 Found 36 hits with Last Name = 'borch' and Initial = 'rf'
Found 36 hits with Last Name = 'borch' and Initial = 'rf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
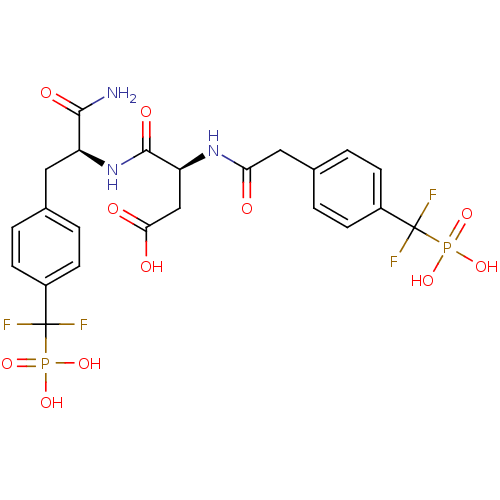
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B expressed in human HepG2 cells |
J Med Chem 50: 856-64 (2007)
Article DOI: 10.1021/jm061146x
BindingDB Entry DOI: 10.7270/Q2B857SK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Mus musculus) | BDBM50107343
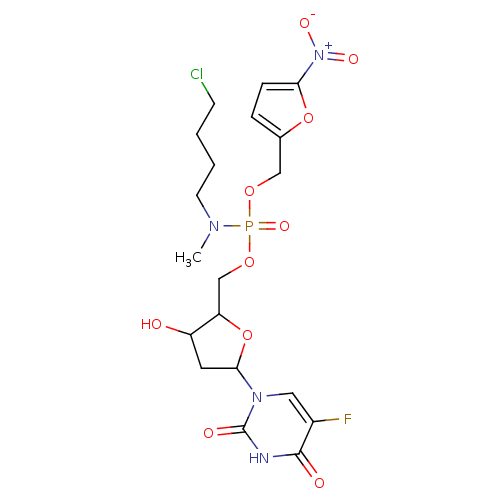
((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCc1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C19H25ClFN4O10P/c1-23(7-3-2-6-20)36(31,32-10-12-4-5-16(34-12)25(29)30)33-11-15-14(26)8-17(35-15)24-9-13(21)18(27)22-19(24)28/h4-5,9,14-15,17,26H,2-3,6-8,10-11H2,1H3,(H,22,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in thymidine kinase deficient /TK cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in L1210 mouse leukemia cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107341

((4-Chloro-butyl)-methyl-phosphoramidic acid 1,4-di...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCC1=CC(=O)c2ccccc2C1=O |t:30| Show InChI InChI=1S/C25H28ClFN3O9P/c1-29(9-5-4-8-26)40(36,37-13-15-10-19(31)16-6-2-3-7-17(16)23(15)33)38-14-21-20(32)11-22(39-21)30-12-18(27)24(34)28-25(30)35/h2-3,6-7,10,12,20-22,32H,4-5,8-9,11,13-14H2,1H3,(H,28,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in L1210 mouse leukemia cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476390
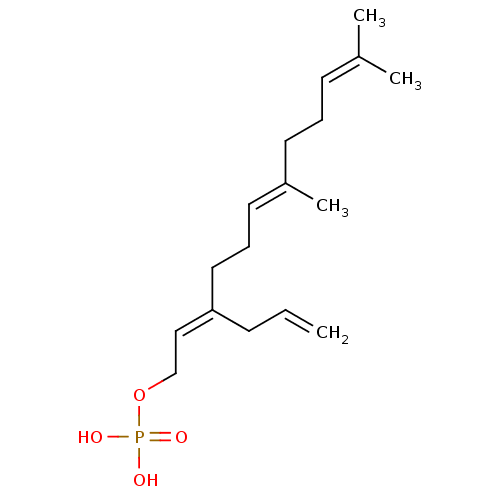
(CHEMBL426360)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6]-[#6]=[#6])=[#6]\[#6]-[#8]P([#8])([#8])=O Show InChI InChI=1S/C17H29O4P/c1-5-8-17(13-14-21-22(18,19)20)12-7-11-16(4)10-6-9-15(2)3/h5,9,11,13H,1,6-8,10,12,14H2,2-4H3,(H2,18,19,20)/b16-11+,17-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase assessed as farnesylation of Dansyl-GCVLS peptide |
J Med Chem 50: 3274-82 (2007)
Article DOI: 10.1021/jm0701829
BindingDB Entry DOI: 10.7270/Q2R49TJB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476389
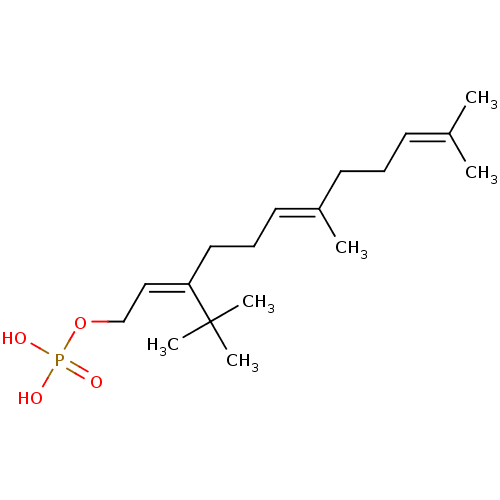
(CHEMBL229376)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](=[#6]\[#6]-[#8]P([#8])([#8])=O)C([#6])([#6])[#6] Show InChI InChI=1S/C18H33O4P/c1-15(2)9-7-10-16(3)11-8-12-17(18(4,5)6)13-14-22-23(19,20)21/h9,11,13H,7-8,10,12,14H2,1-6H3,(H2,19,20,21)/b16-11+,17-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase assessed as farnesylation of Dansyl-GCVLS peptide |
J Med Chem 50: 3274-82 (2007)
Article DOI: 10.1021/jm0701829
BindingDB Entry DOI: 10.7270/Q2R49TJB |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in thymidine kinase deficient /TK cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107341

