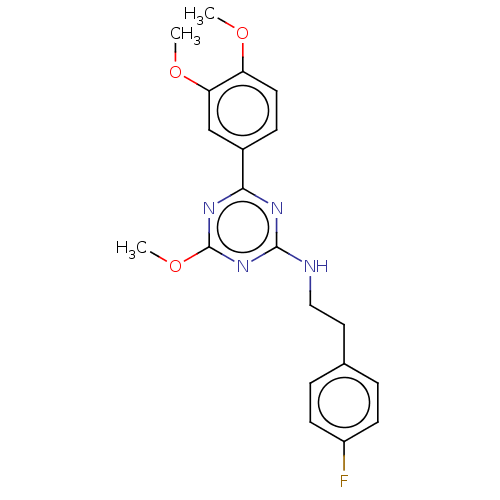
(US9115121, 3)Show SMILES COc1ccc(cc1OC)-c1nc(NCCc2ccc(F)cc2)nc(OC)n1 Show InChI InChI=1S/C20H21FN4O3/c1-26-16-9-6-14(12-17(16)27-2)18-23-19(25-20(24-18)28-3)22-11-10-13-4-7-15(21)8-5-13/h4-9,12H,10-11H2,1-3H3,(H,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176641

(US9115121, 33)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccnc2ccccc12 Show InChI InChI=1S/C21H18FN5O/c1-28-21-26-19(17-11-13-23-18-5-3-2-4-16(17)18)25-20(27-21)24-12-10-14-6-8-15(22)9-7-14/h2-9,11,13H,10,12H2,1H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176601
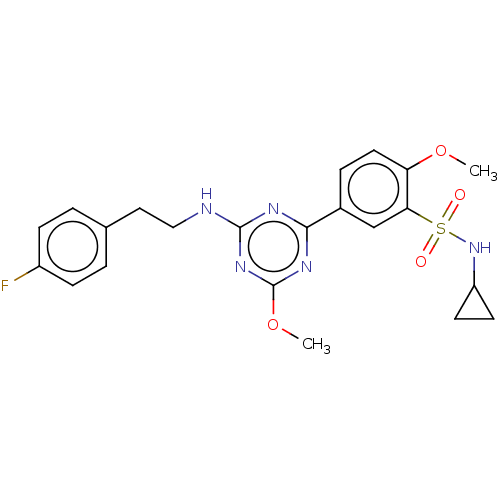
(US9115121, 12)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc(OC)c(c1)S(=O)(=O)NC1CC1 Show InChI InChI=1S/C22H24FN5O4S/c1-31-18-10-5-15(13-19(18)33(29,30)28-17-8-9-17)20-25-21(27-22(26-20)32-2)24-12-11-14-3-6-16(23)7-4-14/h3-7,10,13,17,28H,8-9,11-12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176615
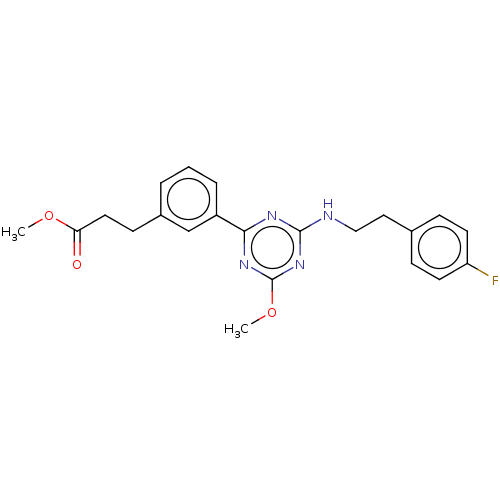
(US9115121, 6)Show SMILES COC(=O)CCc1cccc(c1)-c1nc(NCCc2ccc(F)cc2)nc(OC)n1 Show InChI InChI=1S/C22H23FN4O3/c1-29-19(28)11-8-16-4-3-5-17(14-16)20-25-21(27-22(26-20)30-2)24-13-12-15-6-9-18(23)10-7-15/h3-7,9-10,14H,8,11-13H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176655
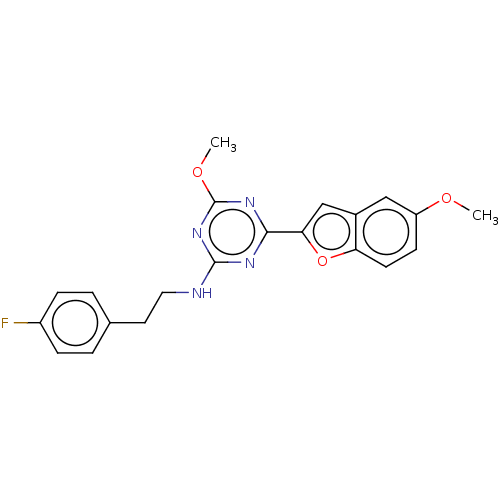
