

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM554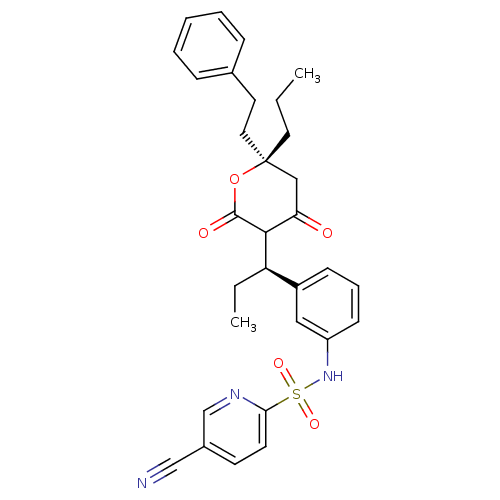 (5-cyano-N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | -63.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM558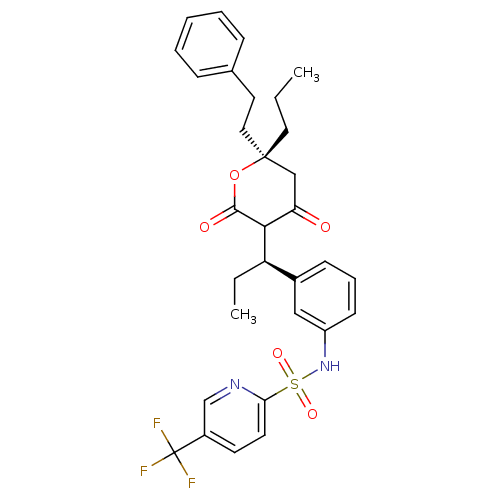 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00800 | -62.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM557 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -60.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM560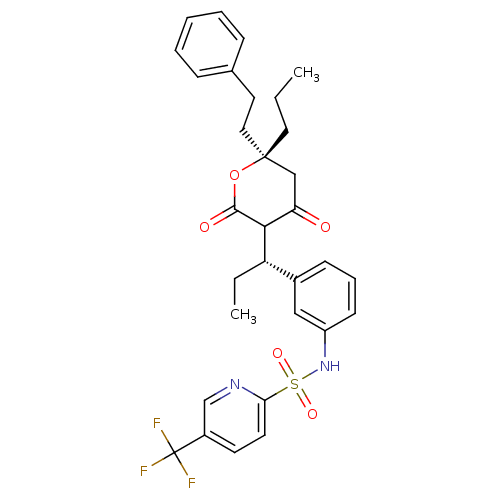 (N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | -59.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM553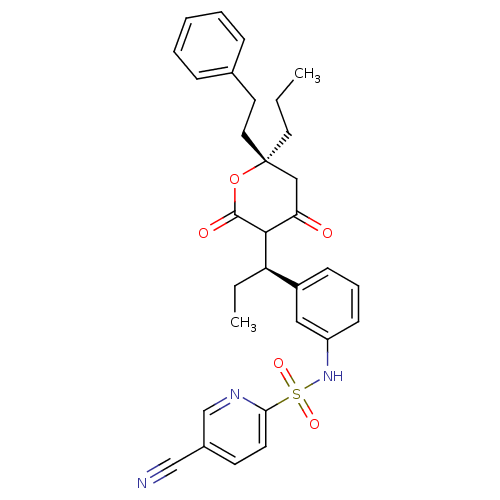 (5-cyano-N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM550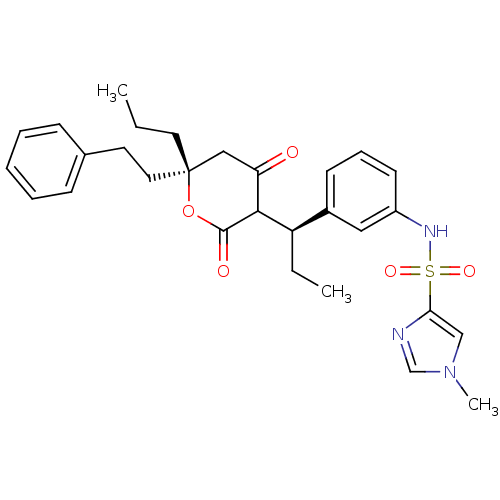 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM556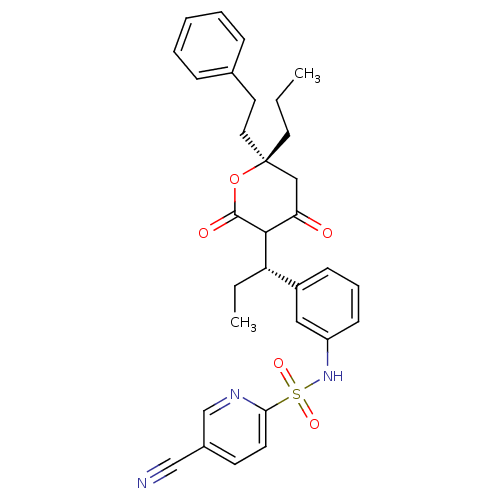 (5-cyano-N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM549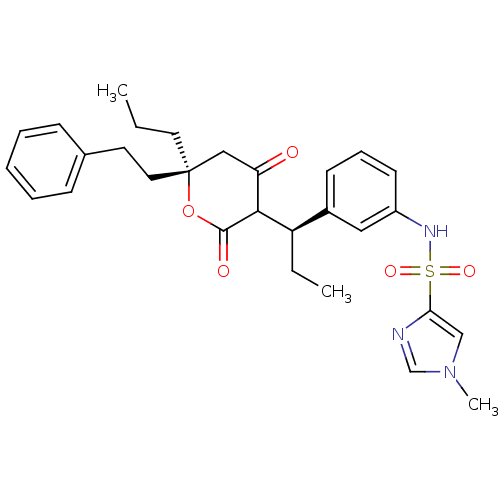 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM555 (5-cyano-N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM559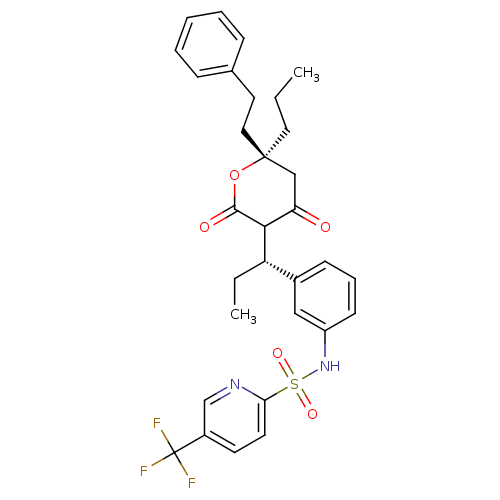 (N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM552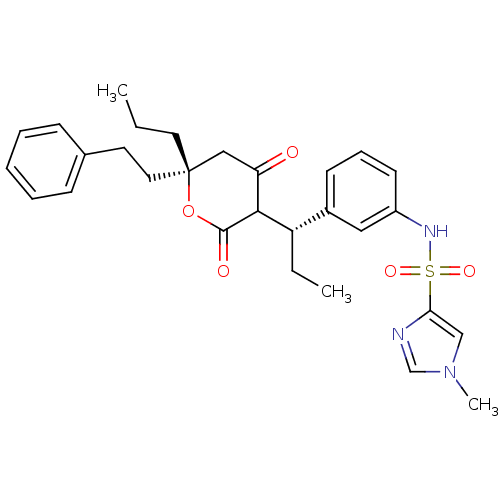 (N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM548 (CHEMBL21188 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM551 (N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM547 (CHEMBL20846 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM546 (4-fluoro-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM545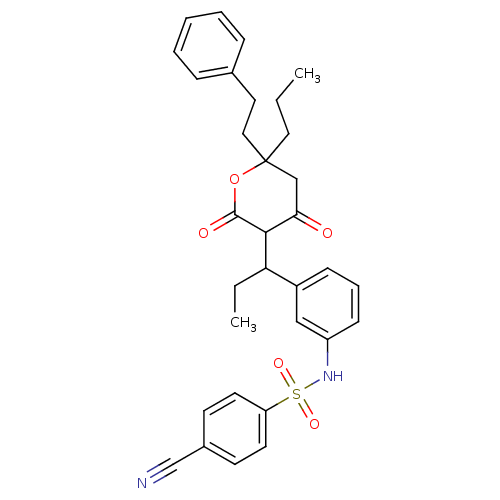 (4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM544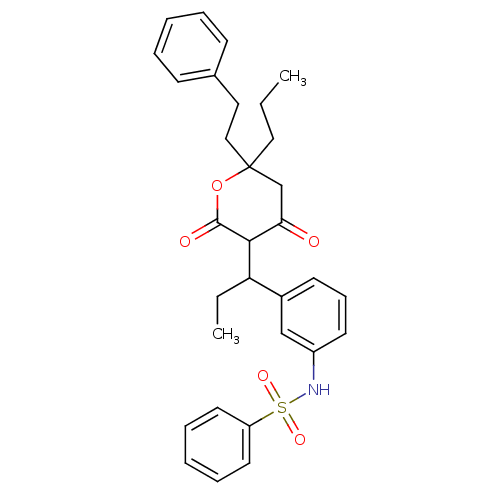 (N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044210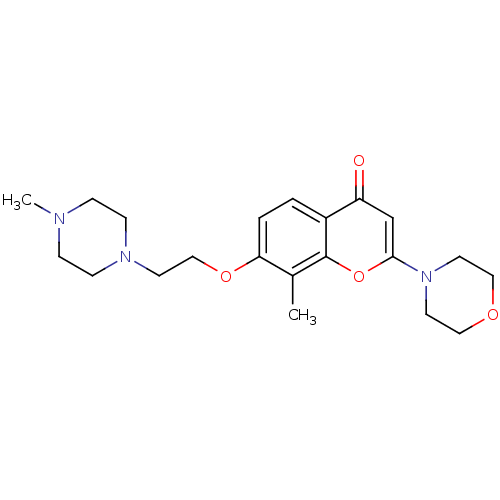 (7-[2-(4-methylpiperazin-1-yl)ethoxy]-8-methyl-2-mo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50228475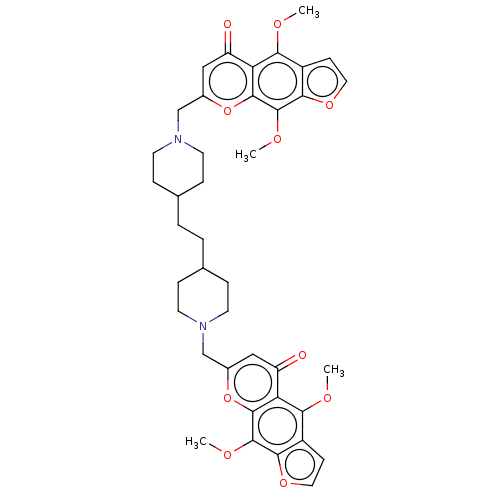 (CHEMBL90348) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase | J Med Chem 33: 2685-7 (1990) BindingDB Entry DOI: 10.7270/Q20867JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044219 (6,8-Dimethyl-7-[2-(4-methyl-piperazin-1-yl)-ethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044221 (7-(1-tert-Butyl-1H-tetrazol-5-ylmethoxy)-8-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044183 (7-(2-Diethylamino-ethoxy)-8-methyl-2-morpholin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044209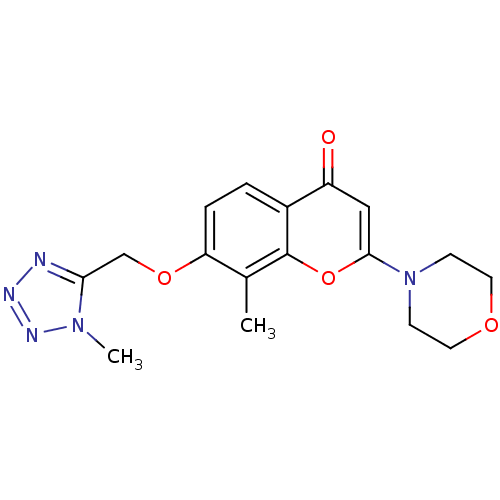 (8-Methyl-7-(1-methyl-1H-tetrazol-5-ylmethoxy)-2-mo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044204 (7-(2-Azepan-1-yl-ethoxy)-8-methyl-2-morpholin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044194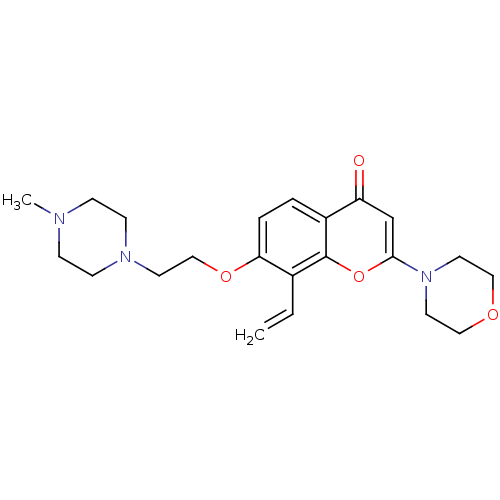 (7-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-2-morpholin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044213 (8-Ethyl-7-[2-(4-methyl-piperazin-1-yl)-ethoxy]-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044208 (8-Methyl-2-morpholin-4-yl-7-(2-piperazin-1-yl-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044216 (8-Methyl-2-morpholin-4-yl-7-(2-morpholin-4-yl-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044215 (8-Methyl-2-morpholin-4-yl-7-(2-pyrrolidin-1-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044188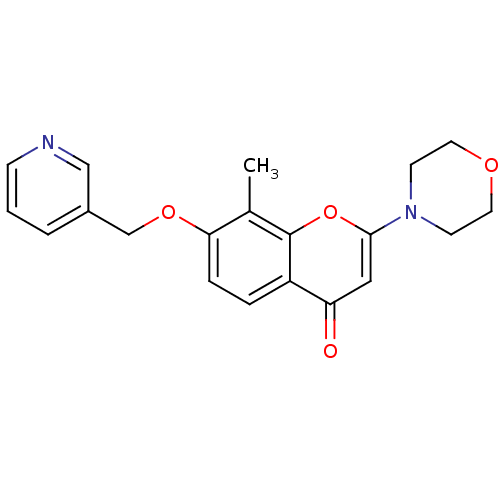 (8-Methyl-2-morpholin-4-yl-7-(pyridin-3-ylmethoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50228476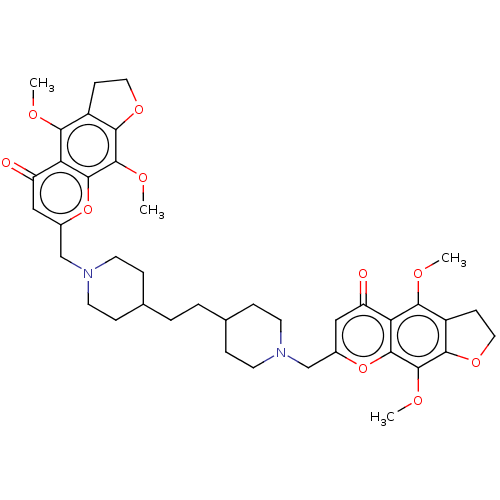 (CHEMBL92580) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase | J Med Chem 33: 2685-7 (1990) BindingDB Entry DOI: 10.7270/Q20867JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044207 (8-Methyl-2-morpholin-4-yl-7-(2-thiomorpholin-4-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044192 (8-Methyl-2-morpholin-4-yl-7-(2-piperidin-1-yl-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50228477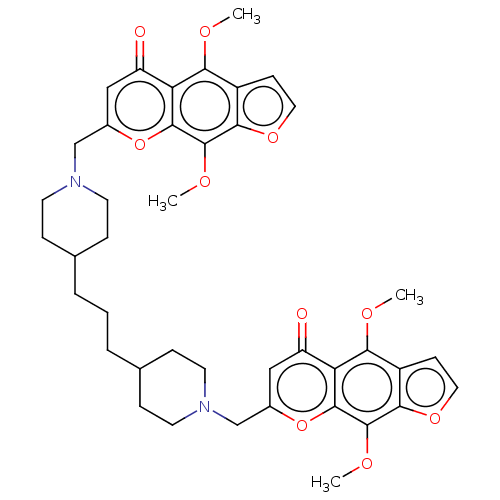 (CHEMBL88725) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase | J Med Chem 33: 2685-7 (1990) BindingDB Entry DOI: 10.7270/Q20867JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044217 (7-(2-Methoxy-ethoxy)-8-methyl-2-morpholin-4-yl-chr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044185 (8-Methyl-2-morpholin-4-yl-7-(1-phenyl-1H-tetrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044200 (8-Allyl-7-[2-(4-methyl-piperazin-1-yl)-ethoxy]-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044186 ((8-Methyl-2-morpholin-4-yl-4-oxo-4H-chromen-7-ylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044193 (7-[2-(4-Benzyl-piperazin-1-yl)-ethoxy]-8-methyl-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044223 (7-{2-[4-(2-Hydroxy-ethyl)-piperazin-1-yl]-ethoxy}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044184 (7-(1-Cyclohexyl-1H-tetrazol-5-ylmethoxy)-8-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044218 (8-Methyl-2-morpholin-4-yl-7-(2-oxo-propoxy)-chrome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044189 (8-Methyl-2-morpholin-4-yl-7-(naphthalen-1-ylmethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50228478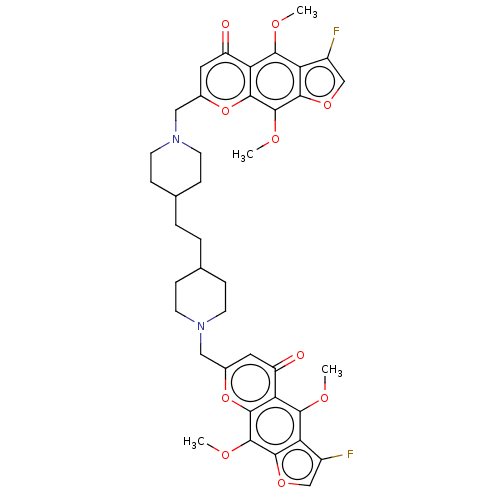 (CHEMBL328228) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase | J Med Chem 33: 2685-7 (1990) BindingDB Entry DOI: 10.7270/Q20867JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044190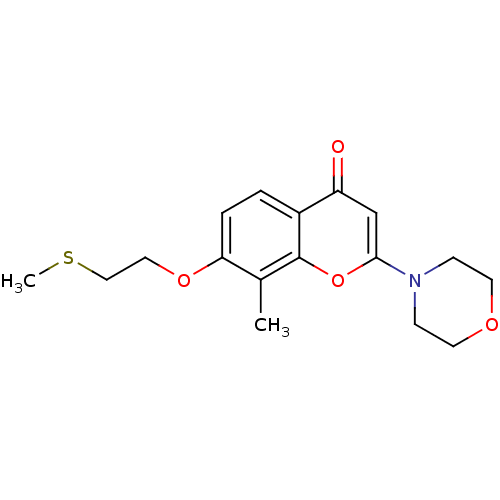 (8-Methyl-7-(2-methylsulfanyl-ethoxy)-2-morpholin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044214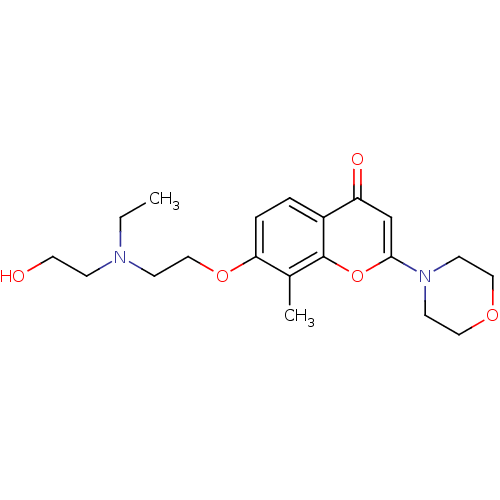 (7-{2-[Ethyl-(2-hydroxy-ethyl)-amino]-ethoxy}-8-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044206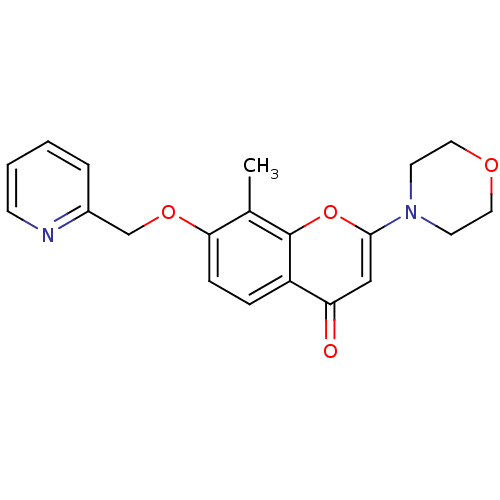 (8-Methyl-2-morpholin-4-yl-7-(pyridin-2-ylmethoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044224 (7-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-2-morpholin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044197 (7-(benzyloxy)-8-methyl-2-morpholino-4H-chromen-4-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50044220 (2-Morpholin-4-yl-7-(naphthalen-1-ylmethoxy)-chrome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against ADP-induced human platelet aggregation | J Med Chem 36: 2026-32 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |