

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50329257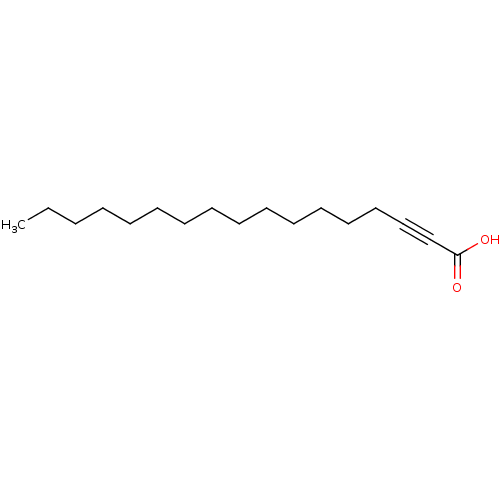 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis inhA | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Non-competitive inhibition of Plasmodium falciparum FabI using NADH as cofactor by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Non-competitive inhibition of Plasmodium falciparum FabI using crotonyl-CoA as substrate by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxyacyl-[acyl-carrier-protein] dehydratase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Competitive inhibition of Plasmodium falciparum FabZ using crotonyl-CoA as substrate by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Non-competitive inhibition of Plasmodium falciparum FabG using NADH as cofactor by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Competitive inhibition of Plasmodium falciparum FabG using acetoacetyl-CoA as substrate by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3414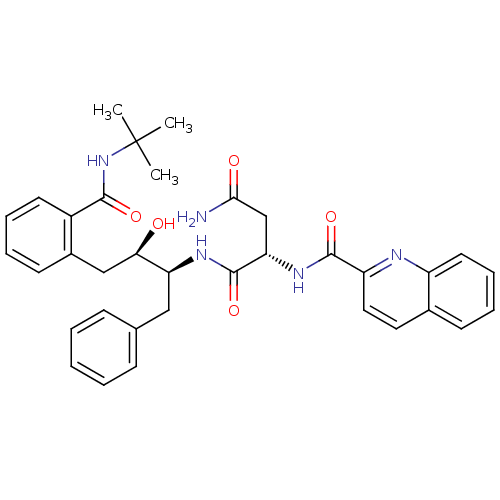 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282667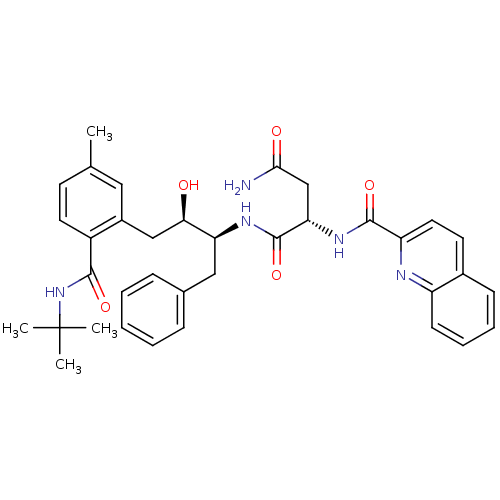 