((4-Chloro-butyl)-methyl-phosphoramidic acid 1,4-di...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCC1=CC(=O)c2ccccc2C1=O |t:30| Show InChI InChI=1S/C25H28ClFN3O9P/c1-29(9-5-4-8-26)40(36,37-13-15-10-19(31)16-6-2-3-7-17(16)23(15)33)38-14-21-20(32)11-22(39-21)30-12-18(27)24(34)28-25(30)35/h2-3,6-7,10,12,20-22,32H,4-5,8-9,11,13-14H2,1H3,(H,28,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in thymidine kinase deficient /TK cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107343

((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCc1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C19H25ClFN4O10P/c1-23(7-3-2-6-20)36(31,32-10-12-4-5-16(34-12)25(29)30)33-11-15-14(26)8-17(35-15)24-9-13(21)18(27)22-19(24)28/h4-5,9,14-15,17,26H,2-3,6-8,10-11H2,1H3,(H,22,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in L1210 mouse leukemia cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107341

((4-Chloro-butyl)-methyl-phosphoramidic acid 1,4-di...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCC1=CC(=O)c2ccccc2C1=O |t:30| Show InChI InChI=1S/C25H28ClFN3O9P/c1-29(9-5-4-8-26)40(36,37-13-15-10-19(31)16-6-2-3-7-17(16)23(15)33)38-14-21-20(32)11-22(39-21)30-12-18(27)24(34)28-25(30)35/h2-3,6-7,10,12,20-22,32H,4-5,8-9,11,13-14H2,1H3,(H,28,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in wild type LM cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in wild type LM cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107343

((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCc1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C19H25ClFN4O10P/c1-23(7-3-2-6-20)36(31,32-10-12-4-5-16(34-12)25(29)30)33-11-15-14(26)8-17(35-15)24-9-13(21)18(27)22-19(24)28/h4-5,9,14-15,17,26H,2-3,6-8,10-11H2,1H3,(H,22,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in wild type LM cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303967

