

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50073120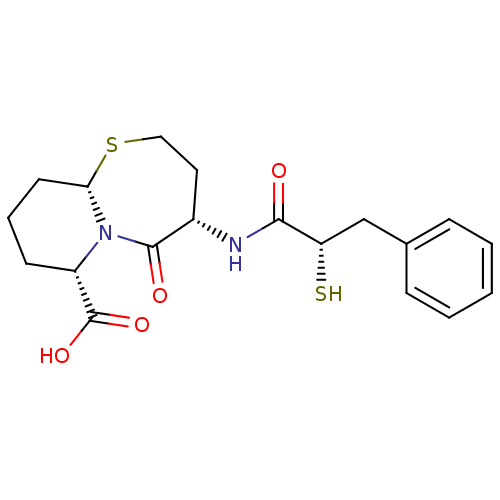 ((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human fully glycosylated ACE N-terminal domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins follo... | J Med Chem 61: 10141-10154 (2018) Article DOI: 10.1021/acs.jmedchem.8b01309 BindingDB Entry DOI: 10.7270/Q2862K4R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50073120 ((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human fully glycosylated ACE C-terminal domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins follo... | J Med Chem 61: 10141-10154 (2018) Article DOI: 10.1021/acs.jmedchem.8b01309 BindingDB Entry DOI: 10.7270/Q2862K4R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50367879 (LISINOPRIL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01924 BindingDB Entry DOI: 10.7270/Q2FT8QZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334215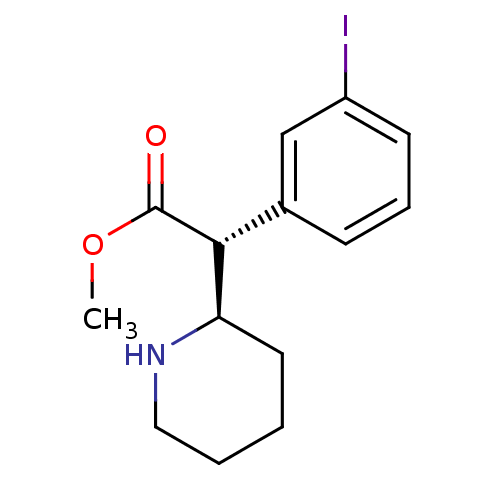 ((+/-)-threo-3-Iodomethylphenidate | CHEMBL1641691) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50367879 (LISINOPRIL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01924 BindingDB Entry DOI: 10.7270/Q2FT8QZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50189452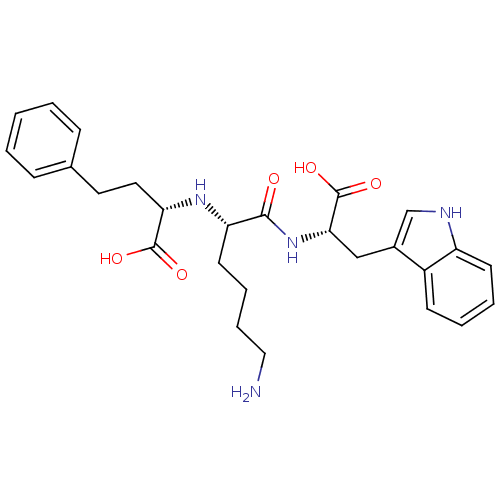 ((S)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01924 BindingDB Entry DOI: 