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(2-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282663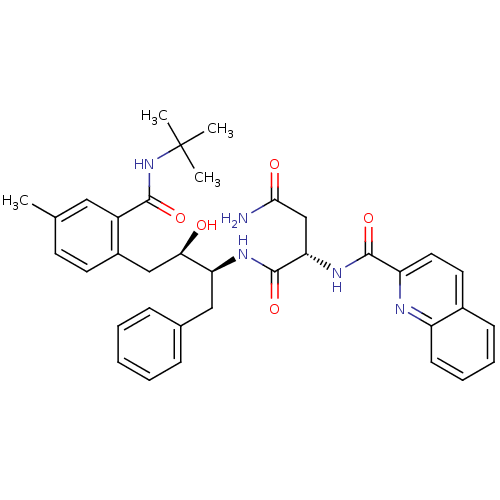 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(2-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282665 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(1-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282664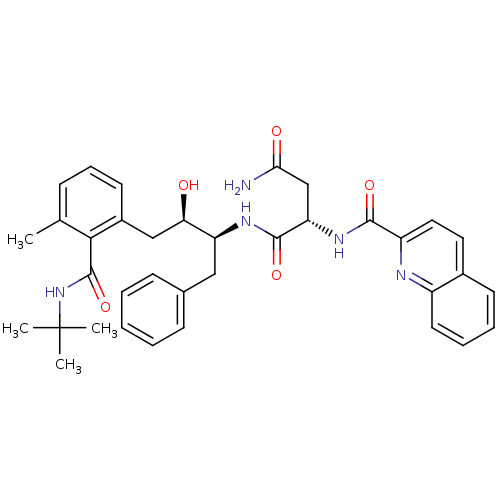 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(2-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282668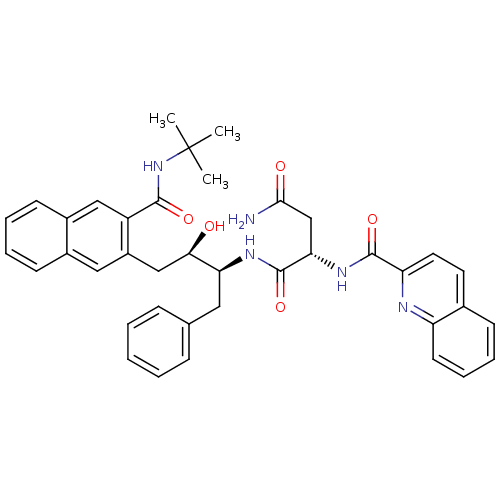 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(3-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099216 ((2S,3S)-1-[2-(3-Benzyloxycarbonyl-phenyl)-acetyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099216 ((2S,3S)-1-[2-(3-Benzyloxycarbonyl-phenyl)-acetyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282666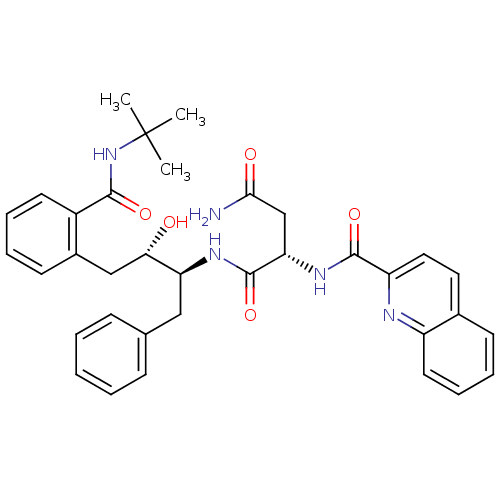 ((S)-N*1*-[(1S,2S)-1-Benzyl-3-(2-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099216 ((2S,3S)-1-[2-(3-Benzyloxycarbonyl-phenyl)-acetyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099216 ((2S,3S)-1-[2-(3-Benzyloxycarbonyl-phenyl)-acetyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099212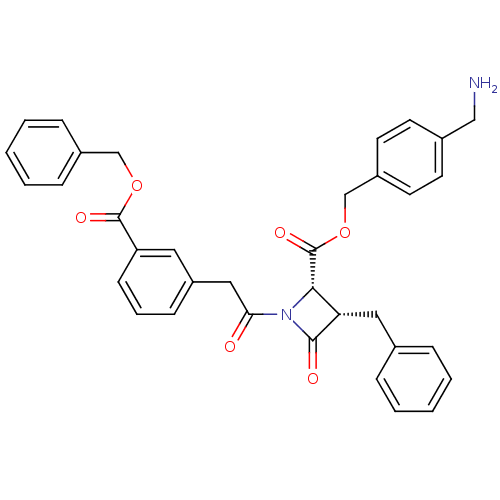 (3-Benzyl-1-[2-(3-benzyloxycarbonyl-phenyl)-acetyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50289630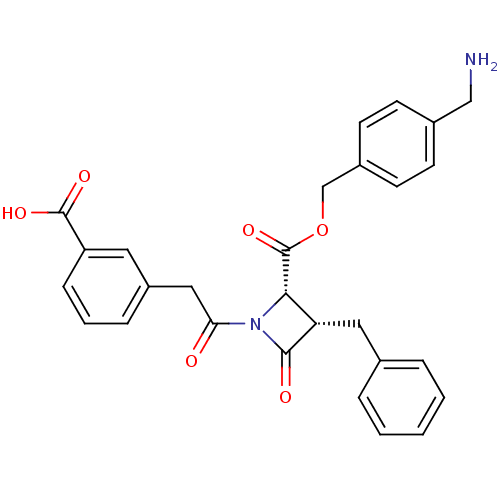 ((2S,3S)-3-Benzyl-1-[2-(3-carboxy-phenyl)-acetyl]-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099218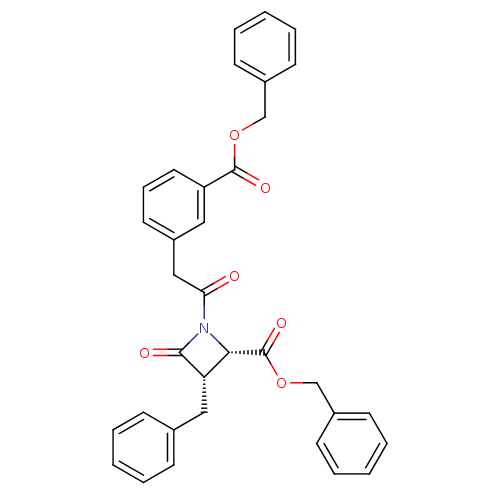 ((2S,3S)-3-Benzyl-1-[2-(3-benzyloxycarbonyl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099218 ((2S,3S)-3-Benzyl-1-[2-(3-benzyloxycarbonyl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099214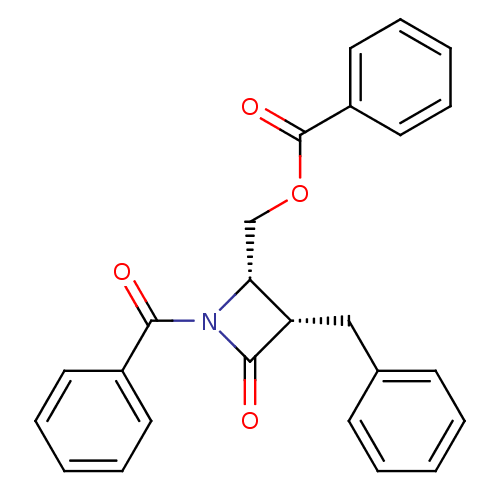 (Benzoic acid 1-benzoyl-3-benzyl-4-oxo-azetidin-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099215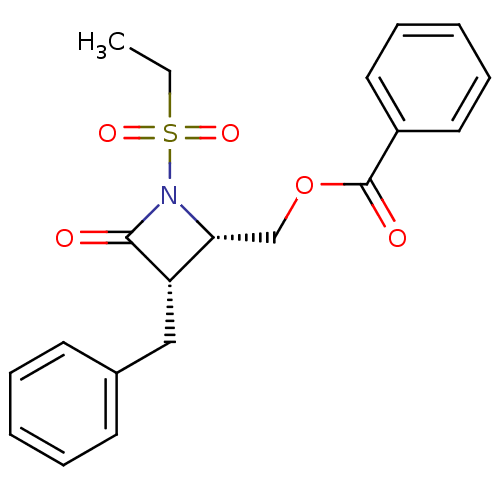 (Benzoic acid 3-benzyl-1-ethanesulfonyl-4-oxo-azeti...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099209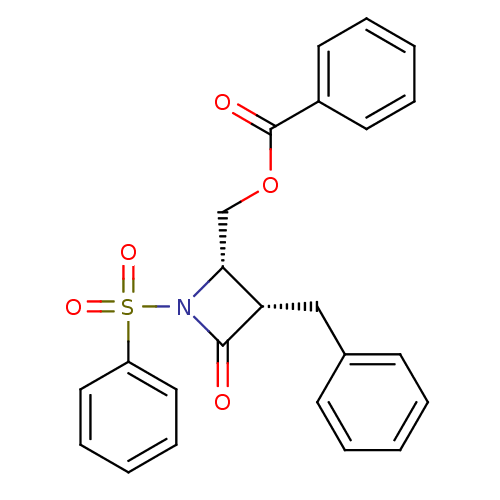 (Benzoic acid 1-benzenesulfonyl-3-benzyl-4-oxo-azet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099221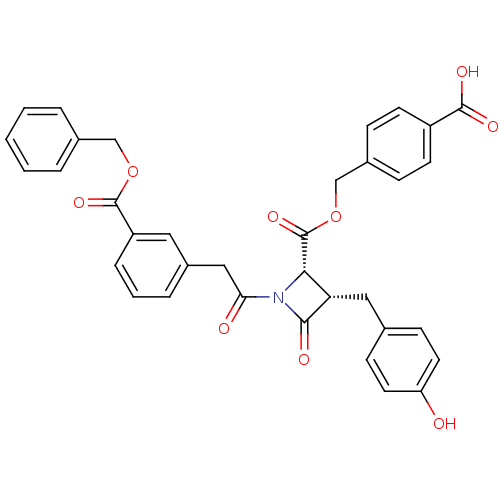 ((2S,3S)-1-[2-(3-Benzyloxycarbonyl-phenyl)-acetyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099221 ((2S,3S)-1-[2-(3-Benzyloxycarbonyl-phenyl)-acetyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099211 (Benzoic acid 3-benzyl-4-oxo-1-(toluene-4-sulfonyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099213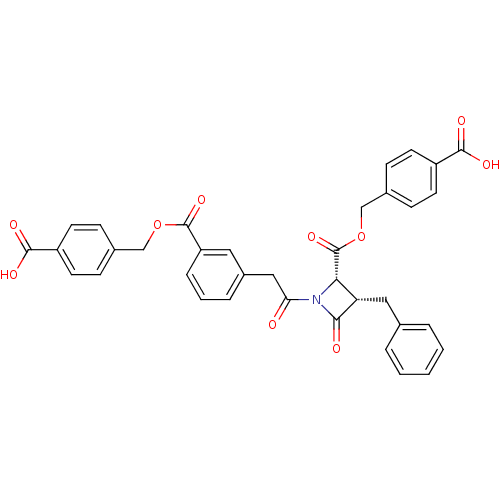 ((2S,3S)-3-Benzyl-1-{2-[3-(4-carboxy-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099213 ((2S,3S)-3-Benzyl-1-{2-[3-(4-carboxy-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099205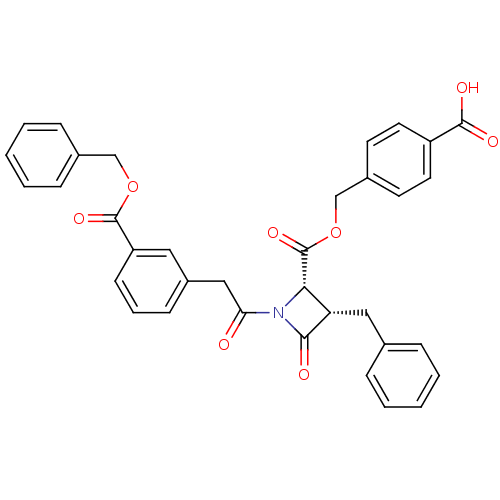 ((2S,3S)-3-Benzyl-1-[2-(3-benzyloxycarbonyl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099205 ((2S,3S)-3-Benzyl-1-[2-(3-benzyloxycarbonyl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099219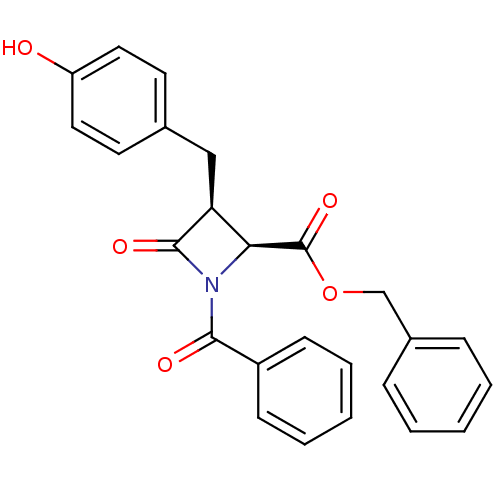 (1-Benzoyl-3-(4-hydroxy-benzyl)-4-oxo-azetidine-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099220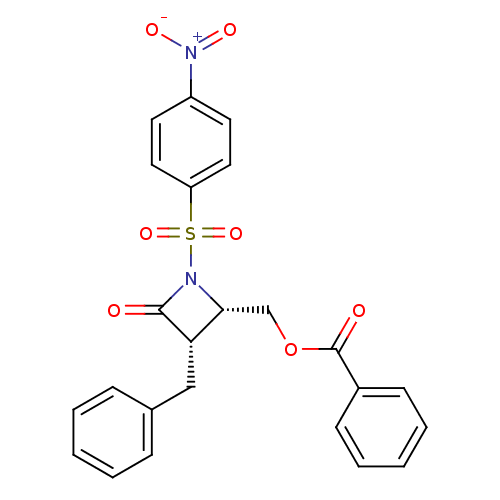 (Benzoic acid 3-benzyl-1-(4-nitro-benzenesulfonyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099217 (1-Benzoyl-3-benzyl-4-oxo-azetidine-2-carboxylic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099206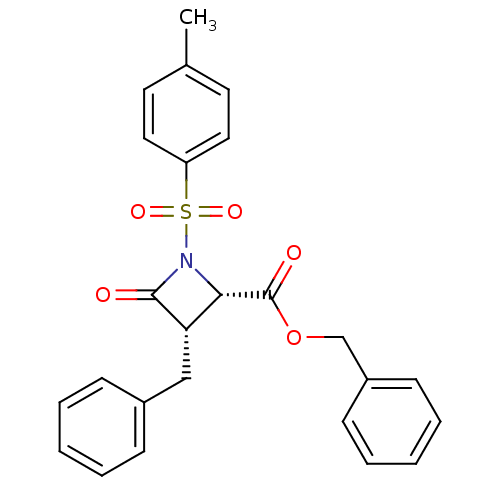 (3-Benzyl-4-oxo-1-(toluene-4-sulfonyl)-azetidine-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099208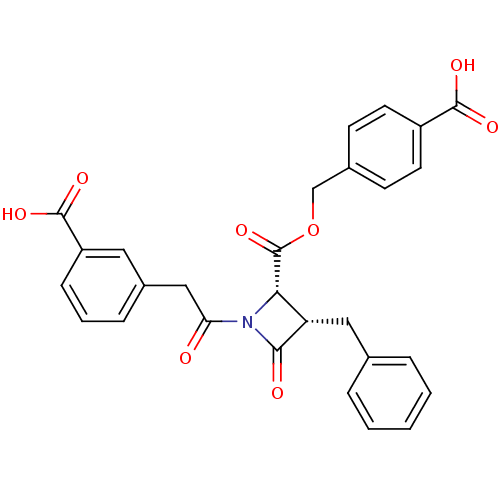 ((2S,3S)-3-Benzyl-1-[2-(3-carboxy-phenyl)-acetyl]-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099208 ((2S,3S)-3-Benzyl-1-[2-(3-carboxy-phenyl)-acetyl]-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of 50% of the cleavage of para-nitroanilide from MeO-Suc-Arg-Pro-Tyr-pNA.HCl by Prostate specific antigen PSA | Bioorg Med Chem Lett 7: 1689-1694 (1997) Article DOI: 10.1016/S0960-894X(97)00285-0 BindingDB Entry DOI: 10.7270/Q2GF0TGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099210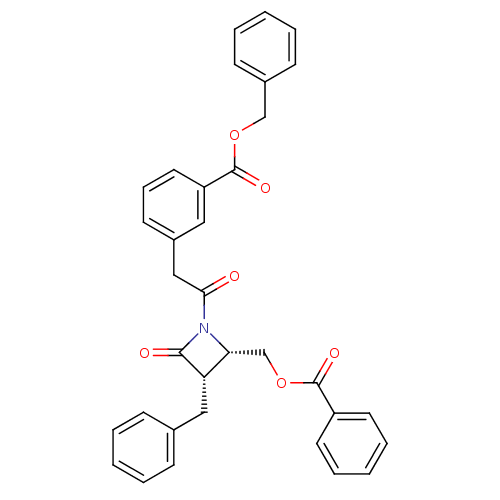 (3-[2-(2-Benzoyloxymethyl-3-benzyl-4-oxo-azetidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50099207 (CHEMBL36215 | Terephthalic acid mono-(1-benzoyl-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Prostate specific antigen PSA (Serine Protease) | J Med Chem 44: 1491-508 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50439266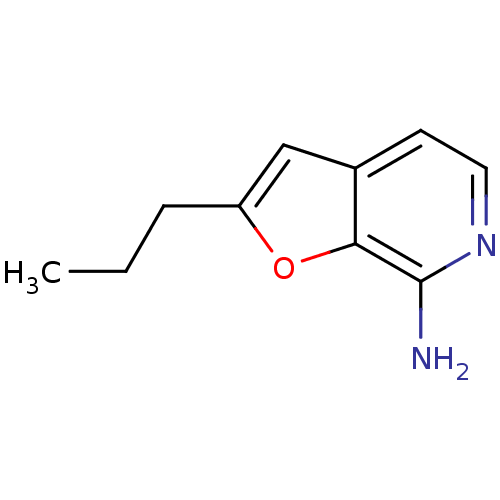 (CHEMBL2419471) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human