((E)-3-(3-Chloro-1,4-naphthoquinon-2-yl)-2-methylpr...)Show SMILES C\C(=C/C1=C(Cl)C(=O)c2ccccc2C1=O)C(O)=O |c:3| Show InChI InChI=1S/C14H9ClO4/c1-7(14(18)19)6-10-11(15)13(17)9-5-3-2-4-8(9)12(10)16/h2-6H,1H3,(H,18,19)/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303973
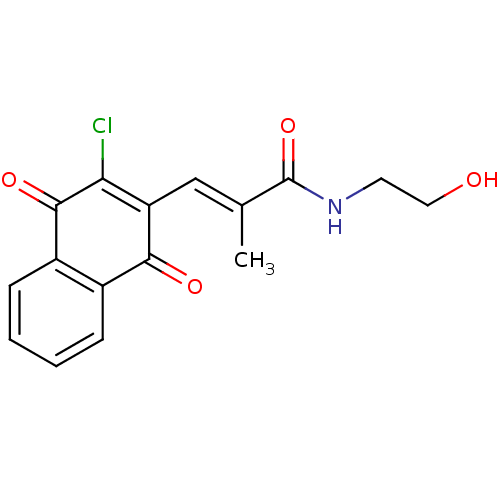
((E)-N-(2-Hydroxyethyl)-3-(3-chloro-1,4-dioxonaphth...)Show SMILES C\C(=C/C1=C(Cl)C(=O)c2ccccc2C1=O)C(=O)NCCO |c:3| Show InChI InChI=1S/C16H14ClNO4/c1-9(16(22)18-6-7-19)8-12-13(17)15(21)11-5-3-2-4-10(11)14(12)20/h2-5,8,19H,6-7H2,1H3,(H,18,22)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303964
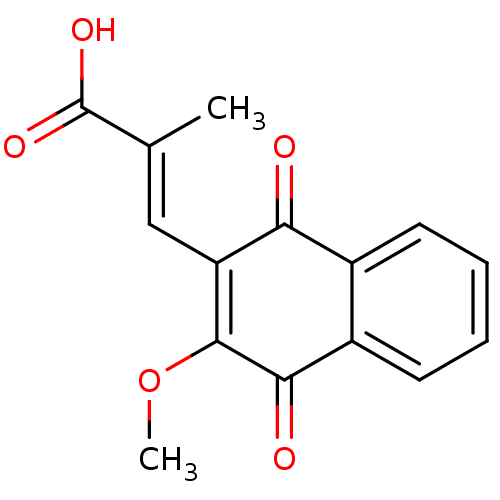
((E)-3-(3-Methoxy-1,4-naphthoquinon-2-yl)-2-methylp...)Show SMILES COC1=C(\C=C(/C)C(O)=O)C(=O)c2ccccc2C1=O |c:2| Show InChI InChI=1S/C15H12O5/c1-8(15(18)19)7-11-12(16)9-5-3-4-6-10(9)13(17)14(11)20-2/h3-7H,1-2H3,(H,18,19)/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303972
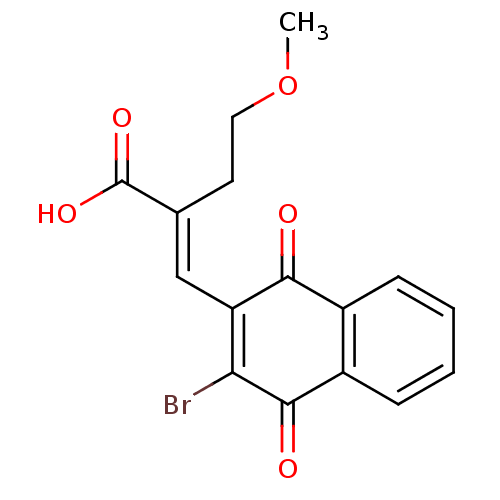
((E)-3-(3-Bromo-1,4-naphthoquinon-2-yl)-2-methoxyet...)Show SMILES COCC\C(=C/C1=C(Br)C(=O)c2ccccc2C1=O)C(O)=O |c:6| Show InChI InChI=1S/C16H13BrO5/c1-22-7-6-9(16(20)21)8-12-13(17)15(19)11-5-3-2-4-10(11)14(12)18/h2-5,8H,6-7H2,1H3,(H,20,21)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303965

((E)-3-(3-Methylthio-1,4-naphthoquinon-2-yl)-2-meth...)Show SMILES CSC1=C(\C=C(/C)C(O)=O)C(=O)c2ccccc2C1=O |c:2| Show InChI InChI=1S/C15H12O4S/c1-8(15(18)19)7-11-12(16)9-5-3-4-6-10(9)13(17)14(11)20-2/h3-7H,1-2H3,(H,18,19)/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107343

((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCc1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C19H25ClFN4O10P/c1-23(7-3-2-6-20)36(31,32-10-12-4-5-16(34-12)25(29)30)33-11-15-14(26)8-17(35-15)24-9-13(21)18(27)22-19(24)28/h4-5,9,14-15,17,26H,2-3,6-8,10-11H2,1H3,(H,22,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in thymidine kinase deficient LM cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303969

