 Found 467 hits with Last Name = 'cheng' and Initial = 'rk'
Found 467 hits with Last Name = 'cheng' and Initial = 'rk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152234

(CHEMBL184061 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES Nc1nc(NCCN2CCN(CC2)c2ccc(F)cc2F)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C20H21F2N9O/c21-13-3-4-15(14(22)12-13)30-9-7-29(8-10-30)6-5-24-19-26-18(23)31-20(27-19)25-17(28-31)16-2-1-11-32-16/h1-4,11-12H,5-10H2,(H3,23,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152234

(CHEMBL184061 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES Nc1nc(NCCN2CCN(CC2)c2ccc(F)cc2F)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C20H21F2N9O/c21-13-3-4-15(14(22)12-13)30-9-7-29(8-10-30)6-5-24-19-26-18(23)31-20(27-19)25-17(28-31)16-2-1-11-32-16/h1-4,11-12H,5-10H2,(H3,23,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152237
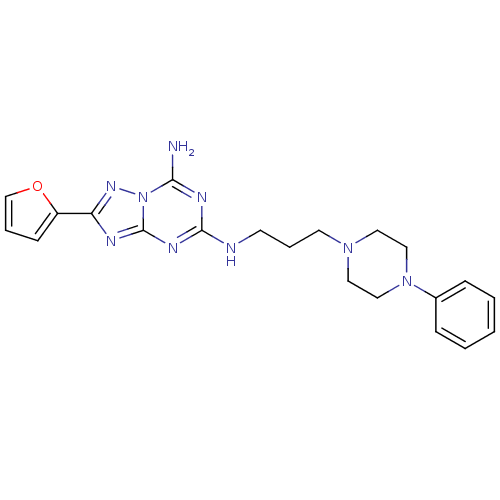
(2-Furan-2-yl-N*5*-[3-(4-phenyl-piperazin-1-yl)-pro...)Show SMILES Nc1nc(NCCCN2CCN(CC2)c2ccccc2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H25N9O/c22-19-25-20(26-21-24-18(27-30(19)21)17-8-4-15-31-17)23-9-5-10-28-11-13-29(14-12-28)16-6-2-1-3-7-16/h1-4,6-8,15H,5,9-14H2,(H3,22,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152237

(2-Furan-2-yl-N*5*-[3-(4-phenyl-piperazin-1-yl)-pro...)Show SMILES Nc1nc(NCCCN2CCN(CC2)c2ccccc2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H25N9O/c22-19-25-20(26-21-24-18(27-30(19)21)17-8-4-15-31-17)23-9-5-10-28-11-13-29(14-12-28)16-6-2-1-3-7-16/h1-4,6-8,15H,5,9-14H2,(H3,22,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50150077
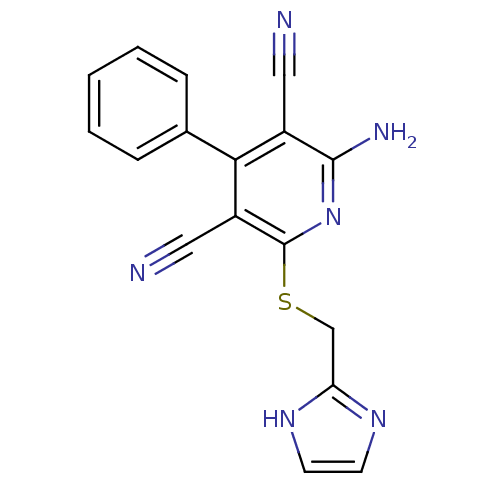
(2-((1H-imidazol-2-yl)methylthio)-6-amino-4-phenylp...)Show InChI InChI=1S/C17H12N6S/c18-8-12-15(11-4-2-1-3-5-11)13(9-19)17(23-16(12)20)24-10-14-21-6-7-22-14/h1-7H,10H2,(H2,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPCPX from human A1 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50532555
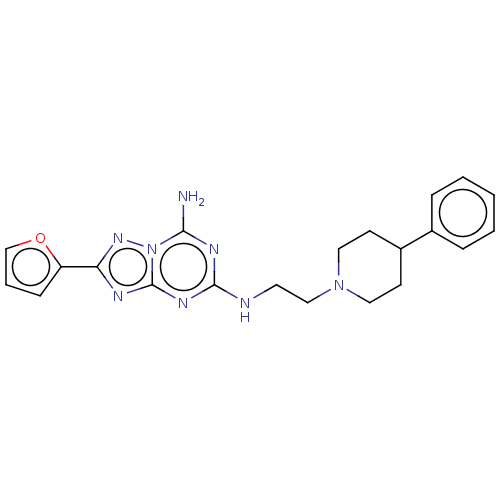
(CHEMBL3937413)Show SMILES Nc1nc(NCCN2CCC(CC2)c2ccccc2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H24N8O/c22-19-25-20(26-21-24-18(27-29(19)21)17-7-4-14-30-17)23-10-13-28-11-8-16(9-12-28)15-5-2-1-3-6-15/h1-7,14,16H,8-13H2,(H3,22,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50532555

