 Found 4513 hits with Last Name = 'smith' and Initial = 'p'
Found 4513 hits with Last Name = 'smith' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931
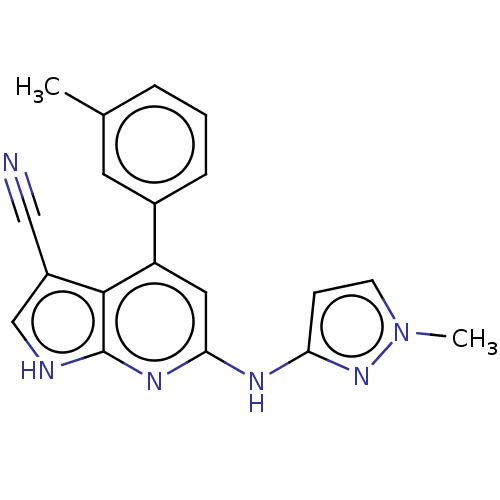
(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165931
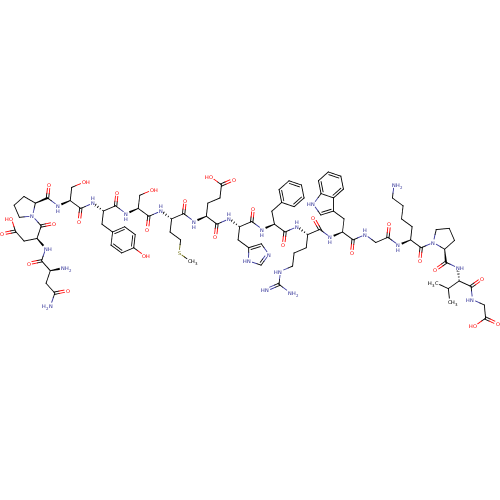
(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165935
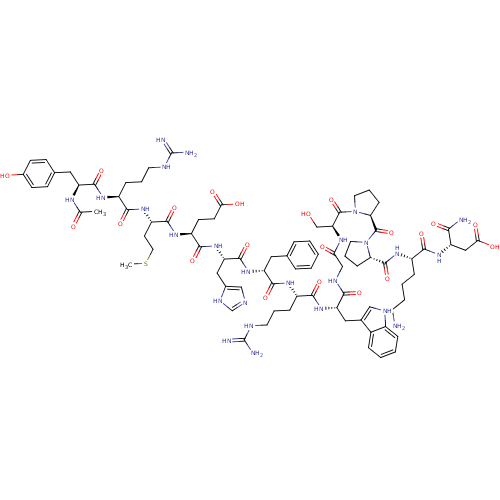
(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582714

(CHEMBL5075978)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cnn(C)c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931

(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165929
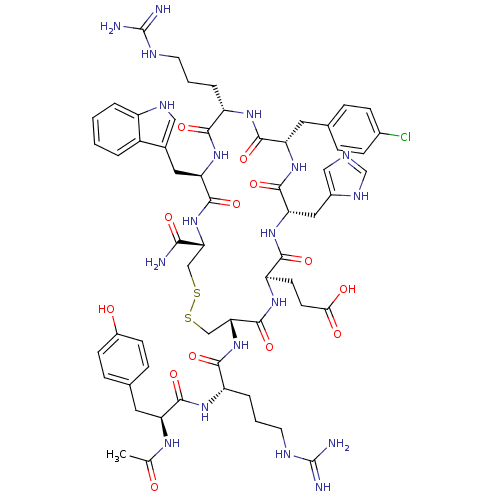
(Ac-YR[CEH(pCl-dF)RWC]-NH2 | CHEMBL415661)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H78ClN19O13S2/c1-31(81)72-43(22-33-12-16-37(82)17-13-33)54(89)73-41(9-5-21-69-60(65)66)52(87)80-48-29-95-94-28-47(50(62)85)79-56(91)45(24-34-26-70-39-7-3-2-6-38(34)39)77-51(86)40(8-4-20-68-59(63)64)74-55(90)44(23-32-10-14-35(61)15-11-32)76-57(92)46(25-36-27-67-30-71-36)78-53(88)42(75-58(48)93)18-19-49(83)84/h2-3,6-7,10-17,26-27,30,40-48,70,82H,4-5,8-9,18-25,28-29H2,1H3,(H2,62,85)(H,67,71)(H,72,81)(H,73,89)(H,74,90)(H,75,93)(H,76,92)(H,77,86)(H,78,88)(H,79,91)(H,80,87)(H,83,84)(H4,63,64,68)(H4,65,66,69)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412441
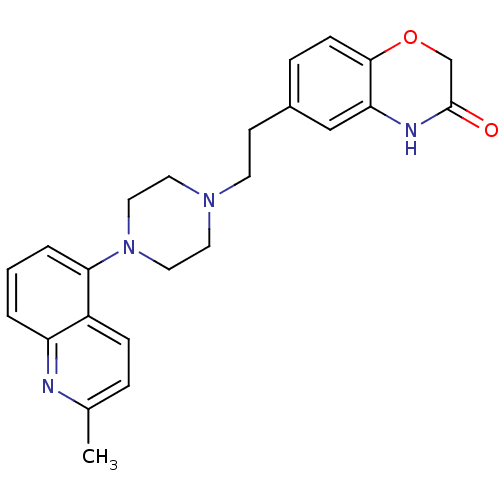
(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human cloned 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5581-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.110
BindingDB Entry DOI: 10.7270/Q2KH0PKZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413698
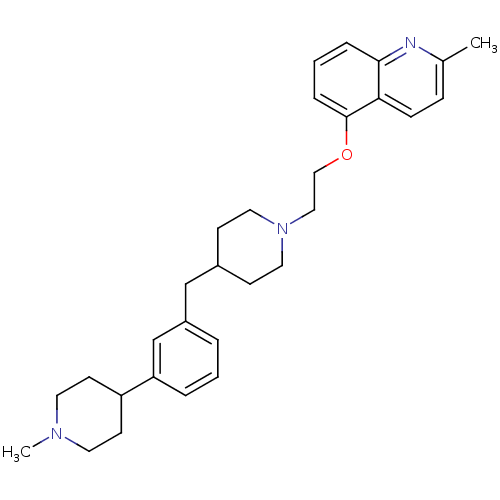
(CHEMBL459282)Show SMILES CN1CCC(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C30H39N3O/c1-23-9-10-28-29(31-23)7-4-8-30(28)34-20-19-33-17-11-24(12-18-33)21-25-5-3-6-27(22-25)26-13-15-32(2)16-14-26/h3-10,22,24,26H,11-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582715

(CHEMBL5091909)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cn[nH]c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165932
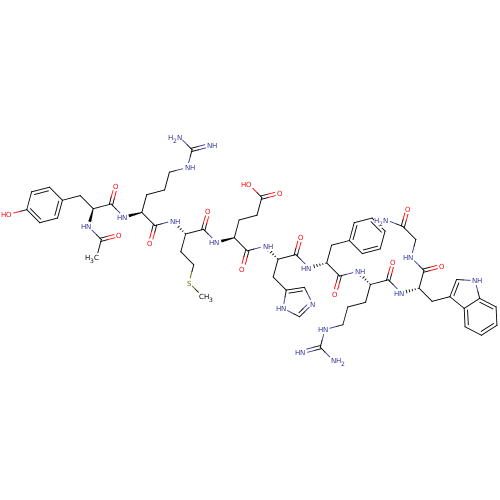
