

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM578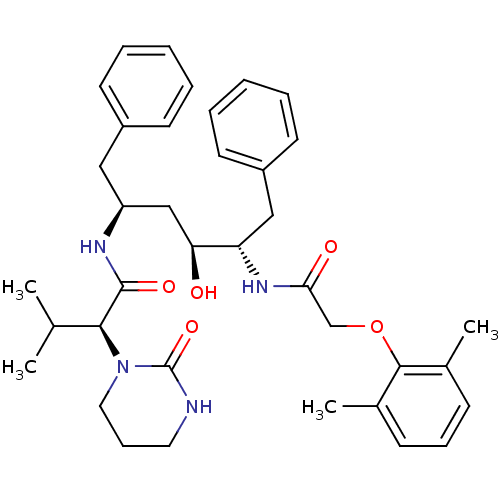 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0190 | -63.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580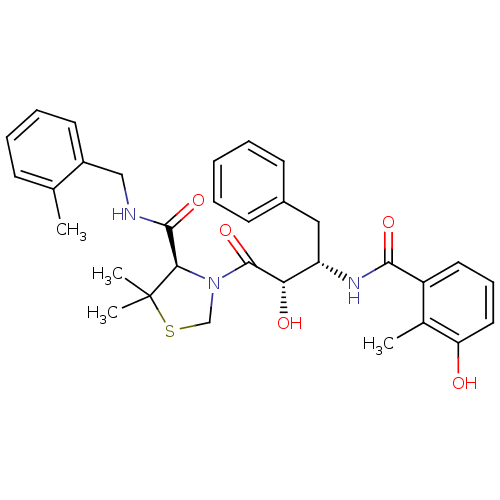 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0210 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0500 | -61.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50153971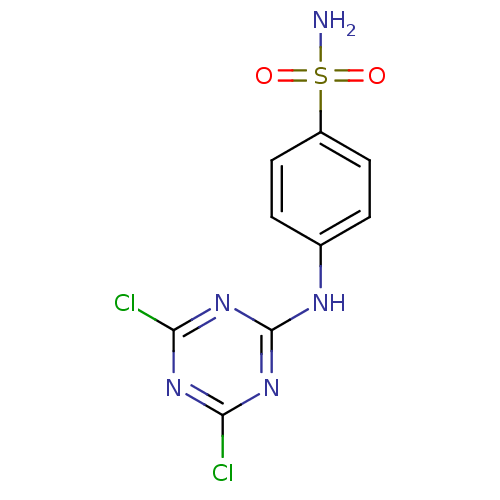 (4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method | Bioorg Med Chem 19: 3105-19 (2011) Article DOI: 10.1016/j.bmc.2011.04.005 BindingDB Entry DOI: 10.7270/Q2FF3SQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.170 | -58.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,I543V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -57.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A] (Homo sapiens (Human)) | BDBM210938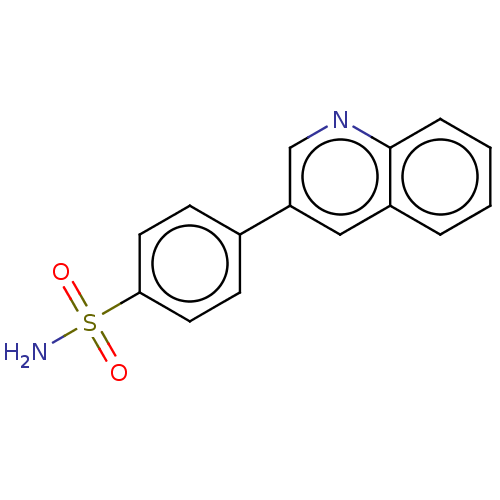 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A] (Homo sapiens (Human)) | BDBM210935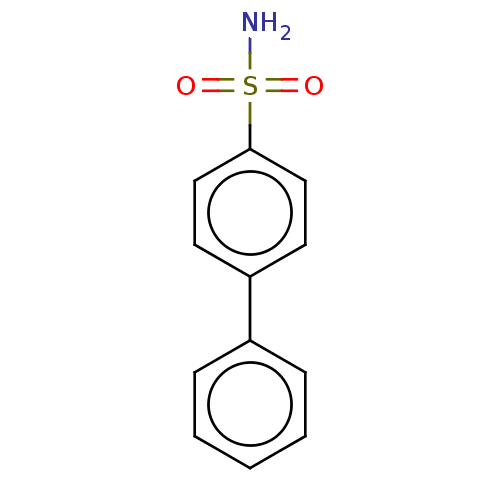 (4-(phenyl)-bezenesulfonamide (4a)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50292219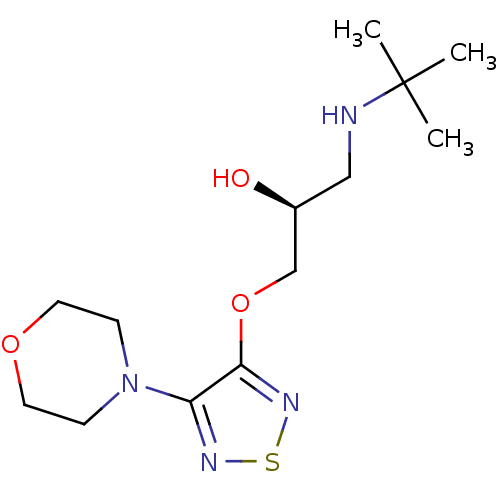 ((-)-3-morpholino-4-(3-tert-butylamino-2-hydroxypro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Displacement of [3H]-CGP12177 from human beta2 ADR expressed in HEK293T cell membrane after 90 mins by scintillation counting | J Med Chem 61: 5380-5394 (2018) Article DOI: 10.1021/acs.jmedchem.8b00625 BindingDB Entry DOI: 10.7270/Q2XS5XX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.240 | -57.