10.7270/Q2FT8QZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50010477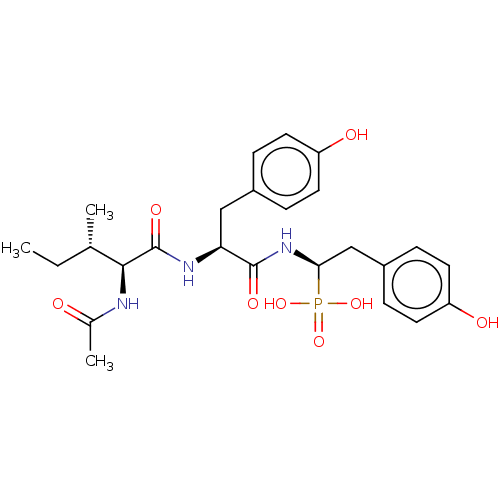 (CHEMBL1233799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry Curated by ChEMBL | Assay Description Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate | ACS Med Chem Lett 5: 346-51 (2014) Article DOI: 10.1021/ml4004588 BindingDB Entry DOI: 10.7270/Q20C4X8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50010479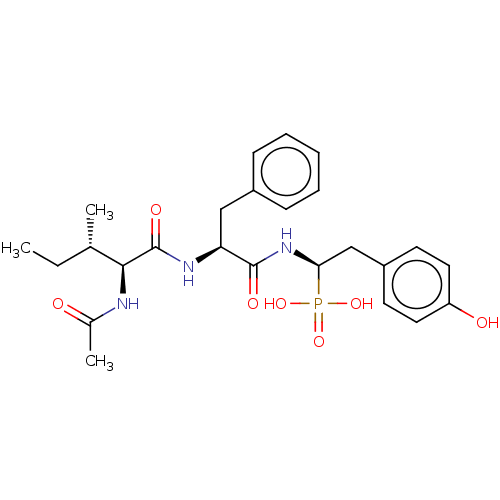 (CHEMBL3264010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry Curated by ChEMBL | Assay Description Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate | ACS Med Chem Lett 5: 346-51 (2014) Article DOI: 10.1021/ml4004588 BindingDB Entry DOI: 10.7270/Q20C4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50327148 ((R)-methyl 2-(4-iodophenyl)-2-((R)-piperidin-2-yl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50085452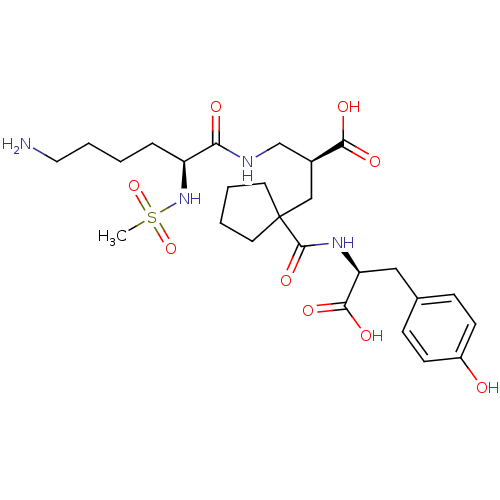 ((S)-2-[((S)-6-Amino-2-methanesulfonylamino-hexanoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human testis ACE C-domain lacking unique O-glycosylated region, transmembrane anchor and cytoplasmic tail expressed in CHO cells using ... | J Med Chem 63: 5488-5500 (2020) Article DOI: 10.1021/acs.jmedchem.0c00441 BindingDB Entry DOI: 10.7270/Q2D2223Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410097 (US10370370, Compound 2-P1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50010478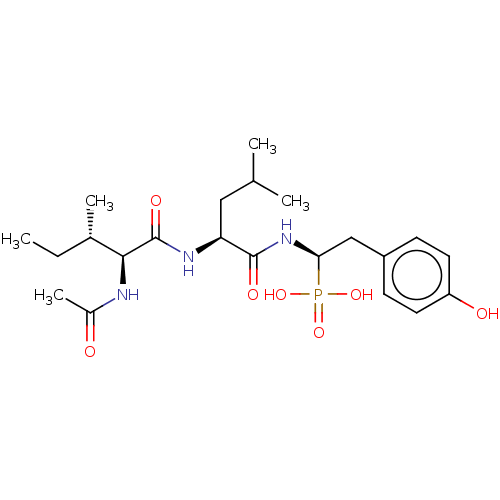 (CHEMBL3264009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry Curated by ChEMBL | Assay Description Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate | ACS Med Chem Lett 5: 346-51 (2014) Article DOI: 10.1021/ml4004588 BindingDB Entry DOI: 10.7270/Q20C4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50062915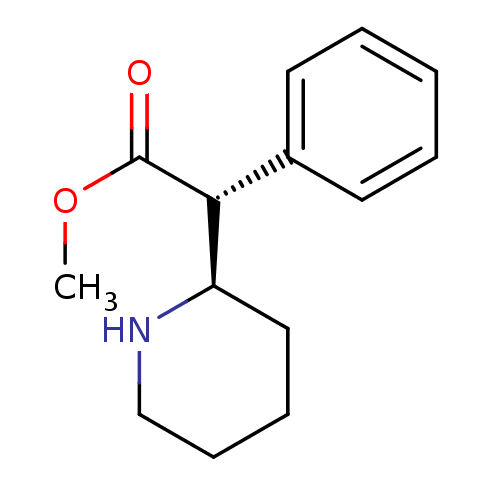 (CHEMBL827 | METHYLPHENIDATE | methyl (2R)-phenyl[(...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410113 (US10370370, Compound 9-P2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410139 (US10370370, Compound (R)-31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322432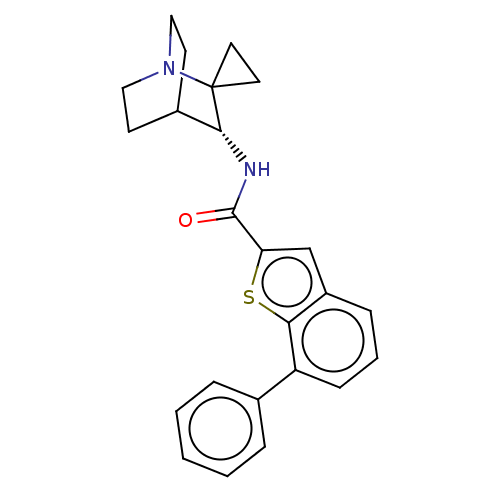 ((R)-7-phenyl-N-(1'-azaspiro[cyclopropane-1,2'-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410109 (US10370370, Compound 7-P2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410099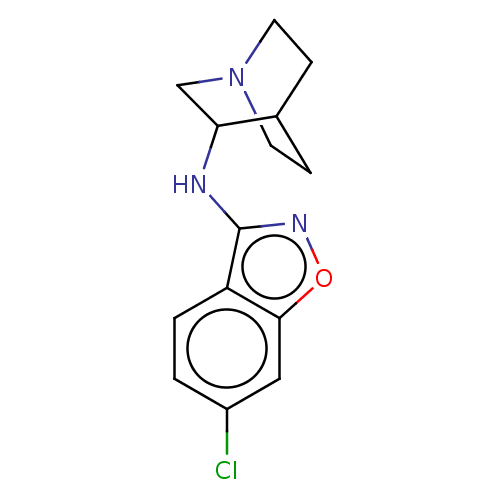 (US10370370, Compound 3-P1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50010478 (CHEMBL3264009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry Curated by ChEMBL | Assay Description Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate | ACS Med Chem Lett 5: 346-51 (2014) Article DOI: 10.1021/ml4004588 BindingDB Entry DOI: 10.7270/Q20C4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50583450 (CHEMBL5077796) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01924 BindingDB Entry DOI: 10.7270/Q2FT8QZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322406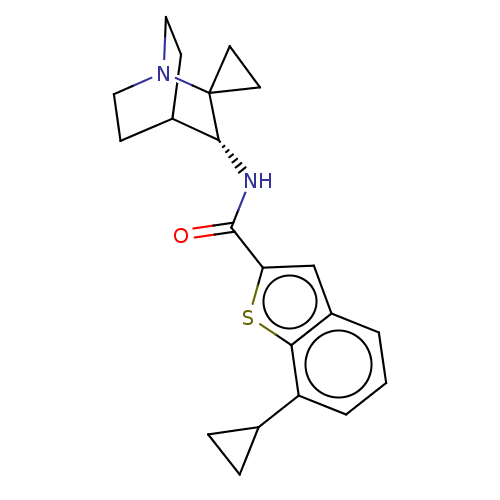 ((R)-7-cyclopropyl-N-(1'-azaspiro[cyclopropane-1,2'...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322274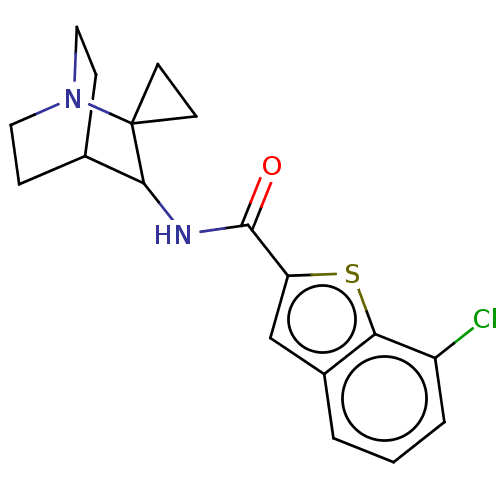 (7-chloro-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410112 (US10370370, Compound 9-P1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 43.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410152 (US10370370, Compound (R)-43) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410148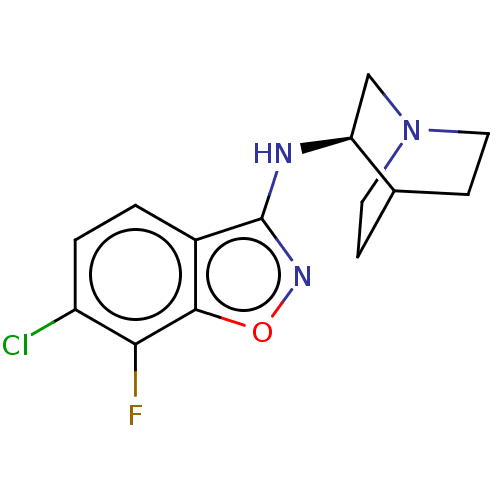 (US10370370, Compound (R)-41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322452 ((R)-6-chloro-7-methyl-N-(1'-azaspiro[cyclopropane-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322274 (7-chloro-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322387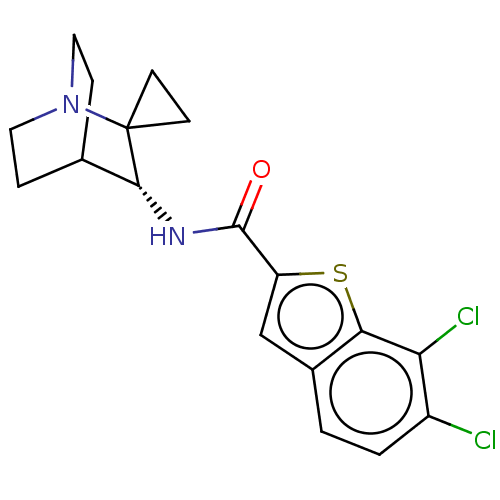 ((R)-6,7-dichloro-N-(1'-azaspiro[cyclopropane-1,2'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 54.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410101 (US10370370, Compound (R)-3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322402 ((R)-7-methoxy-N-(1'-azaspiro[cyclopropane-1,2'-bic...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410112 (US10370370, Compound 9-P1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 59.