((E)-3-(3-Bromo-1,4-naphthoquinon-2-yl)-2-methylpro...)Show SMILES C\C(=C/C1=C(Br)C(=O)c2ccccc2C1=O)C(O)=O |c:3| Show InChI InChI=1S/C14H9BrO4/c1-7(14(18)19)6-10-11(15)13(17)9-5-3-2-4-8(9)12(10)16/h2-6H,1H3,(H,18,19)/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303968

((Z)-3-(3-Chloro-1,4-naphthoquinon-2-yl)-2-methylpr...)Show SMILES C\C(=C\C1=C(Cl)C(=O)c2ccccc2C1=O)C(O)=O |c:3| Show InChI InChI=1S/C14H9ClO4/c1-7(14(18)19)6-10-11(15)13(17)9-5-3-2-4-8(9)12(10)16/h2-6H,1H3,(H,18,19)/b7-6- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303962

((E)-3-(1,4-Naphthoquinon-2-yl)-2-methylpropenoic a...)Show InChI InChI=1S/C14H10O4/c1-8(14(17)18)6-9-7-12(15)10-4-2-3-5-11(10)13(9)16/h2-7H,1H3,(H,17,18)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303959

((E)-3-(3-Chloro-5,6-dimethoxy-1,4-dioxocyclohexa-2...)Show SMILES COC1=C(OC)C(=O)C(\C=C(/C)C(O)=O)=C(Cl)C1=O |c:2,t:14| Show InChI InChI=1S/C12H11ClO6/c1-5(12(16)17)4-6-7(13)9(15)11(19-3)10(18-2)8(6)14/h4H,1-3H3,(H,16,17)/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303957

((E)-3-(5,6-Dimethoxy-3-methyl-1,4-dioxocyclohexa-2...)Show SMILES COC1=C(OC)C(=O)C(C=C(C)C(O)=O)=C(C)C1=O |w:9.8,c:2,t:14| Show InChI InChI=1S/C13H14O6/c1-6(13(16)17)5-8-7(2)9(14)11(18-3)12(19-4)10(8)15/h5H,1-4H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303971
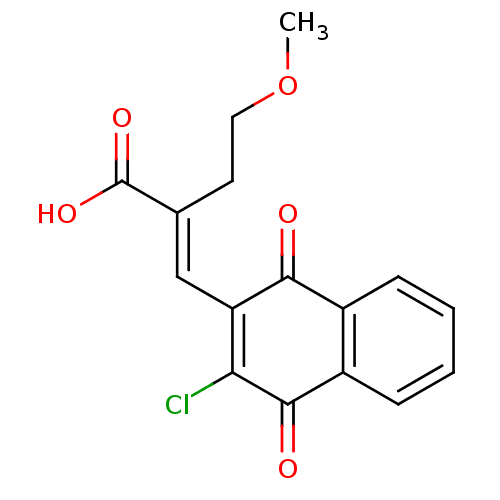
((E)-2-((3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2...)Show SMILES COCC\C(=C/C1=C(Cl)C(=O)c2ccccc2C1=O)C(O)=O |c:6| Show InChI InChI=1S/C16H13ClO5/c1-22-7-6-9(16(20)21)8-12-13(17)15(19)11-5-3-2-4-10(11)14(12)18/h2-5,8H,6-7H2,1H3,(H,20,21)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303966