(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human cloned 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5581-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.110
BindingDB Entry DOI: 10.7270/Q2KH0PKZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412126
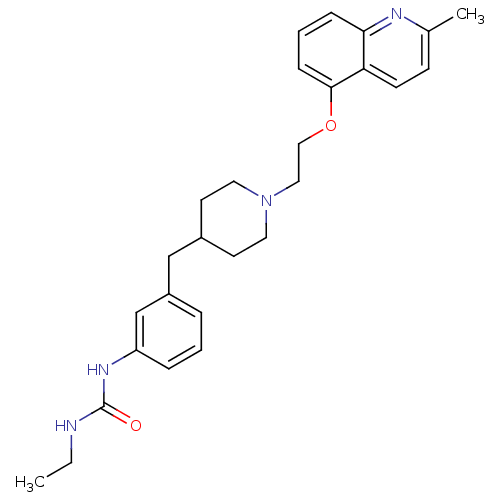
(CHEMBL525362)Show SMILES CCNC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H34N4O2/c1-3-28-27(32)30-23-7-4-6-22(19-23)18-21-12-14-31(15-13-21)16-17-33-26-9-5-8-25-24(26)11-10-20(2)29-25/h4-11,19,21H,3,12-18H2,1-2H3,(H2,28,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165927
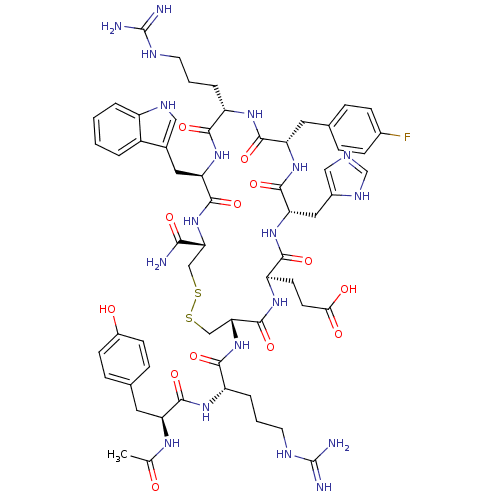
(Ac-YR[CEH(pF-dF)RWC]-NH2 | CHEMBL407809)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H78FN19O13S2/c1-31(81)72-43(22-33-12-16-37(82)17-13-33)54(89)73-41(9-5-21-69-60(65)66)52(87)80-48-29-95-94-28-47(50(62)85)79-56(91)45(24-34-26-70-39-7-3-2-6-38(34)39)77-51(86)40(8-4-20-68-59(63)64)74-55(90)44(23-32-10-14-35(61)15-11-32)76-57(92)46(25-36-27-67-30-71-36)78-53(88)42(75-58(48)93)18-19-49(83)84/h2-3,6-7,10-17,26-27,30,40-48,70,82H,4-5,8-9,18-25,28-29H2,1H3,(H2,62,85)(H,67,71)(H,72,81)(H,73,89)(H,74,90)(H,75,93)(H,76,92)(H,77,86)(H,78,88)(H,79,91)(H,80,87)(H,83,84)(H4,63,64,68)(H4,65,66,69)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582714

(CHEMBL5075978)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cnn(C)c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human GST tagged truncated LRRK2 G2019S mutant incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165924

(Ac-YR[CEH(d-2alpha-Nal)RWC]-NH2 | CHEMBL412523)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C64H81N19O13S2/c1-34(84)75-47(25-35-15-18-41(85)19-16-35)58(92)76-45(13-7-23-72-64(68)69)56(90)83-52-32-98-97-31-51(54(65)88)82-60(94)49(27-39-29-73-43-11-5-4-10-42(39)43)80-55(89)44(12-6-22-71-63(66)67)77-59(93)48(26-36-14-17-37-8-2-3-9-38(37)24-36)79-61(95)50(28-40-30-70-33-74-40)81-57(91)46(78-62(52)96)20-21-53(86)87/h2-5,8-11,14-19,24,29-30,33,44-52,73,85H,6-7,12-13,20-23,25-28,31-32H2,1H3,(H2,65,88)(H,70,74)(H,75,84)(H,76,92)(H,77,93)(H,78,96)(H,79,95)(H,80,89)(H,81,91)(H,82,94)(H,83,90)(H,86,87)(H4,66,67,71)(H4,68,69,72)/t44-,45-,46+,47-,48-,49+,50-,51+,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
