

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM26113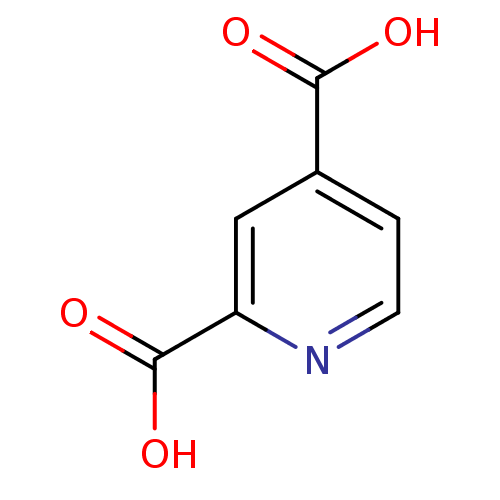 (2,4 PDCA | cid_10365 | pyridine carboxylate, 6a | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151392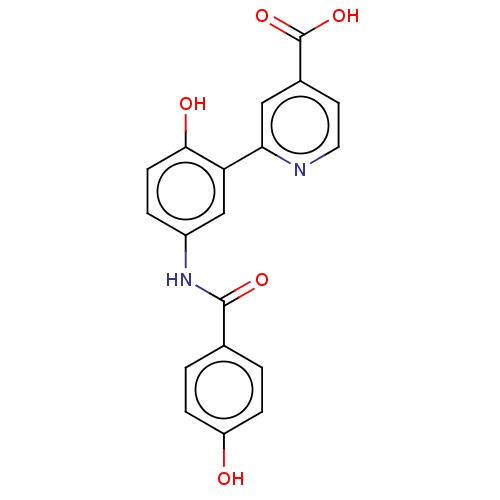 (CHEMBL3775262) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151399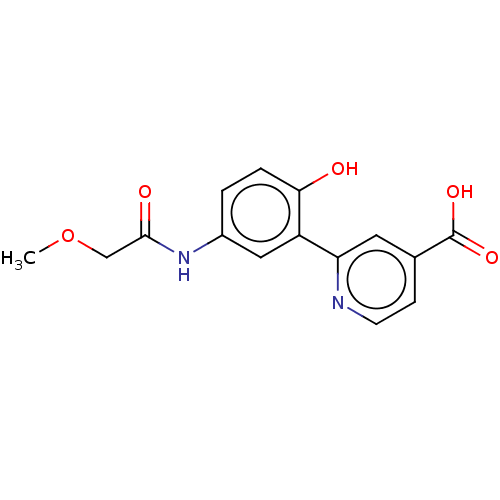 (CHEMBL3775380) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A [1-801] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50453140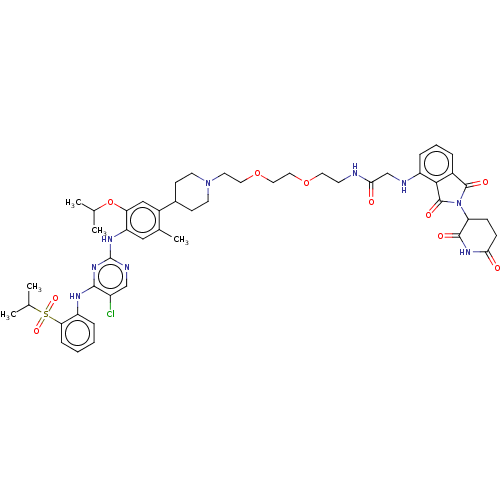 (CHEMBL4213986) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Induction of ALK degradation in human NCI-H3122 cells after 16 hrs by immunoblot method | J Med Chem 61: 4249-4255 (2018) Article DOI: 10.1021/acs.jmedchem.7b01655 BindingDB Entry DOI: 10.7270/Q2XS5XZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50453141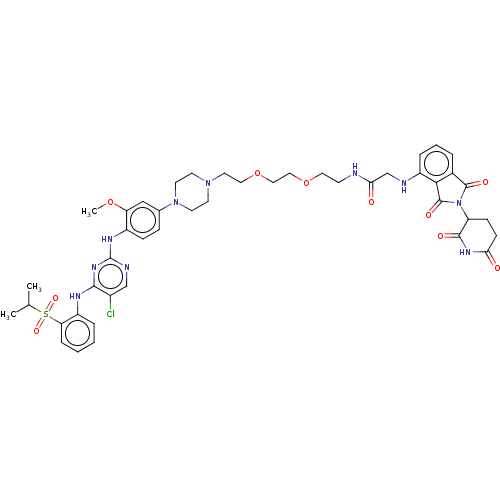 (CHEMBL4217325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Induction of ALK degradation in human NCI-H3122 cells after 16 hrs by immunoblot method | J Med Chem 61: 4249-4255 (2018) Article DOI: 10.1021/acs.jmedchem.7b01655 BindingDB Entry DOI: 10.7270/Q2XS5XZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C [1-765] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D [1-775] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223320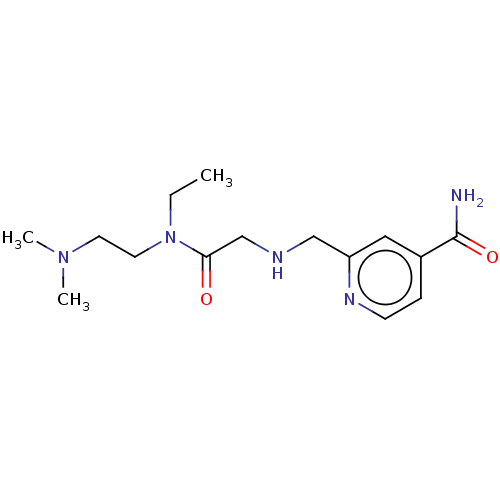 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5A [1-801] (Homo sapiens (Human)) | BDBM195612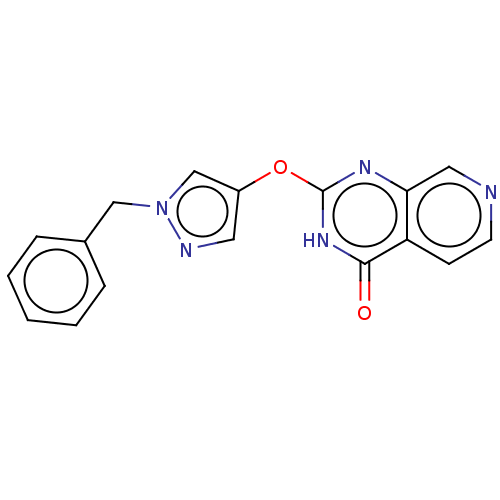 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM195612 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50148605 (CHEMBL3769729) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-conjugated JQ1 from wildtype BRD4 BD1 (unknown origin) expressed in Escherichia coli BL21-DE3 rosetta cells incubated for 15 min... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00958 BindingDB Entry DOI: 10.7270/Q23N278B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50453140 (CHEMBL4213986) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Induction of ALK degradation in human KARPAS299 cells after 16 hrs by immunoblot method | J Med Chem 61: 4249-4255 (2018) Article DOI: 10.1021/acs.jmedchem.7b01655 BindingDB Entry DOI: 10.7270/Q2XS5XZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191599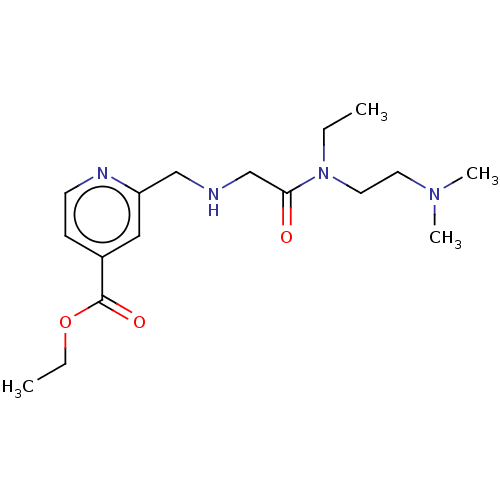 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-conjugated JQ1 from wildtype BRD4 BD1 (unknown origin) expressed in Escherichia coli BL21-DE3 rosetta cells incubated for 15 min... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00958 BindingDB Entry DOI: 10.7270/Q23N278B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50453140 (CHEMBL4213986) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Induction of ALK degradation in human Kelly cells after 16 hrs by immunoblot method | J Med Chem 61: 4249-4255 (2018) Article DOI: 10.1021/acs.jmedchem.7b01655 BindingDB Entry DOI: 10.7270/Q2XS5XZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50453141 (CHEMBL4217325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Induction of ALK degradation in human Kelly cells after 16 hrs by immunoblot method | J Med Chem 61: 4249-4255 (2018) Article DOI: 10.1021/acs.jmedchem.7b01655 BindingDB Entry DOI: 10.7270/Q2XS5XZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151392 (CHEMBL3775262) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using biotinylated ... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151393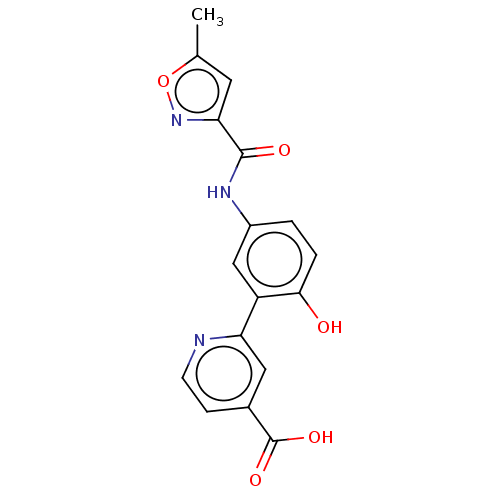 (CHEMBL3775867) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using biotinylated ... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C [1-765] (Homo sapiens (Human)) | BDBM195612 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151396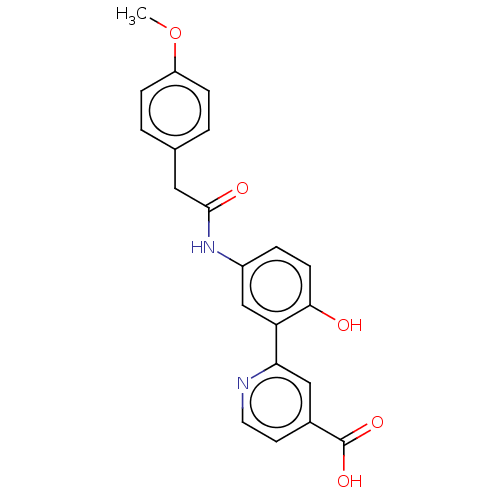 (CHEMBL3775563) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using biotinylated ... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5D [1-775] (Homo sapiens (Human)) | BDBM195612 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-conjugated JQ1 from BRD4 BD2 (unknown origin) expressed in Escherichia coli BL21-DE3 rosetta cells incubated for 15 mins by comp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00958 BindingDB Entry DOI: 10.7270/Q23N278B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151399 (CHEMBL3775380) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using biotinylated ... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151397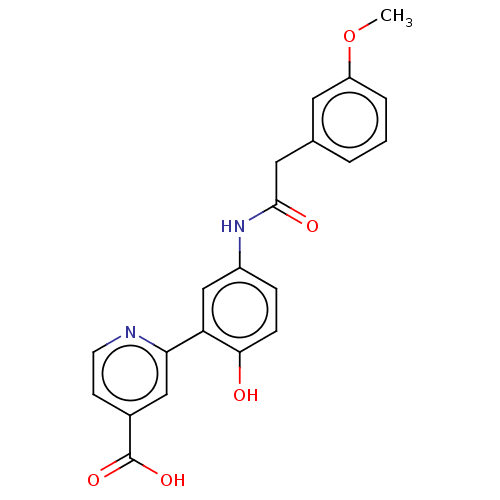 (CHEMBL3775272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using biotinylated ... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 6B (Homo sapiens (Human)) | BDBM60875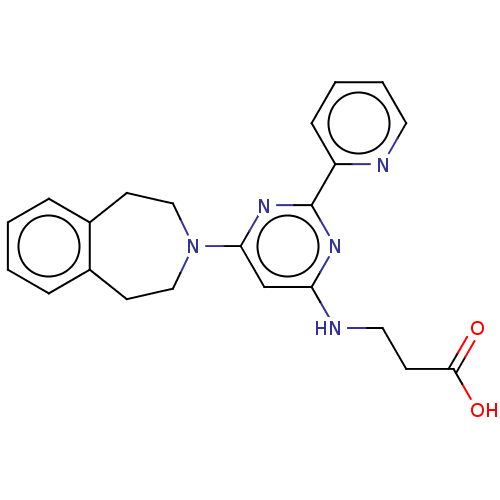 (3-((6-(4,5-Dihydro-1H-benzo[d]azepin-3(2H)-yl)-2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151395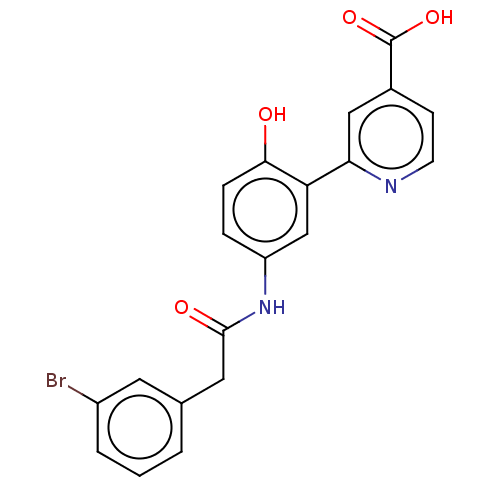 (CHEMBL3775162) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using biotinylated ... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50453141 (CHEMBL4217325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Induction of ALK degradation in human KARPAS299 cells after 16 hrs by immunoblot method | J Med Chem 61: 4249-4255 (2018) Article DOI: 10.1021/acs.jmedchem.7b01655 BindingDB Entry DOI: 10.7270/Q2XS5XZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM26113 (2,4 PDCA | cid_10365 | pyridine carboxylate, 6a | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using biotinylated ... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 339 total ) | Next | Last >> |