((E)-3-(3-Fluoro-1,4-naphthoquinon-2-yl)-2-methylpr...)Show SMILES C\C(=C/C1=C(F)C(=O)c2ccccc2C1=O)C(O)=O |c:3| Show InChI InChI=1S/C14H9FO4/c1-7(14(18)19)6-10-11(15)13(17)9-5-3-2-4-8(9)12(10)16/h2-6H,1H3,(H,18,19)/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303974
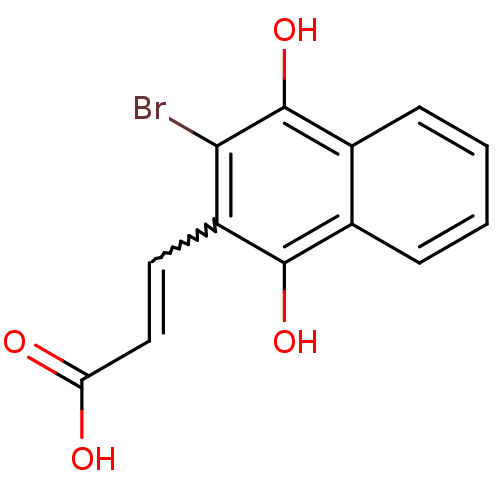
(3-(3-Bromo-1,4-naphthoquinon-2-yl)-propionic Acid ...)Show InChI InChI=1S/C13H9BrO4/c14-11-9(5-6-10(15)16)12(17)7-3-1-2-4-8(7)13(11)18/h1-6,17-18H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476391
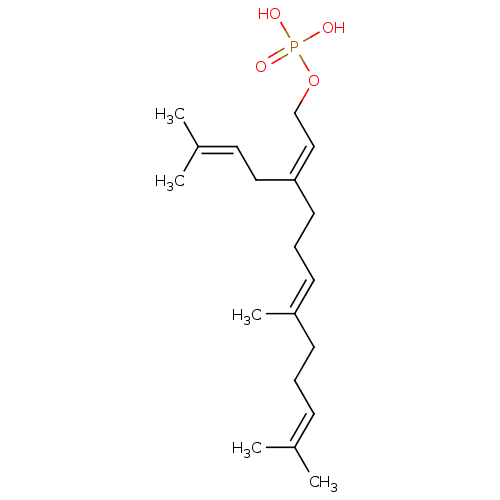
(CHEMBL447845)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]\[#6]-[#8]P([#8])([#8])=O Show InChI InChI=1S/C19H33O4P/c1-16(2)8-6-9-18(5)10-7-11-19(13-12-17(3)4)14-15-23-24(20,21)22/h8,10,12,14H,6-7,9,11,13,15H2,1-5H3,(H2,20,21,22)/b18-10+,19-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase assessed as farnesylation of Dansyl-GCVLS peptide |
J Med Chem 50: 3274-82 (2007)
Article DOI: 10.1021/jm0701829
BindingDB Entry DOI: 10.7270/Q2R49TJB |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in thymidine kinase deficient LM cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107341

((4-Chloro-butyl)-methyl-phosphoramidic acid 1,4-di...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCC1=CC(=O)c2ccccc2C1=O |t:30| Show InChI InChI=1S/C25H28ClFN3O9P/c1-29(9-5-4-8-26)40(36,37-13-15-10-19(31)16-6-2-3-7-17(16)23(15)33)38-14-21-20(32)11-22(39-21)30-12-18(27)24(34)28-25(30)35/h2-3,6-7,10,12,20-22,32H,4-5,8-9,11,13-14H2,1H3,(H,28,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in thymidine kinase deficient LM cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303960