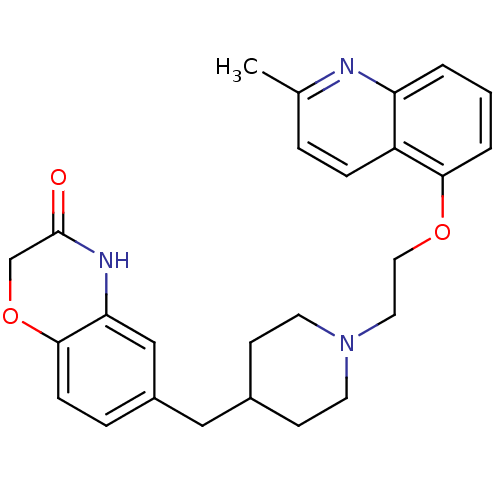
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412121
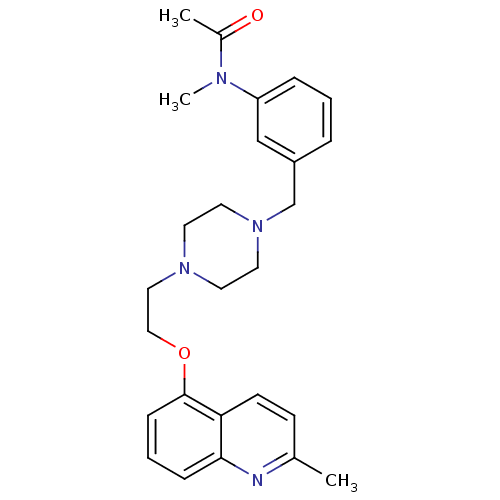
(CHEMBL506942)Show SMILES CN(C(C)=O)c1cccc(CN2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C26H32N4O2/c1-20-10-11-24-25(27-20)8-5-9-26(24)32-17-16-29-12-14-30(15-13-29)19-22-6-4-7-23(18-22)28(3)21(2)31/h4-11,18H,12-17,19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165936
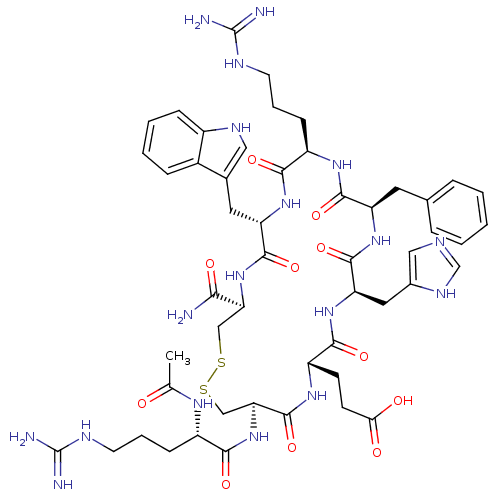
(Ac-dR[CEHdFRWC]-NH2 | CHEMBL267900)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34+,35-,36+,37-,38+,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412124
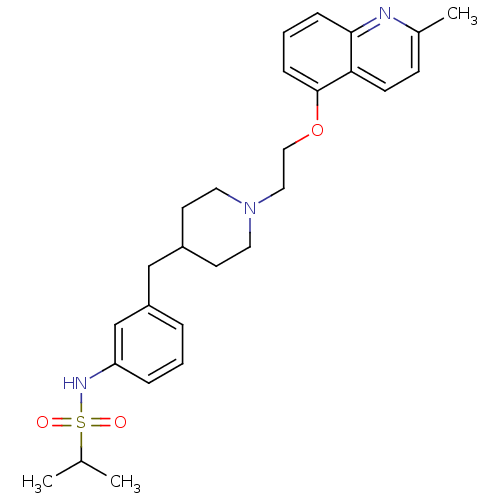
(CHEMBL495213)Show SMILES CC(C)S(=O)(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H35N3O3S/c1-20(2)34(31,32)29-24-7-4-6-23(19-24)18-22-12-14-30(15-13-22)16-17-33-27-9-5-8-26-25(27)11-10-21(3)28-26/h4-11,19-20,22,29H,12-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50477395
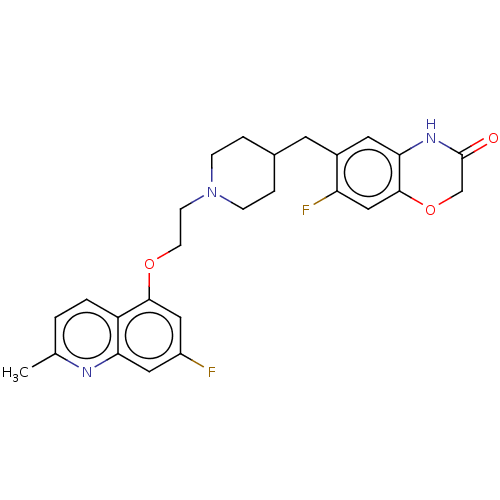
(CHEMBL238520)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc5NC(=O)COc5cc4F)CC3)cc(F)cc2n1 Show InChI InChI=1S/C26H27F2N3O3/c1-16-2-3-20-22(29-16)12-19(27)13-24(20)33-9-8-31-6-4-17(5-7-31)10-18-11-23-25(14-21(18)28)34-15-26(32)30-23/h2-3,11-14,17H,4-10,15H2,1H3,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50477399
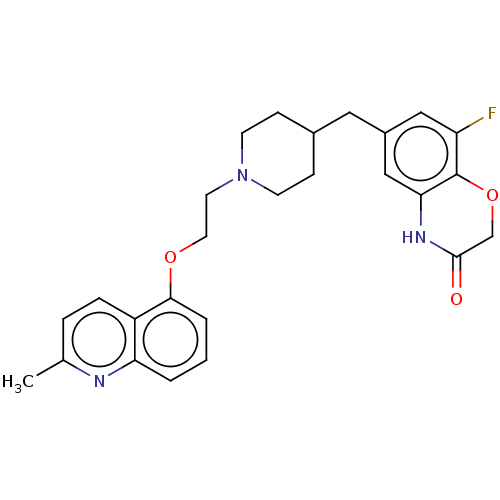
(CHEMBL241463)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc(F)c5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H28FN3O3/c1-17-5-6-20-22(28-17)3-2-4-24(20)32-12-11-30-9-7-18(8-10-30)13-19-14-21(27)26-23(15-19)29-25(31)16-33-26/h2-6,14-15,18H,7-13,16H2,1H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human cloned 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582715

(CHEMBL5091909)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cn[nH]c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human GST tagged truncated LRRK2 G2019S mutant incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582707
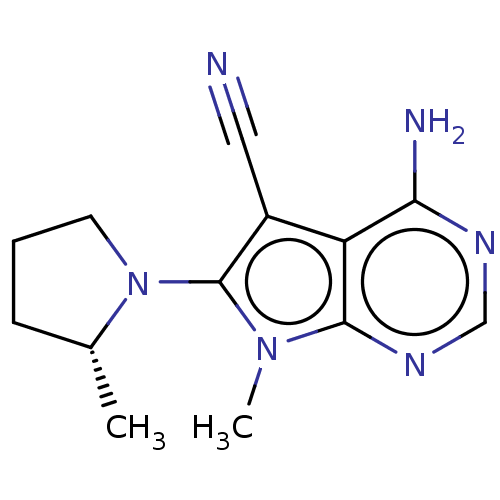
(CHEMBL5086513) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363990
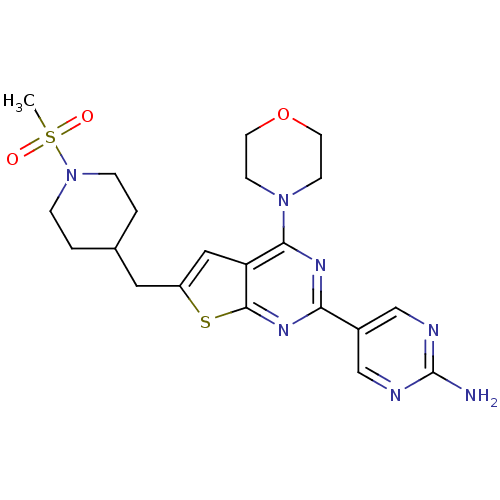
