

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-10 (Rattus norvegicus) | BDBM50366779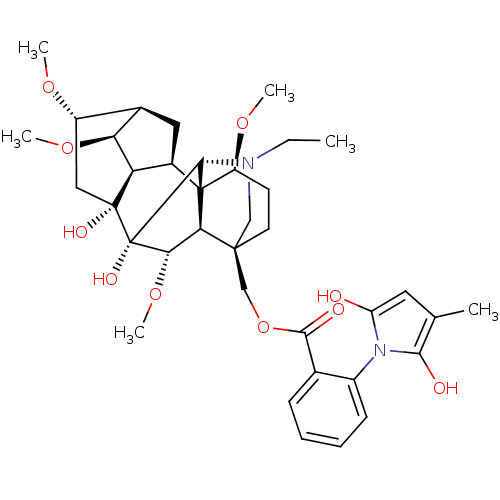 (METHYLLYCACONITINE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Binding affinity towards alpha3-beta4 neuronal nicotinic acetylcholine receptor | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine as radioligand for Dopamine receptor D2 in rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7262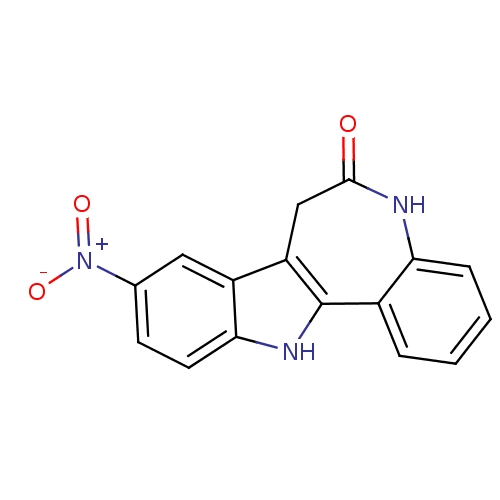 (14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GSK-3beta incubated with substrate in presence of ATP measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7262 (14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GSK-3beta preincubated for 3 hrs followed by ATP and substrate addition measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine binding to Dopamine receptor D2 of rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50369323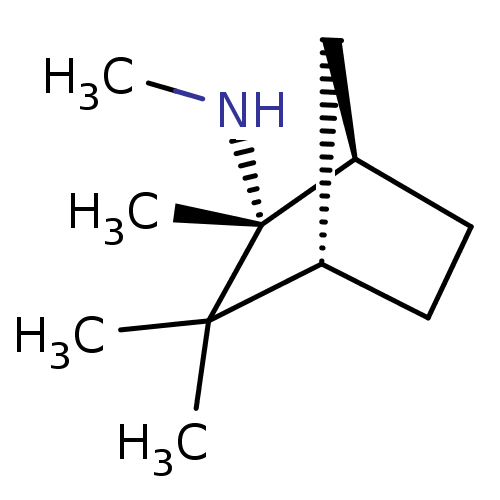 (MECAMYLAMINE) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Inhibitory activity against nicotinic acetylcholine receptor alpha3-beta4 | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50571206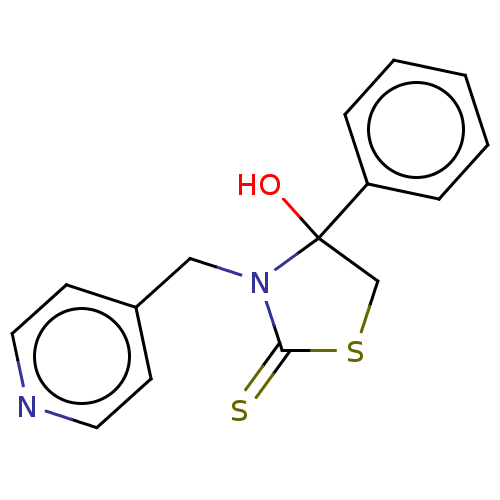 (CHEMBL4861930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GSK-3beta preincubated for 3 hrs followed by ATP and substrate addition measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50166940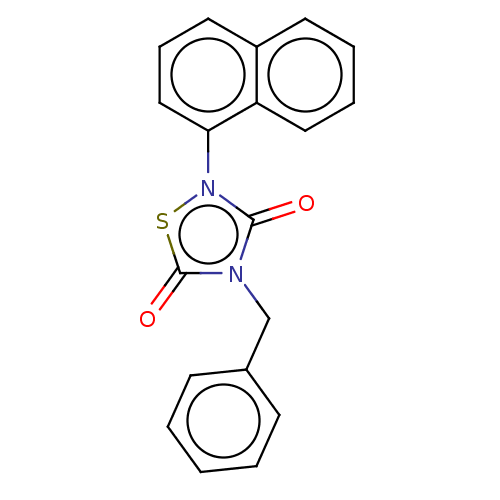 (NP-031112 | NP-12 | Tideglusib | US20230414581, Co...