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50562972 (CHEMBL4799997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CAH7 expressed in Escherichia coli BL21 (DE3) by stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02077 BindingDB Entry DOI: 10.7270/Q2C25158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11625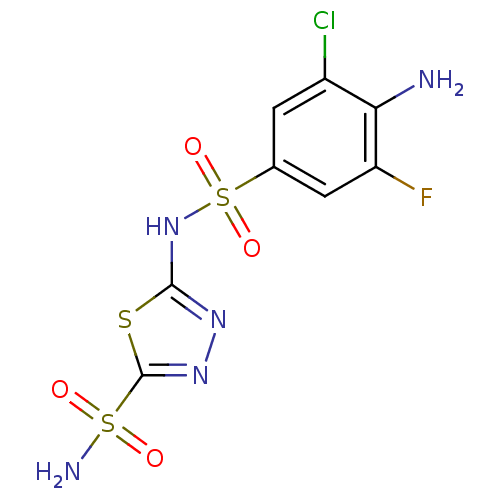 (2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem Lett 20: 4376-81 (2010) Article DOI: 10.1016/j.bmcl.2010.06.082 BindingDB Entry DOI: 10.7270/Q2C24XCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50153971 (4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method | Bioorg Med Chem 19: 3105-19 (2011) Article DOI: 10.1016/j.bmc.2011.04.005 BindingDB Entry DOI: 10.7270/Q2FF3SQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50547697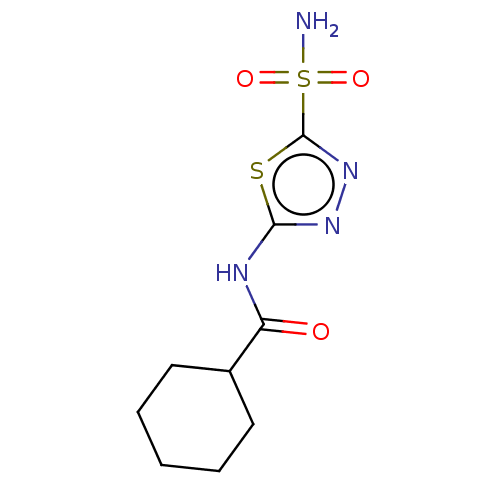 (CHEMBL4739913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50331834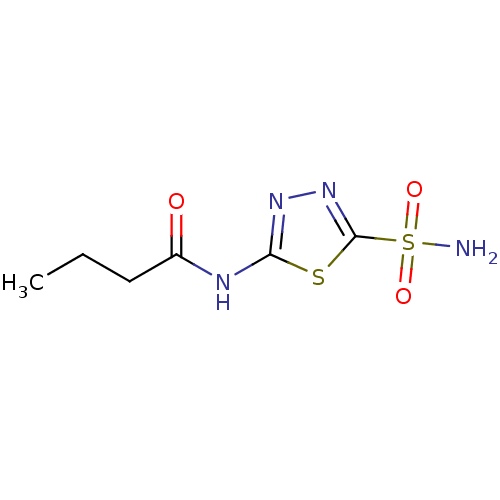 (5-butyramido-2-sulfamoyl-1,3,4-thiadiazole | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.400 | -55.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM16668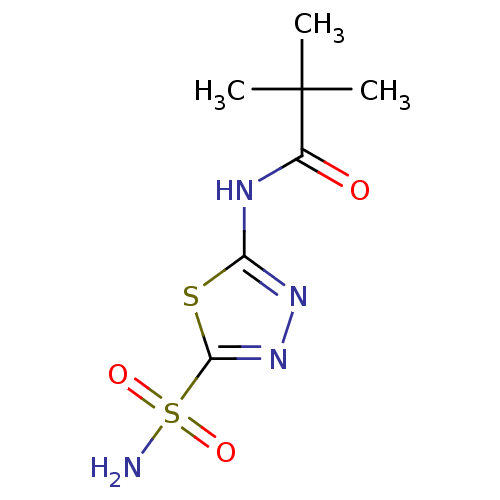 (2,2-dimethyl-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50331835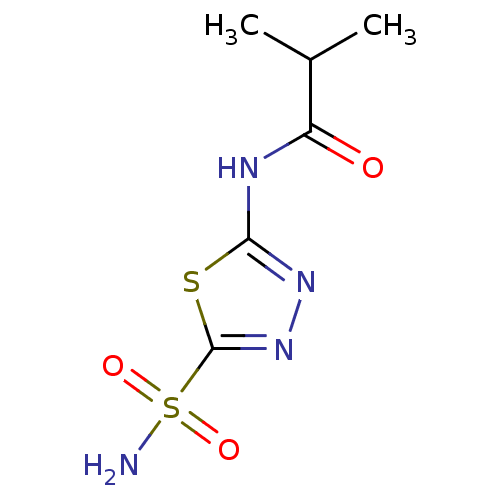 (5-(2-methyl-propylamido)-2-sulfamoyl-1,3,4-thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50564139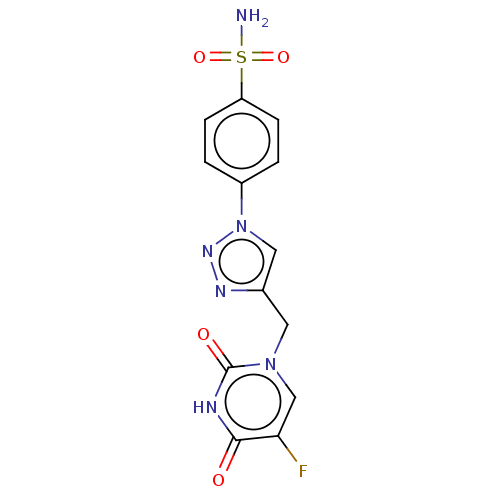 (CHEMBL4785560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA9 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112112 BindingDB Entry DOI: 10.7270/Q2J96B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.480 | -55.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50334361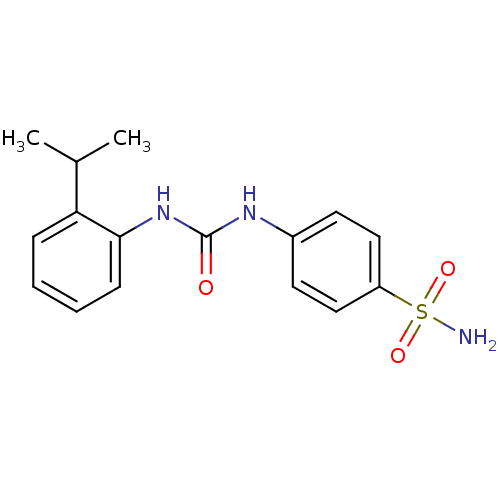 (4-(3-(2-isopropylphenyl)ureido)benzenesulfonamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,L579M] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,M535I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50331834 (5-butyramido-2-sulfamoyl-1,3,4-thiadiazole | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.520 | -55.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50547697 (CHEMBL4739913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,I573V,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.560 | -54.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50331835 (5-(2-methyl-propylamido)-2-sulfamoyl-1,3,4-thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50564133 (CHEMBL4786026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112112 BindingDB Entry DOI: 10.7270/Q2J96B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50540944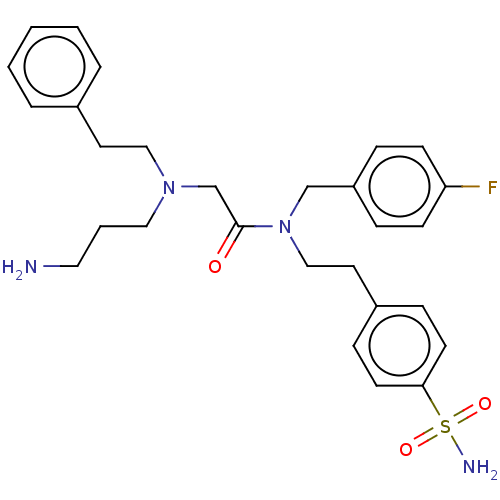 (CHEMBL4637053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Firenze Curated by ChEMBL | Assay Description Inhibition of human CA12 preincubated