(CHEMBL1949915)Show SMILES CS(=O)(=O)N1CCC(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-4-2-14(3-5-28)10-16-11-17-19(27-6-8-31-9-7-27)25-18(26-20(17)32-16)15-12-23-21(22)24-13-15/h11-14H,2-10H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165941

(Ac-R[CEHdFRWC]-NH2 | CHEMBL408257)Show SMILES CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34-,35+,36-,37+,38-,39+,40+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254929
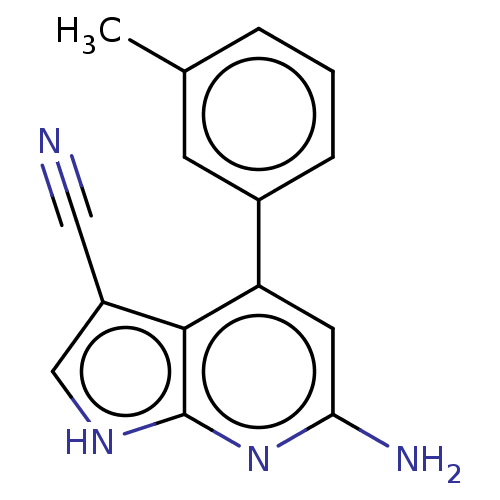
(US9499542, 12 | US9675594, 12)Show InChI InChI=1S/C15H12N4/c1-9-3-2-4-10(5-9)12-6-13(17)19-15-14(12)11(7-16)8-18-15/h2-6,8H,1H3,(H3,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50376788
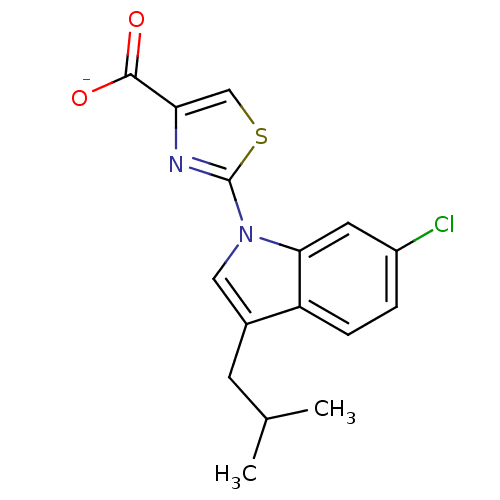
(CHEMBL257997)Show SMILES CC(C)Cc1cn(-c2nc(cs2)C([O-])=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C16H15ClN2O2S/c1-9(2)5-10-7-19(14-6-11(17)3-4-12(10)14)16-18-13(8-22-16)15(20)21/h3-4,6-9H,5H2,1-2H3,(H,20,21)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP1 receptor expressed in CHOK1 cells assessed as inhibition of PGE2-induced intracellular calcium mobilization by ... |
Bioorg Med Chem Lett 18: 2684-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.018
BindingDB Entry DOI: 10.7270/Q2PC338K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413691
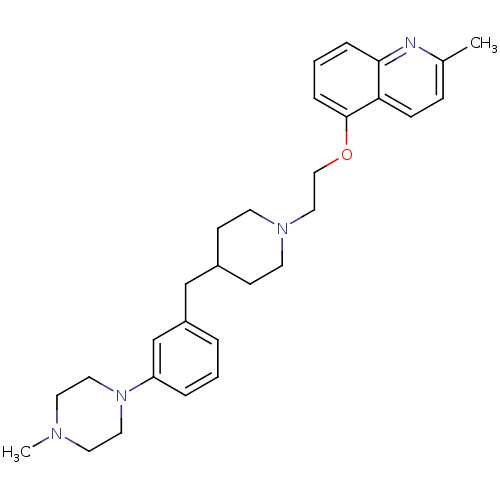
(CHEMBL458199)Show SMILES CN1CCN(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C29H38N4O/c1-23-9-10-27-28(30-23)7-4-8-29(27)34-20-19-32-13-11-24(12-14-32)21-25-5-3-6-26(22-25)33-17-15-31(2)16-18-33/h3-10,22,24H,11-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
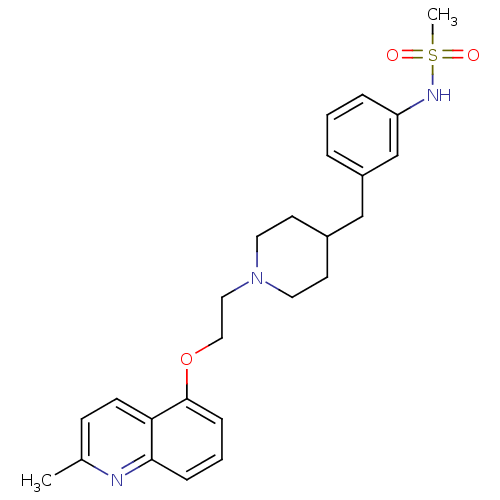
(Homo sapiens (Human)) | BDBM50412132
(CHEMBL497963)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(NS(C)(=O)=O)c4)CC3)cccc2n1 Show InChI InChI=1S/C25H31N3O3S/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-13-11-20(12-14-28)17-21-5-3-6-22(18-21)27-32(2,29)30/h3-10,18,20,27H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human cloned 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5581-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.110
BindingDB Entry DOI: 10.7270/Q2KH0PKZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