(CHEMBL3937413)Show SMILES Nc1nc(NCCN2CCC(CC2)c2ccccc2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H24N8O/c22-19-25-20(26-21-24-18(27-29(19)21)17-7-4-14-30-17)23-10-13-28-11-8-16(9-12-28)15-5-2-1-3-6-15/h1-7,14,16H,8-13H2,(H3,22,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150077

(2-((1H-imidazol-2-yl)methylthio)-6-amino-4-phenylp...)Show InChI InChI=1S/C17H12N6S/c18-8-12-15(11-4-2-1-3-5-11)13(9-19)17(23-16(12)20)24-10-14-21-6-7-22-14/h1-7H,10H2,(H2,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50582415
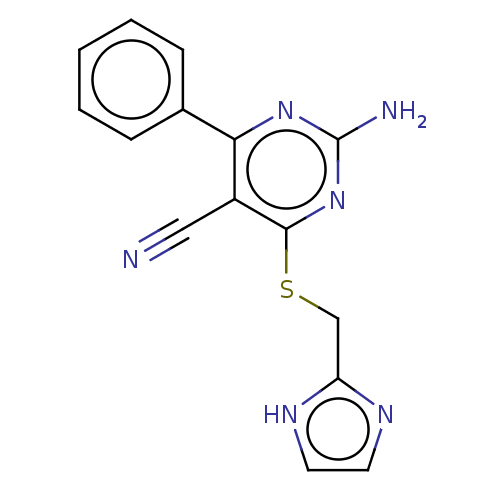
(CHEMBL5093654) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50532554
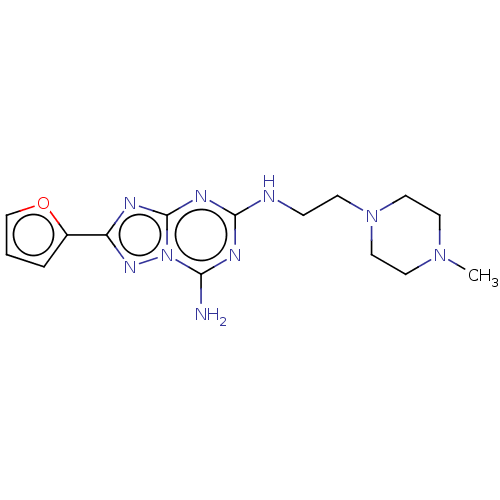
(CHEMBL3934661)Show InChI InChI=1S/C15H21N9O/c1-22-6-8-23(9-7-22)5-4-17-14-19-13(16)24-15(20-14)18-12(21-24)11-3-2-10-25-11/h2-3,10H,4-9H2,1H3,(H3,16,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50532554

(CHEMBL3934661)Show InChI InChI=1S/C15H21N9O/c1-22-6-8-23(9-7-22)5-4-17-14-19-13(16)24-15(20-14)18-12(21-24)11-3-2-10-25-11/h2-3,10H,4-9H2,1H3,(H3,16,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50150077