for 15 mins by stopped-flow CO2 hydration kinetic assay based Cheng-Prusoff equation analysis | J Med Chem 63: 7422-7444 (2020) Article DOI: 10.1021/acs.jmedchem.0c00733 BindingDB Entry DOI: 10.7270/Q2BV7M64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM210937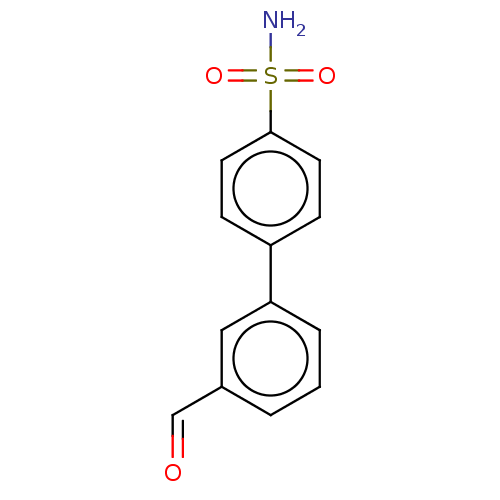 (4-(3-formylphenyl)-benzenesulfonamide (4e)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16668 (2,2-dimethyl-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50564139 (CHEMBL4785560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112112 BindingDB Entry DOI: 10.7270/Q2J96B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,V572F,L579M] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50540941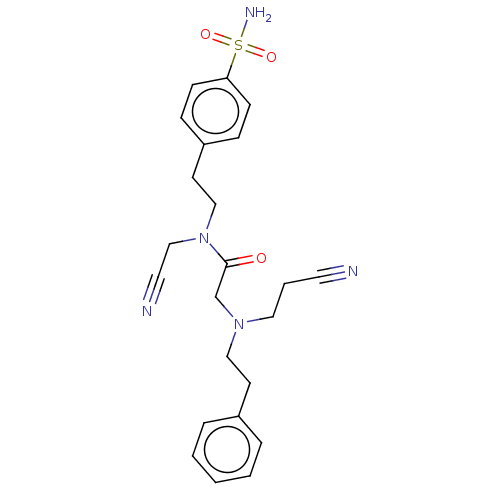 (CHEMBL4633228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Firenze Curated by ChEMBL | Assay Description Inhibition of human CA2 preincubated for 15 mins by stopped-flow CO2 hydration kinetic assay based Cheng-Prusoff equation analysis | J Med Chem 63: 7422-7444 (2020) Article DOI: 10.1021/acs.jmedchem.0c00733 BindingDB Entry DOI: 10.7270/Q2BV7M64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50382706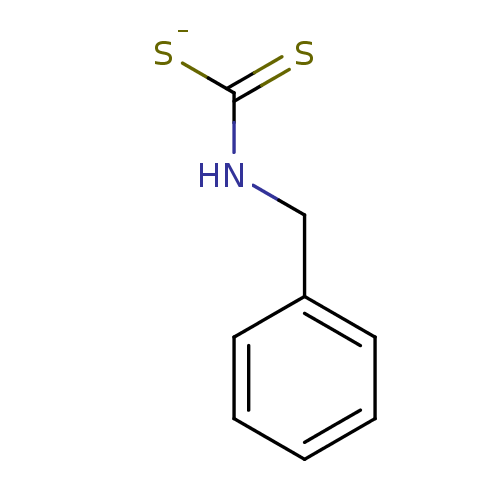 (Benzyldithiocarbamate sodium salt (I) | CHEMBL2023...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human CA2 pre-incubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 1721-30 (2012) Article DOI: 10.1021/jm300031j BindingDB Entry DOI: 