((E)-3-(5,6-Dimethoxy-3-methyl-1,4-dioxocyclohexa-2...)Show SMILES COCCC(=CC1=C(C)C(=O)C(OC)=C(OC)C1=O)C(O)=O |w:5.5,c:6,t:13| Show InChI InChI=1S/C15H18O7/c1-8-10(7-9(15(18)19)5-6-20-2)12(17)14(22-4)13(21-3)11(8)16/h7H,5-6H2,1-4H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303970
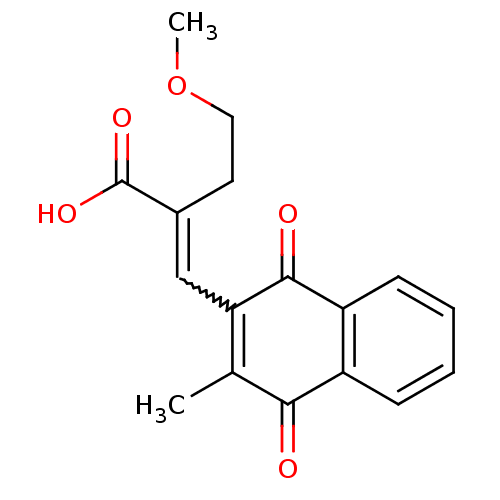
((E)-3-(3-Methyl-1,4-naphthoquinon-2-yl)-2-methoxye...)Show SMILES COCCC(=CC1=C(C)C(=O)c2ccccc2C1=O)C(O)=O |w:5.5,c:6| Show InChI InChI=1S/C17H16O5/c1-10-14(9-11(17(20)21)7-8-22-2)16(19)13-6-4-3-5-12(13)15(10)18/h3-6,9H,7-8H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303955
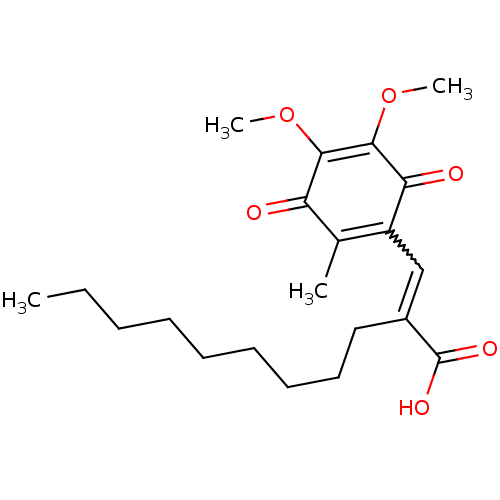
((E)-3-(5,6-Dimethoxy-3-methyl-14-dioxocyclohexa-25...)Show SMILES CCCCCCCCCC(=CC1=C(C)C(=O)C(OC)=C(OC)C1=O)C(O)=O |w:10.10,c:11,t:18| Show InChI InChI=1S/C21H30O6/c1-5-6-7-8-9-10-11-12-15(21(24)25)13-16-14(2)17(22)19(26-3)20(27-4)18(16)23/h13H,5-12H2,1-4H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303961
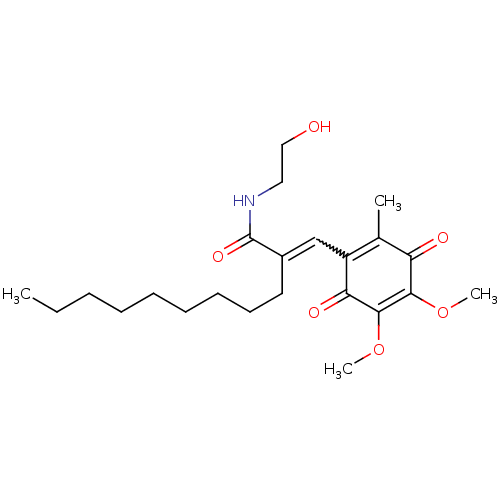
((E)-N-(2-Hydroxyethyl)-3-(5,6-dimethoxy-3-methyl-1...)Show SMILES CCCCCCCCCC(=CC1=C(C)C(=O)C(OC)=C(OC)C1=O)C(=O)NCCO |w:10.10,c:11,t:18| Show InChI InChI=1S/C23H35NO6/c1-5-6-7-8-9-10-11-12-17(23(28)24-13-14-25)15-18-16(2)19(26)21(29-3)22(30-4)20(18)27/h15,25H,5-14H2,1-4H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303958

((E)-3-(5,6-Dimethoxy-3-methyl-1,4-dioxocyclohexa-2...)Show SMILES CCCCC(=CC1=C(C)C(=O)C(OC)=C(OC)C1=O)C(O)=O |w:5.5,c:6,t:13| Show InChI InChI=1S/C16H20O6/c1-5-6-7-10(16(19)20)8-11-9(2)12(17)14(21-3)15(22-4)13(11)18/h8H,5-7H2,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303963

((E)-3-(3-Methyl-1,4-naphthoquinon-2-yl)-2-methylpr...)Show SMILES CC(=CC1=C(C)C(=O)c2ccccc2C1=O)C(O)=O |w:2.2,c:3| Show InChI InChI=1S/C15H12O4/c1-8(15(18)19)7-12-9(2)13(16)10-5-3-4-6-11(10)14(12)17/h3-7H,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50303956

((E)-3-(5,6-Dimethoxy-3-methyl-1,4-dioxocyclohexa-2...)Show SMILES COC1=C(OC)C(=O)C(C=CC(O)=O)=C(C)C1=O |w:9.8,c:2,t:13| Show InChI InChI=1S/C12H12O6/c1-6-7(4-5-8(13)14)10(16)12(18-3)11(17-2)9(6)15/h4-5H,1-3H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Ape1/ref-1 redox activity in presence of 0.02 mM DTT and human Hey-C2 cells nuclear extracts by EMSA |
J Med Chem 53: 1200-10 (2010)
Article DOI: 10.1021/jm9014857
BindingDB Entry DOI: 10.7270/Q2HD7VR8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data