(2-((1H-imidazol-2-yl)methylthio)-6-amino-4-phenylp...)Show InChI InChI=1S/C17H12N6S/c18-8-12-15(11-4-2-1-3-5-11)13(9-19)17(23-16(12)20)24-10-14-21-6-7-22-14/h1-7H,10H2,(H2,20,23)(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-603 from human A2B receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50150077

(2-((1H-imidazol-2-yl)methylthio)-6-amino-4-phenylp...)Show InChI InChI=1S/C17H12N6S/c18-8-12-15(11-4-2-1-3-5-11)13(9-19)17(23-16(12)20)24-10-14-21-6-7-22-14/h1-7H,10H2,(H2,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-11 from human A3 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50582413
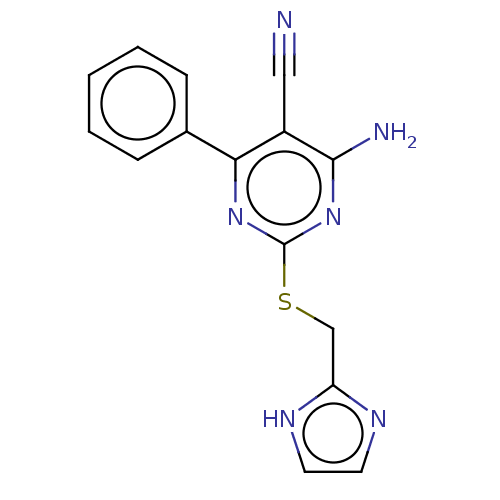
(CHEMBL5078507) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50582415

(CHEMBL5093654) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPCPX from human A1 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50582413

(CHEMBL5078507) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPCPX from human A1 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50582417

(CHEMBL5093743) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPCPX from human A1 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50582414
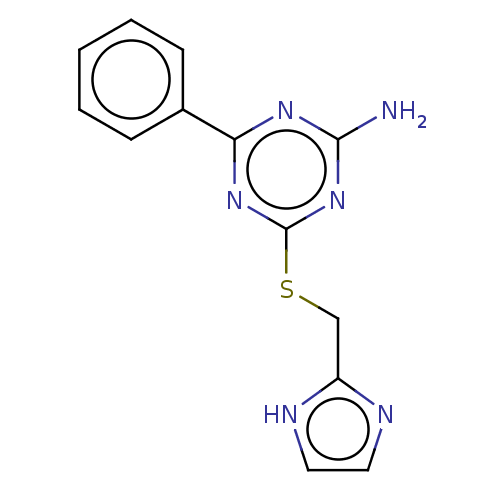
(CHEMBL5091876) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPCPX from human A1 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50582413

(CHEMBL5078507) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-11 from human A3 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50582416
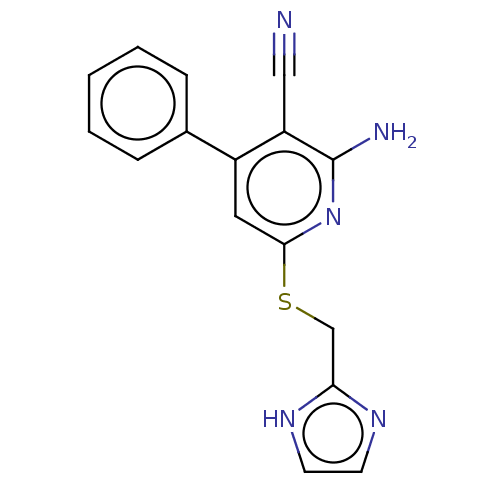
(CHEMBL5088305) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-603 from human A2B receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50582416

(CHEMBL5088305) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50582416

(CHEMBL5088305) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPCPX from human A1 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50582414

(CHEMBL5091876) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50582417

(CHEMBL5093743) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50582415

(CHEMBL5093654) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-11 from human A3 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50582417

(CHEMBL5093743) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-11 from human A3 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50582416