(US9115121, 48)Show SMILES COc1ccc2oc(cc2c1)-c1nc(NCCc2ccc(F)cc2)nc(OC)n1 Show InChI InChI=1S/C21H19FN4O3/c1-27-16-7-8-17-14(11-16)12-18(29-17)19-24-20(26-21(25-19)28-2)23-10-9-13-3-5-15(22)6-4-13/h3-8,11-12H,9-10H2,1-2H3,(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176657
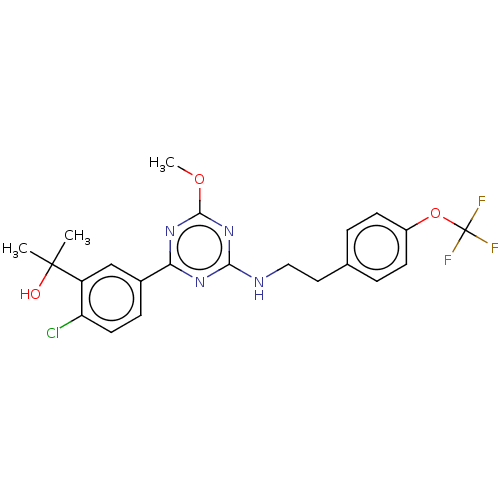
(US9115121, 50)Show SMILES COc1nc(NCCc2ccc(OC(F)(F)F)cc2)nc(n1)-c1ccc(Cl)c(c1)C(C)(C)O Show InChI InChI=1S/C22H22ClF3N4O3/c1-21(2,31)16-12-14(6-9-17(16)23)18-28-19(30-20(29-18)32-3)27-11-10-13-4-7-15(8-5-13)33-22(24,25)26/h4-9,12,31H,10-11H2,1-3H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176603
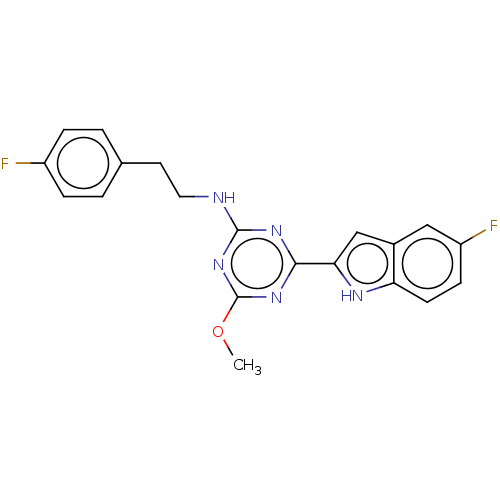
(US9115121, 20)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1cc2cc(F)ccc2[nH]1 Show InChI InChI=1S/C20H17F2N5O/c1-28-20-26-18(17-11-13-10-15(22)6-7-16(13)24-17)25-19(27-20)23-9-8-12-2-4-14(21)5-3-12/h2-7,10-11,24H,8-9H2,1H3,(H,23,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM176637
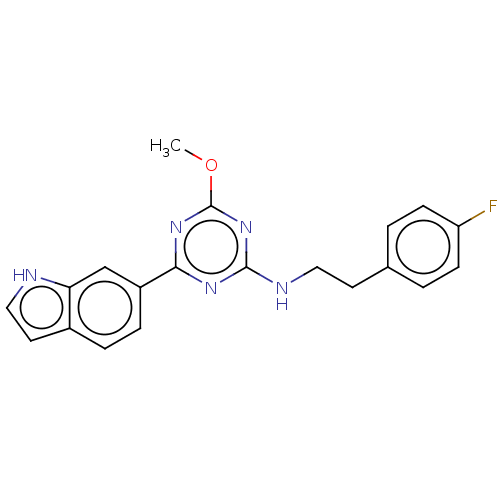
(US9115121, 29)Show SMILES COc1nc(NCCc2ccc(F)cc2)nc(n1)-c1ccc2cc[nH]c2c1 Show InChI InChI=1S/C20H18FN5O/c1-27-20-25-18(15-5-4-14-9-11-22-17(14)12-15)24-19(26-20)23-10-8-13-2-6-16(21)7-3-13/h2-7,9,11-12,22H,8,10H2,1H3,(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... |
US Patent US9115121 (2015)
BindingDB Entry DOI: 10.7270/Q23N225X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data