TLR8 expressed in HEK293 cells co-transfected with MD2 and sAP assessed as induction of NF-kappaB activity by reporter gene... | J Med Chem 56: 6871-85 (2013) Article DOI: 10.1021/jm400694d BindingDB Entry DOI: 10.7270/Q2FB54CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50439267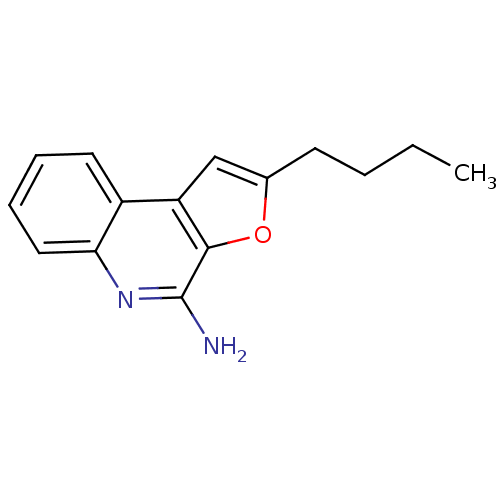 (CHEMBL2419475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human TLR8 expressed in HEK293 cells co-transfected with MD2 and sAP assessed as induction of NF-kappaB activity by reporter gene... | J Med Chem 56: 6871-85 (2013) Article DOI: 10.1021/jm400694d BindingDB Entry DOI: 10.7270/Q2FB54CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50439265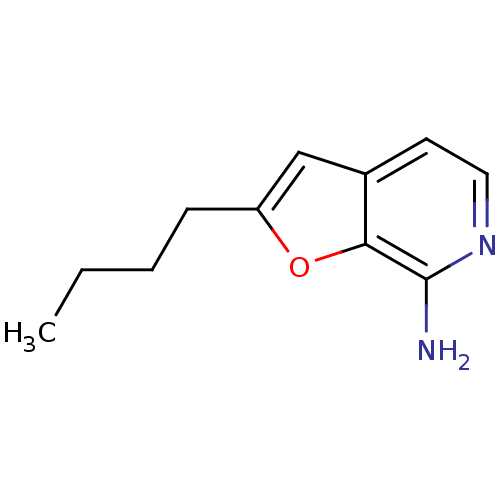 (CHEMBL2419274) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.62E+4 | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human TLR8 expressed in HEK293 cells co-transfected with MD2 and sAP assessed as induction of NF-kappaB activity by reporter gene... | J Med Chem 56: 6871-85 (2013) Article DOI: 10.1021/jm400694d BindingDB Entry DOI: 10.7270/Q2FB54CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||