(CHEMBL5088305) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-11 from human A3 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50582414

(CHEMBL5091876) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PSB-11 from human A3 receptor expressed in CHO cell membranes measured after 3 hrs by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501754

(CHEMBL4071396)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O4/c1-19(31)26(27(32)29-33)28-25-4-2-3-23-17-21(11-12-24(23)25)8-5-20-6-9-22(10-7-20)18-30-13-15-34-16-14-30/h6-7,9-12,17,19,25-26,28,31,33H,2-4,13-16,18H2,1H3,(H,29,32)/t19-,25?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501764
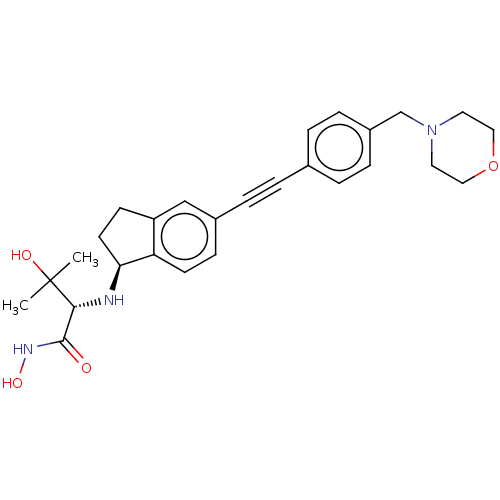
(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501755
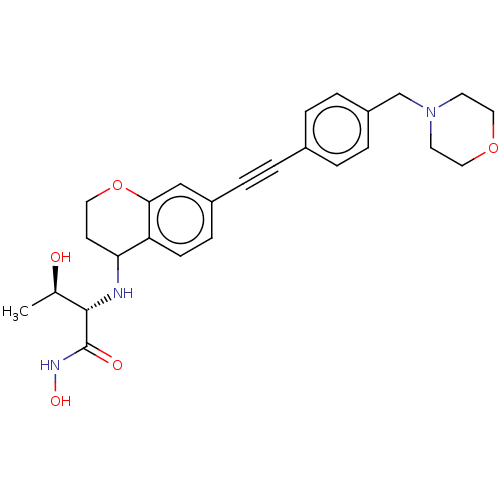
(CHEMBL4077947)Show SMILES C[C@@H](O)[C@H](NC1CCOc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-23-10-13-34-24-16-20(8-9-22(23)24)5-2-19-3-6-21(7-4-19)17-29-11-14-33-15-12-29/h3-4,6-9,16,18,23,25,27,30,32H,10-15,17H2,1H3,(H,28,31)/t18-,23?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501779
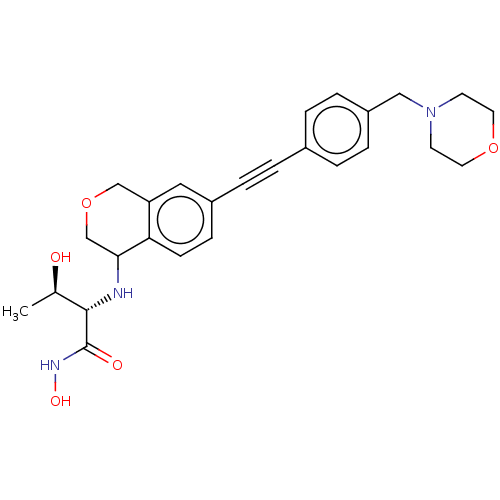
(CHEMBL4102207)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-24-17-34-16-22-14-20(8-9-23(22)24)5-2-19-3-6-21(7-4-19)15-29-10-12-33-13-11-29/h3-4,6-9,14,18,24-25,27,30,32H,10-13,15-17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501782