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322271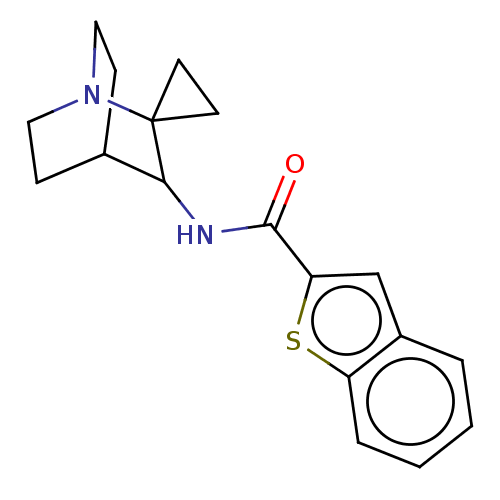 (N-(1''-azaspiro[cyclopropane-1,2''-bicyclo[2.2.2]o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410144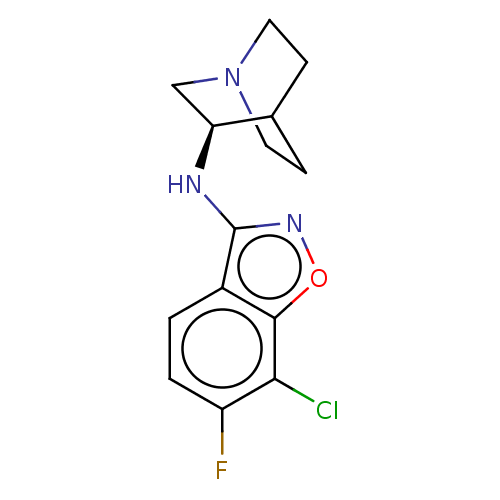 (US10370370, Compound (R)-35) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410096 (US10370370, Compound 1-P2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410130 (US10370370, Compound (R)-23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322275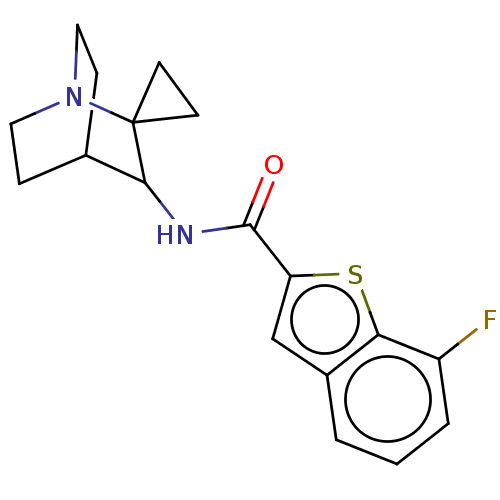 (7-fluoro-N-(1''-azaspiro[cyclopropane-1,2''-bicycl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50010477 (CHEMBL1233799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry Curated by ChEMBL | Assay Description Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate | ACS Med Chem Lett 5: 346-51 (2014) Article DOI: 10.1021/ml4004588 BindingDB Entry DOI: 10.7270/Q20C4X8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322440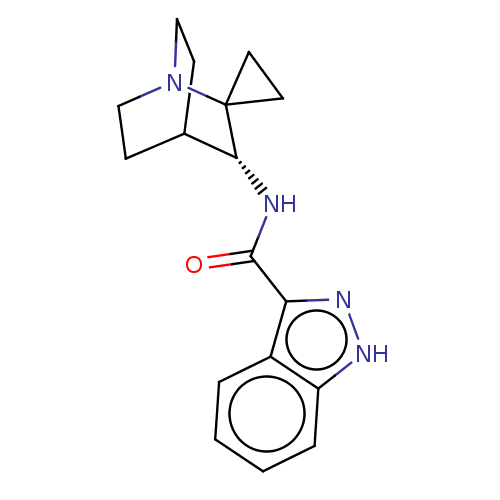 ((R)-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[2.2.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322427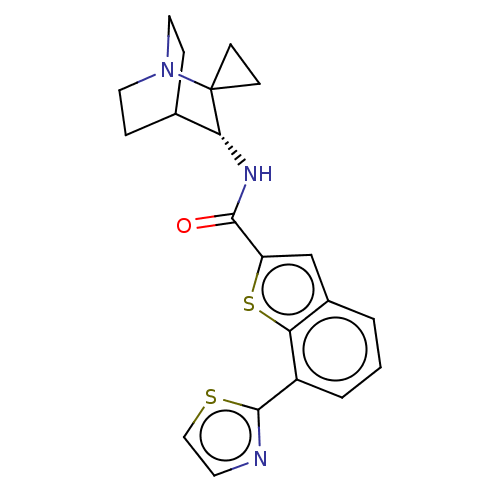 ((R)-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[2.2.