10.7270/Q2GQ6ZSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 13 (Homo sapiens (Human)) | BDBM11625 (2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 13 after 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem Lett 20: 4376-81 (2010) Article DOI: 10.1016/j.bmcl.2010.06.082 BindingDB Entry DOI: 10.7270/Q2C24XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50564137 (CHEMBL4796488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112112 BindingDB Entry DOI: 10.7270/Q2J96B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50066126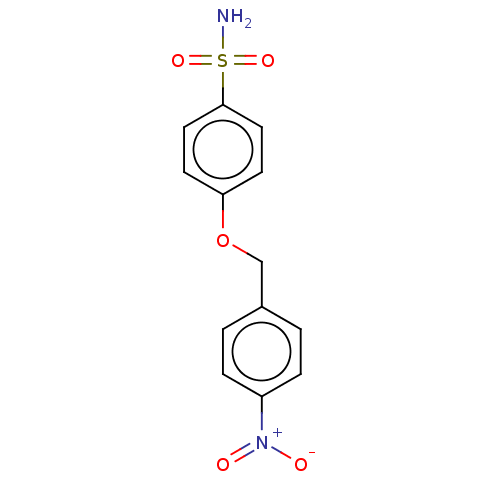 (CHEMBL3402966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant CA-12 after 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 23: 1828-40 (2015) Article DOI: 10.1016/j.bmc.2015.02.027 BindingDB Entry DOI: 10.7270/Q2KS6T6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50349850 (CHEMBL1738787) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human CA9 by stopped-flow CO2 hydrase assay | Bioorg Med Chem 21: 6674-80 (2013) Article DOI: 10.1016/j.bmc.2013.08.011 BindingDB Entry DOI: 10.7270/Q2V40Z44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50066128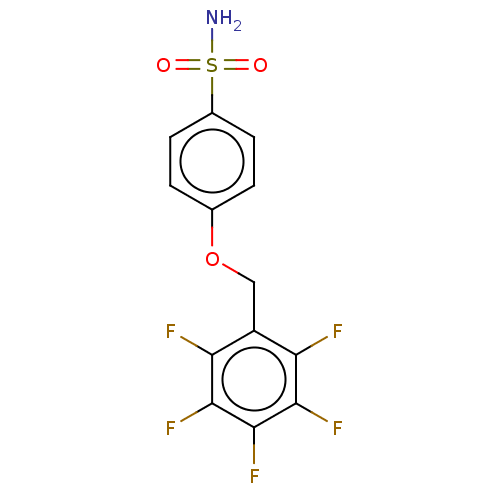 (CHEMBL3402964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant CA-12 after 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 23: 1828-40 (2015) Article DOI: 10.1016/j.bmc.2015.02.027 BindingDB Entry DOI: 10.7270/Q2KS6T6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2525 total ) | Next | Last >> |