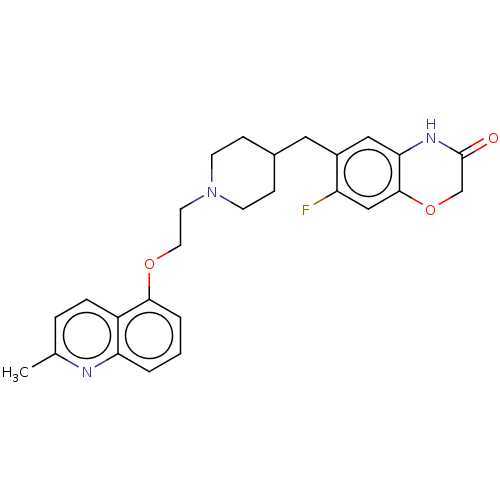
(Homo sapiens (Human)) | BDBM50477400
(CHEMBL239168)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc5NC(=O)COc5cc4F)CC3)cccc2n1 Show InChI InChI=1S/C26H28FN3O3/c1-17-5-6-20-22(28-17)3-2-4-24(20)32-12-11-30-9-7-18(8-10-30)13-19-14-23-25(15-21(19)27)33-16-26(31)29-23/h2-6,14-15,18H,7-13,16H2,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50192018

(CHEMBL3350037)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H](CO)[C@@H](C)O)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39+,40+,41+,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using [125I][Tyr3]-octreotide radioligand |
Bioorg Med Chem Lett 8: 1207-10 (1999)
BindingDB Entry DOI: 10.7270/Q2DB834V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
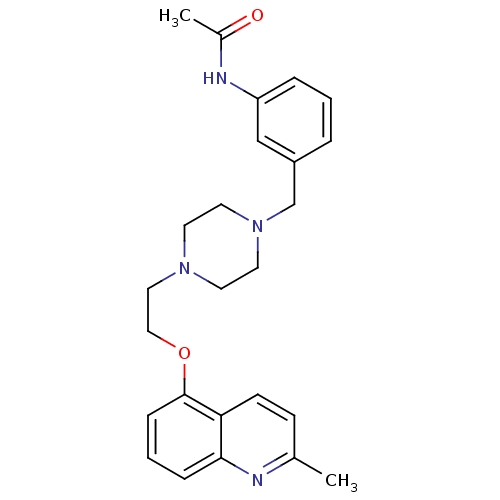
(Homo sapiens (Human)) | BDBM50412118
(CHEMBL494806)Show SMILES CC(=O)Nc1cccc(CN2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C25H30N4O2/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-11-13-29(14-12-28)18-21-5-3-6-22(17-21)27-20(2)30/h3-10,17H,11-16,18H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412134

(CHEMBL525712)Show SMILES CC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C26H31N3O2/c1-19-9-10-24-25(27-19)7-4-8-26(24)31-16-15-29-13-11-21(12-14-29)17-22-5-3-6-23(18-22)28-20(2)30/h3-10,18,21H,11-17H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412132

(CHEMBL497963)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(NS(C)(=O)=O)c4)CC3)cccc2n1 Show InChI InChI=1S/C25H31N3O3S/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-13-11-20(12-14-28)17-21-5-3-6-22(18-21)27-32(2,29)30/h3-10,18,20,27H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
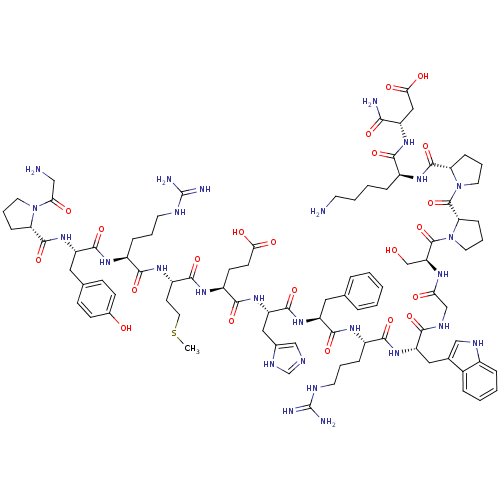
(Homo sapiens (Human)) | BDBM50165933
(CHEMBL428326 | GPYRMEHFRWGSPPKD-NH2)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C89H127N27O22S/c1-139-37-30-60(107-76(127)57(19-9-32-98-88(93)94)104-82(133)63(39-50-24-26-53(118)27-25-50)113-84(135)67-21-11-34-114(67)71(120)43-91)80(131)106-59(28-29-72(121)122)79(130)112-65(41-52-45-97-48-102-52)83(134)110-62(38-49-14-3-2-4-15-49)81(132)105-58(20-10-33-99-89(95)96)78(129)111-64(40-51-44-100-55-17-6-5-16-54(51