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322385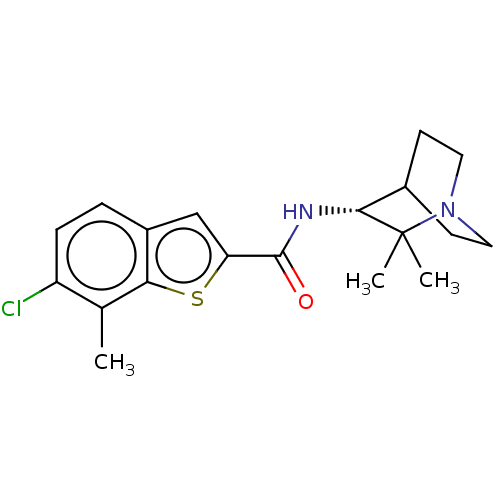 ((R)-6-chloro-N-(2,2-dimethylquinuclidin-3-yl)-7-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322280 (N-(1''-azaspiro[cyclopropane-1,2''-bicyclo[2.2.2]o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322368 ((R)-N-(2,2-dimethylquinuclidin-3-yl)-7-phenylbenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322451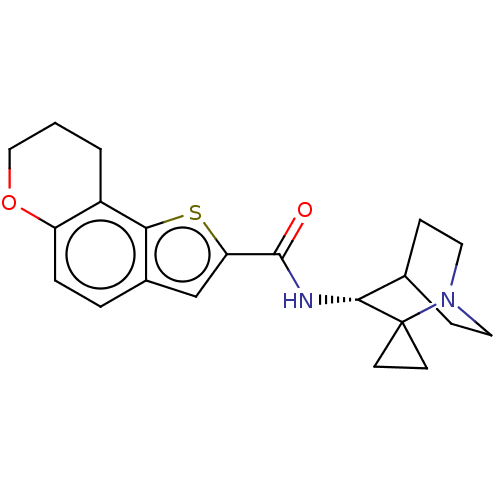 ((R)-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[2.2.2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50583449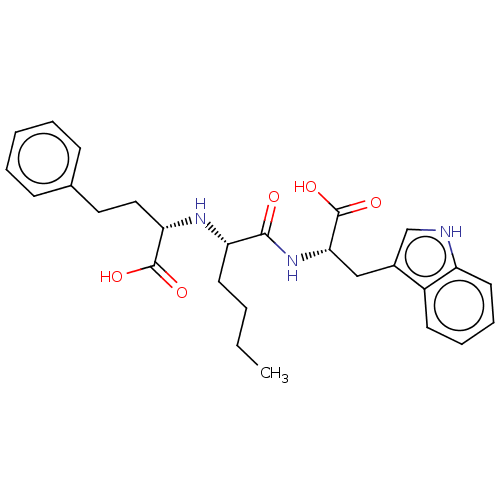 (CHEMBL5075705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01924 BindingDB Entry DOI: 10.7270/Q2FT8QZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322283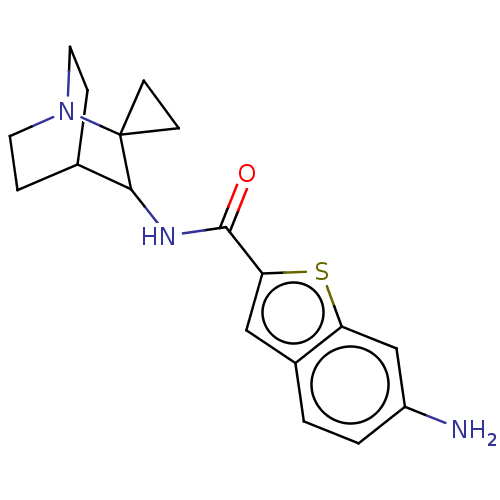 (6-amino-N-(1''-azaspiro[cyclopropane-1,2''-bicyclo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322401 ((R)-7-cyano-N-(1'-azaspiro[cyclopropane-1,2'-bicyc...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322193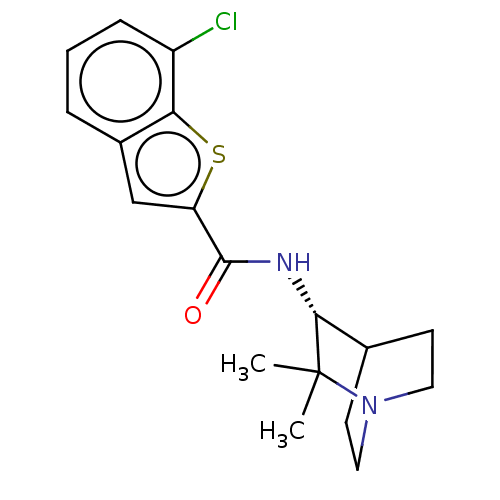 ((R)-7-chloro-N-(2,2-dimethylquinuclidin-3-yl)benzo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322430 ((R)-7-(tert-butyl)-N-(1'-azaspiro[cyclopropane-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322277 (N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[2.2.2]oct...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322275 (7-fluoro-N-(1''-azaspiro[cyclopropane-1,2''-bicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 889 total ) | Next | Last >> |