(CHEMBL4087371)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H24N2O3/c1-15(25)21(22(26)24-27)23-20-9-5-8-18-14-17(12-13-19(18)20)11-10-16-6-3-2-4-7-16/h2-4,6-7,12-15,20-21,23,25,27H,5,8-9H2,1H3,(H,24,26)/t15-,20?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501780
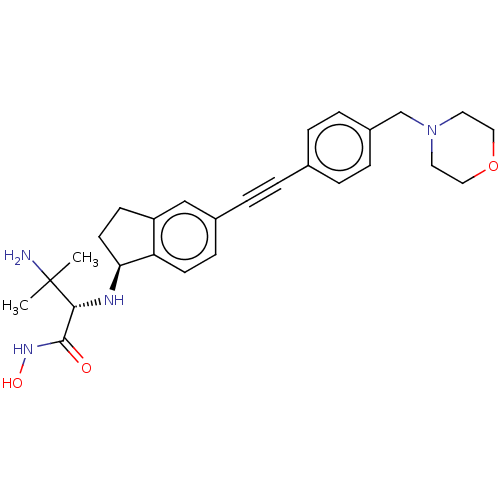
(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501764

(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501762

(CHEMBL4081478)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2C)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H28N4O3/c1-17(31)25(26(32)29-33)28-24-12-10-22-15-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)16-30-14-13-27-18(30)2/h4-5,7-9,11,13-15,17,24-25,28,31,33H,10,12,16H2,1-2H3,(H,29,32)/t17-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501752
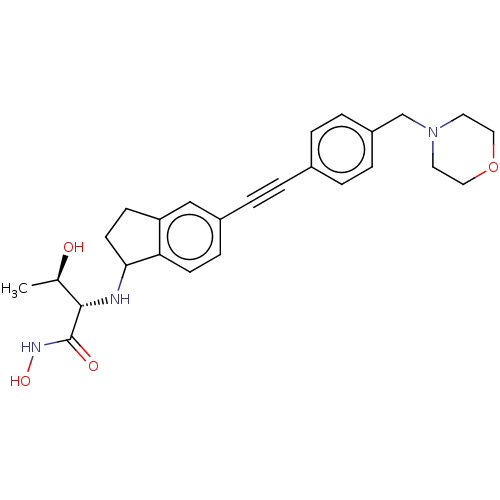
(CHEMBL4095059)Show SMILES C[C@@H](O)[C@H](NC1CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501754

(CHEMBL4071396)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O4/c1-19(31)26(27(32)29-33)28-25-4-2-3-23-17-21(11-12-24(23)25)8-5-20-6-9-22(10-7-20)18-30-13-15-34-16-14-30/h6-7,9-12,17,19,25-26,28,31,33H,2-4,13-16,18H2,1H3,(H,29,32)/t19-,25?,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501768
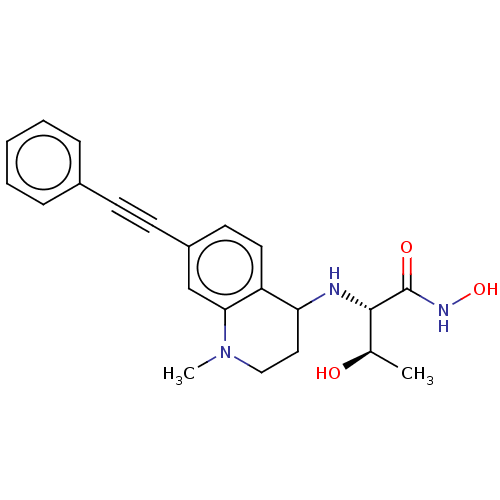
(CHEMBL4102818)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H25N3O3/c1-15(26)21(22(27)24-28)23-19-12-13-25(2)20-14-17(10-11-18(19)20)9-8-16-6-4-3-5-7-16/h3-7,10-11,14-15,19,21,23,26,28H,12-13H2,1-2H3,(H,24,27)/t15-,19?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501778