)55)75(126)101-46-70(119)103-66(47-117)86(137)116-36-13-23-69(116)87(138)115-35-12-22-68(115)85(136)108-56(18-7-8-31-90)77(128)109-61(74(92)125)42-73(123)124/h2-6,14-17,24-27,44-45,48,56-69,100,117-118H,7-13,18-23,28-43,46-47,90-91H2,1H3,(H2,92,125)(H,97,102)(H,101,126)(H,103,119)(H,104,133)(H,105,132)(H,106,131)(H,107,127)(H,108,136)(H,109,128)(H,110,134)(H,111,129)(H,112,130)(H,113,135)(H,121,122)(H,123,124)(H4,93,94,98)(H4,95,96,99)/t56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165930
(Ac-YR[CEHdFRWC]-NH2 | CHEMBL264352)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H79N19O13S2/c1-32(80)71-43(24-34-15-17-37(81)18-16-34)54(88)72-41(14-8-22-68-60(64)65)52(86)79-48-30-94-93-29-47(50(61)84)78-56(90)45(25-35-27-69-39-12-6-5-11-38(35)39)76-51(85)40(13-7-21-67-59(62)63)73-55(89)44(23-33-9-3-2-4-10-33)75-57(91)46(26-36-28-66-31-70-36)77-53(87)42(74-58(48)92)19-20-49(82)83/h2-6,9-12,15-18,27-28,31,40-48,69,81H,7-8,13-14,19-26,29-30H2,1H3,(H2,61,84)(H,66,70)(H,71,80)(H,72,88)(H,73,89)(H,74,92)(H,75,91)(H,76,85)(H,77,87)(H,78,90)(H,79,86)(H,82,83)(H4,62,63,67)(H4,64,65,68)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165926

(Ac-YR[CEHdFRWC]SPPKD-NH2 | CHEMBL2373515)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O |wU:103.110,32.96,27.27,4.3,72.76,114.121,61.64,50.53,wD:110.118,94.99,16.16,123.130,36.37,82.87,(-1.2,-14.32,;-.65,-12.88,;.87,-12.64,;-1.63,-11.7,;-1.07,-10.25,;-2.62,-10.24,;-3.96,-9.47,;-5.2,-10.39,;-6.61,-9.78,;-6.79,-8.24,;-8.2,-7.63,;-5.55,-7.32,;-4.14,-7.93,;.45,-10.01,;1.41,-11.19,;.99,-8.56,;.02,-7.37,;1.51,-6.97,;3.03,-6.87,;3.9,-8.14,;5.44,-8.04,;6.29,-9.31,;7.83,-9.22,;5.61,-10.7,;-1.49,-7.62,;-2.04,-9.06,;-2.48,-6.43,;-1.91,-4.99,;-.42,-5.33,;1.11,-5.27,;2.58,-4.78,;3.85,-3.92,;4.85,-2.75,;5.49,-1.36,;5.74,.16,;7.28,.13,;5.56,1.69,;7.06,2.08,;8.14,3.18,;9.06,4.42,;10.51,3.93,;10.5,2.38,;11.63,1.35,;11.28,-.15,;9.82,-.62,;8.7,.44,;9.03,1.94,;4.98,3.11,;4.04,4.33,;5.12,5.43,;2.81,5.24,;3.54,6.6,;5.09,6.63,;5.82,7.98,;7.36,8,;8.1,9.36,;9.64,9.4,;7.3,10.67,;1.37,5.79,;-.16,5.95,;-.23,7.47,;-1.68,5.67,;-2.16,7.12,;-1.14,8.26,;.38,7.93,;1.39,9.08,;.91,10.56,;-.6,10.86,;-1.62,9.71,;-3.06,4.97,;-4.21,3.95,;-5.38,4.96,;-5.04,2.66,;-6.44,3.31,;-6.58,4.84,;-5.42,5.85,;-6.02,7.26,;-7.56,7.12,;-7.89,5.62,;-5.48,1.19,;-5.52,-.35,;-7.05,-.52,;-5.13,-1.84,;-6.56,-2.42,;-7.8,-1.48,;-9.21,-2.06,;-10.42,-1.11,;-9.42,-3.59,;-4.38,-3.17,;-3.27,-4.25,;-4.19,-5.49,;6.15,-3.59,;6.08,-5.12,;7.51,-2.88,;8.81,-3.7,;8.73,-5.24,;10.04,-6.07,;10.17,-3,;10.25,-1.46,;11.47,-3.71,;10.78,-2.02,;11.57,-.8,;12.9,-1.58,;12.83,-3.12,;14.14,-3.95,;14.07,-5.49,;15.34,-3.36,;14.94,-4.86,;15.22,-6.01,;16.72,-5.61,;16.79,-4.06,;18.17,-3.36,;18.22,-1.83,;19.46,-4.2,;20.82,-3.48,;20.9,-1.95,;22.25,-1.24,;22.33,.3,;23.7,1,;23.77,2.55,;22.11,-4.31,;22.04,-5.85,;23.49,-3.61,;24.79,-4.43,;24.71,-5.98,;26.01,-6.8,;27.51,-7.2,;25.94,-8.33,;26.15,-3.73,;27.45,-4.56,;26.22,-2.19,)| Show InChI InChI=1S/C83H115N25O21S2/c1-44(110)95-57(34-46-22-24-49(111)25-23-46)73(121)96-54(19-10-30-92-83(88)89)71(119)105-62-41-130-131-42-63(78(126)104-61(40-109)80(128)108-32-12-21-65(108)81(129)107-31-11-20-64(107)79(127)99-52(17-7-8-28-84)69(117)100-56(68(85)116)37-67(114)115)106-75(123)59(35-47-38-93-51-16-6-5-15-50(47)51)102-70(118)53(18-9-29-91-82(86)87)97-74(122)58(33-45-13-3-2-4-14-45)101-76(124)60(36-48-39-90-43-94-48)103-72(120)55(98-77(62)125)26-27-66(112)113/h2-6,13-16,22-25,38-39,43,52-65,93,109,111H,7-12,17-21,26-37,40-42,84H2,1H3,(H2,85,116)(H,90,94)(H,95,110)(H,96,121)(H,97,122)(H,98,125)(H,99,127)(H,100,117)(H,101,124)(H,102,118)(H,103,120)(H,104,126)(H,105,119)(H,106,123)(H,112,113)(H,114,115)(H4,86,87,91)(H4,88,89,92)/t52-,53-,54?,55+,56-,57-,58-,59+,60-,61-,62+,63+,64-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165932

(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165936

(Ac-dR[CEHdFRWC]-NH2 | CHEMBL267900)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34+,35-,36+,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165926

(Ac-YR[CEHdFRWC]SPPKD-NH2 | CHEMBL2373515)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O |wU:103.110,32.96,27.27,4.3,72.76,114.121,61.64,50.53,wD:110.118,94.99,16.16,123.130,36.37,82.87,(-1.2,-14.32,;-.65,-12.88,;.87,-12.64,;-1.63,-11.7,;-1.07,-10.25,;-2.62,-10.24,;-3.96,-9.47,;-5.2,-10.39,;-6.61,-9.78,;-6.79,-8.24,;-8.2,-7.63,;-5.55,-7.32,;-4.14,-7.93,;.45,-10.01,;1.41,-11.19,;.99,-8.56,;.02,-7.37,;1.51,-6.97,;3.03,-6.87,;3.9,-8.14,;5.44,-8.04,;6.29,-9.31,;7.83,-9.22,;5.61,-10.7,;-1.49,-7.62,;-2.04,-9.06,;-2.48,-6.43,;-1.91,-4.99,;-.42,-5.33,;1.11,-5.27,;2.58,-4.78,;3.85,-3.92,;4.85,-2.75,;5.49,-1.36,;5.74,.16,;7.28,.13,;5.56,1.69,;7.06,2.08,;8.14,3.18,;9.06,4.42,;10.51,3.93,;10.5,2.38,;11.63,1.35,;11.28,-.15,;9.82,-.62,;8.7,.44,;9.03,1.94,;4.98,3.11,;4.04,4.33,;5.12,5.43,;2.81,5.24,;3.54,6.6,;5.09,6.63,;5.82,7.98,;7.36,8,;8.1,9.36,;9.64,9.4,;7.3,10.67,;1.37,5.79,;-.16,5.95,;-.23,7.47,;-1.68,5.67,;-2.16,7.12,;-1.14,8.26,;.38,7.93,;1.39,9.08,;.91,10.56,;-.6,10.86,;-1.62,9.71,;-3.06,4.97,;-4.21,3.95,;-5.38,4.96,;-5.04,2.66,;-6.44,3.31,;-6.58,4.84,;-5.42,5.85,;-6.02,7.26,;-7.56,7.12,;-7.89,5.62,;-5.48,1.19,;-5.52,-.35,;-7.05,-.52,;-5.13,-1.84,;-6.56,-2.42,;-7.8,-1.48,;-9.21,-2.06,;-10.42,-1.11,;-9.42,-3.59,;-4.38,-3.17,;-3.27,-4.25,;-4.19,-5.49,;6.15,-3.59,;6.08,-5.12,;7.51,-2.88,;8.81,-3.7,;8.73,-5.24,;10.04,-6.07,;10.17,-3,;10.25,-1.46,;11.47,-3.71,;10.78,-2.02,;11.57,-.8,;12.9,-1.58,;12.83,-3.12,;14.14,-3.95,;14.07,-5.49,;15.34,-3.36,;14.94,-4.86,;15.22,-6.01,;16.72,-5.61,;16.79,-4.06,;18.17,-3.36,;18.22,-1.83,;19.46,-4.2,;20.82,-3.48,;20.9,-1.95,;22.25,-1.24,;22.33,.3,;23.7,1,;23.77,2.55,;22.11,-4.31,;22.04,-5.85,;23.49,-3.61,;24.79,-4.43,;24.71,-5.98,;26.01,-6.8,;27.51,-7.2,;25.94,-8.33,;26.15,-3.73,;27.45,-4.56,;26.22,-2.19,)| Show InChI InChI=1S/C83H115N25O21S2/c1-44(110)95-57(34-46-22-24-49(111)25-23-46)73(121)96-54(19-10-30-92-83(88)89)71(119)105-62-41-130-131-42-63(78(126)104-61(40-109)80(128)108-32-12-21-65(108)81(129)107-31-11-20-64(107)79(127)99-52(17-7-8-28-84)69(117)100-56(68(85)116)37-67(114)115)106-75(123)59(35-47-38-93-51-16-6-5-15-50(47)51)102-70(118)53(18-9-29-91-82(86)87)97-74(122)58(33-45-13-3-2-4-14-45)101-76(124)60(36-48-39-90-43-94-48)103-72(120)55(98-77(62)125)26-27-66(112)113/h2-6,13-16,22-25,38-39,43,52-65,93,109,111H,7-12,17-21,26-37,40-42,84H2,1H3,(H2,85,116)(H,90,94)(H,95,110)(H,96,121)(H,97,122)(H,98,125)(H,99,127)(H,100,117)(H,101,124)(H,102,118)(H,103,120)(H,104,126)(H,105,119)(H,106,123)(H,112,113)(H,114,115)(H4,86,87,91)(H4,88,89,92)/t52-,53-,54?,55+,56-,57-,58-,59+,60-,61-,62+,63+,64-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data