(CHEMBL4098674)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O4/c1-14(24)20(21(25)23-26)22-19-13-27-12-17-11-16(9-10-18(17)19)8-7-15-5-3-2-4-6-15/h2-6,9-11,14,19-20,22,24,26H,12-13H2,1H3,(H,23,25)/t14-,19?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501770
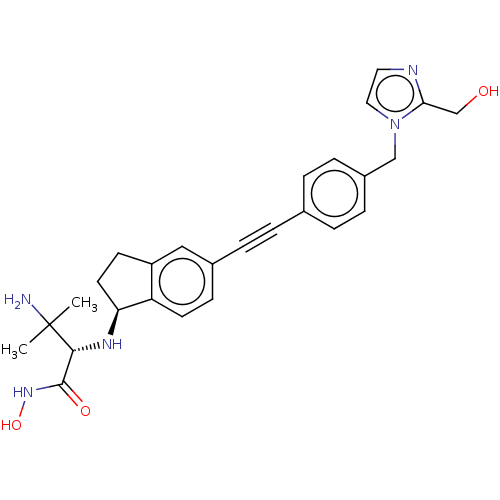
(CHEMBL4092719)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H31N5O3/c1-27(2,28)25(26(34)31-35)30-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-29-24(32)17-33/h4-5,7-9,11,13-15,23,25,30,33,35H,10,12,16-17,28H2,1-2H3,(H,31,34)/t23-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501756
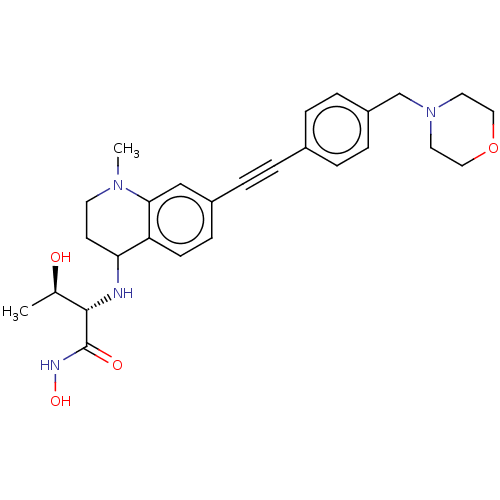
(CHEMBL4066982)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-19(32)26(27(33)29-34)28-24-11-12-30(2)25-17-21(9-10-23(24)25)6-3-20-4-7-22(8-5-20)18-31-13-15-35-16-14-31/h4-5,7-10,17,19,24,26,28,32,34H,11-16,18H2,1-2H3,(H,29,33)/t19-,24?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501780

(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501761

(CHEMBL4068010)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCC(CC2)OC)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C28H35N3O4/c1-19(32)27(28(33)30-34)29-26-12-10-23-17-21(9-11-25(23)26)6-3-20-4-7-22(8-5-20)18-31-15-13-24(35-2)14-16-31/h4-5,7-9,11,17,19,24,26-27,29,32,34H,10,12-16,18H2,1-2H3,(H,30,33)/t19-,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50420474
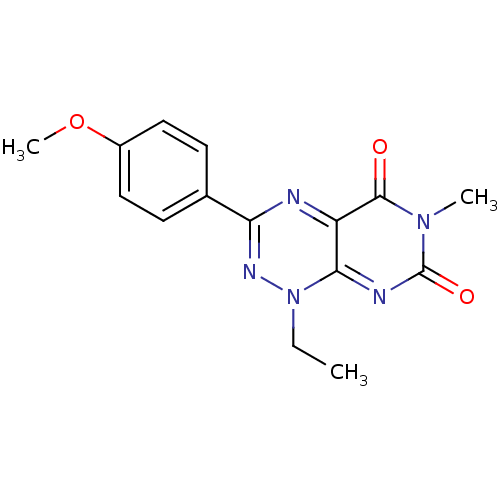
(CHEMBL2086889 | US9073941, 607)Show InChI InChI=1S/C15H15N5O3/c1-4-20-13-11(14(21)19(2)15(22)17-13)16-12(18-20)9-5-7-10(23-3)8-6-9/h5-8H,4H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of caspase3 |
J Med Chem 55: 1021-46 (2012)
Article DOI: 10.1021/jm201310y
BindingDB Entry DOI: 10.7270/Q2CN7560 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data