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GSK-3beta preincubated for 3 hrs followed by ATP and substrate addition measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM81924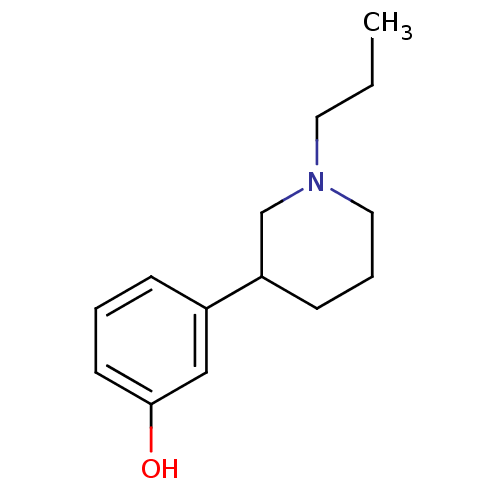 ((R)-3-(1-Propyl-piperidin-3-yl)-phenol | 3PPP(+/-)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine binding to Dopamine receptor D2 of rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM81924 ((R)-3-(1-Propyl-piperidin-3-yl)-phenol | 3PPP(+/-)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010580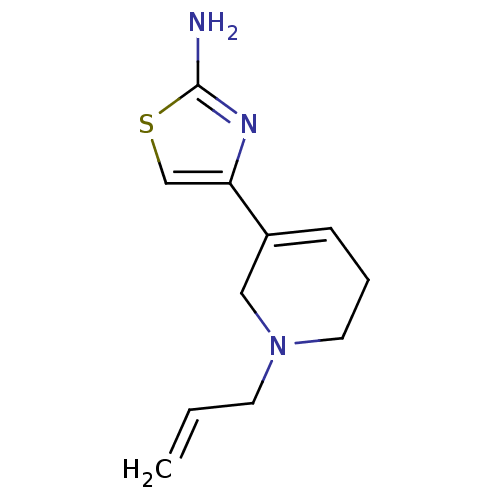 (4-(1-Allyl-1,2,5,6-tetrahydro-pyridin-3-yl)-thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine binding to Dopamine receptor D2 of rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50571206 (CHEMBL4861930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GSK-3beta incubated with substrate in presence of ATP measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM50571206 (CHEMBL4861930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type GSK-3beta incubated with substrate in presence of ATP measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SCH-23390 to Dopamine receptor D1 was determined | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010573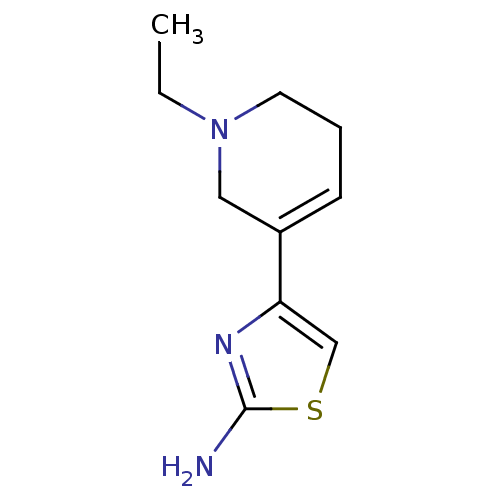 (4-(1-Ethyl-1,2,5,6-tetrahydro-pyridin-3-yl)-thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine binding to Dopamine receptor D2 of rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010580 (4-(1-Allyl-1,2,5,6-tetrahydro-pyridin-3-yl)-thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM81924 ((R)-3-(1-Propyl-piperidin-3-yl)-phenol | 3PPP(+/-)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007573 (4-(1-Propyl-1,2,5,6-tetrahydro-pyridin-3-yl)-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 596 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50129131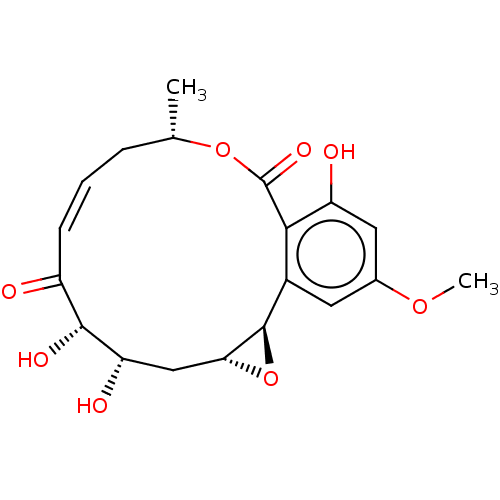 (CHEBI:83275 | Hypothemycin | US10434085, Compound ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 663 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GSK-3beta preincubated for 3 hrs followed by ATP and substrate addition measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367946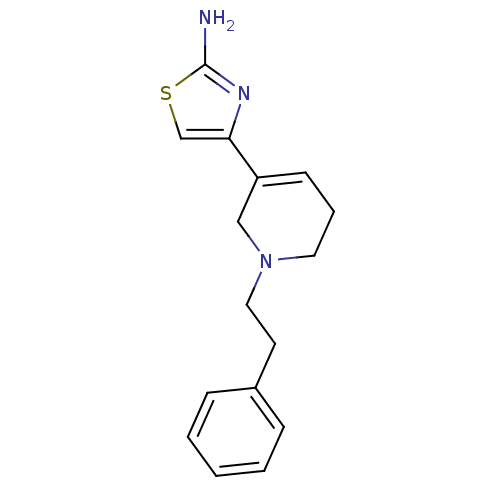 (CHEMBL1203001) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 911 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine binding to Dopamine receptor D2 of rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM81924 ((R)-3-(1-Propyl-piperidin-3-yl)-phenol | 3PPP(+/-)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010573 (4-(1-Ethyl-1,2,5,6-tetrahydro-pyridin-3-yl)-thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 945 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007573 (4-(1-Propyl-1,2,5,6-tetrahydro-pyridin-3-yl)-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 958 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010571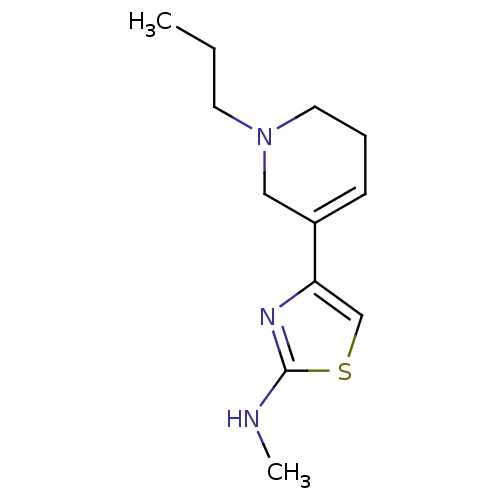 (CHEMBL108682 | Methyl-[4-(1-propyl-1,2,5,6-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. (25% inhibition at 10 e-6 M) | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010575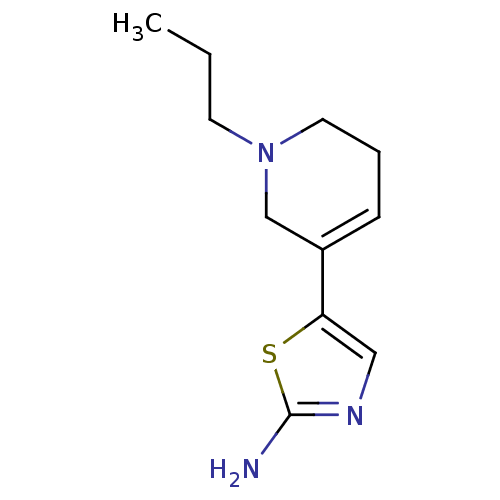 (5-(1-Propyl-1,2,5,6-tetrahydro-pyridin-3-yl)-3H-1l...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. (2% inhibition at 10 e-6 M) | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367941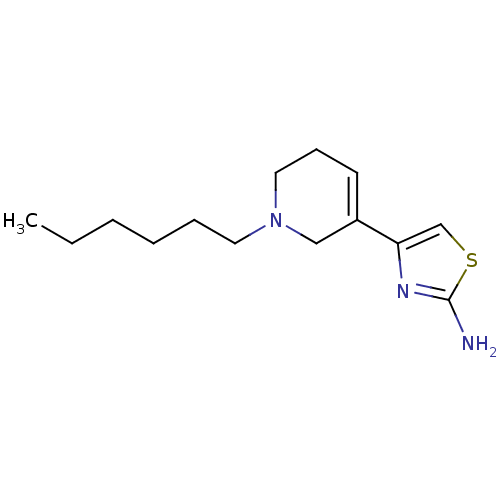 (CHEMBL1203003) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. (36%inhibition at 10 e-6 M) | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010571 (CHEMBL108682 | Methyl-[4-(1-propyl-1,2,5,6-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine binding to Dopamine receptor D2 of rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367938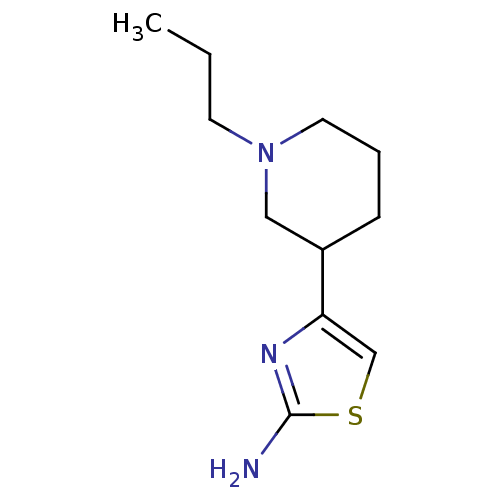 (CHEMBL1203009) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. (15% inhibition at 10 e-6 M) | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367947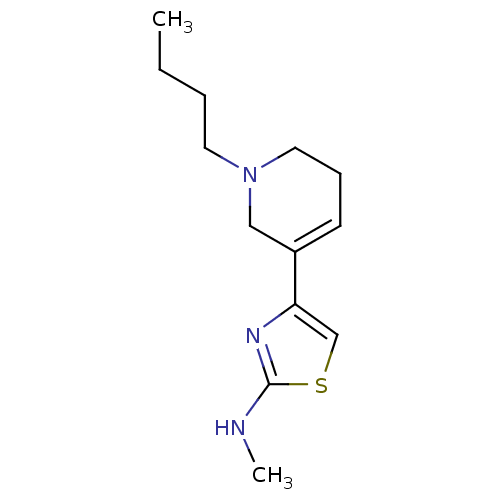 (CHEMBL1203005) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. (25% inhibition at 10 e-6 M) | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367942 (CHEMBL1203006) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. (41% inhibition at 10 e-6 M) | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367945 (CHEMBL1203008) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367946 (CHEMBL1203001) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50451971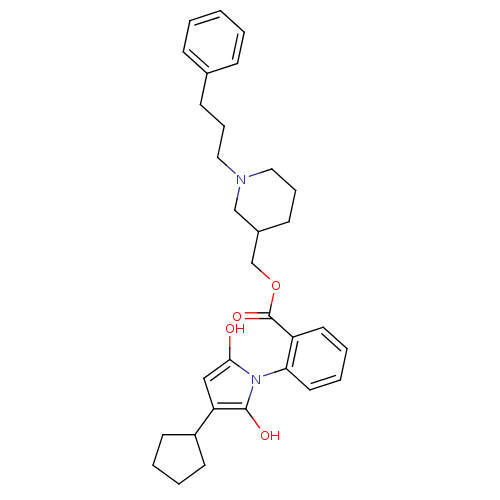 (CHEMBL330867) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50451972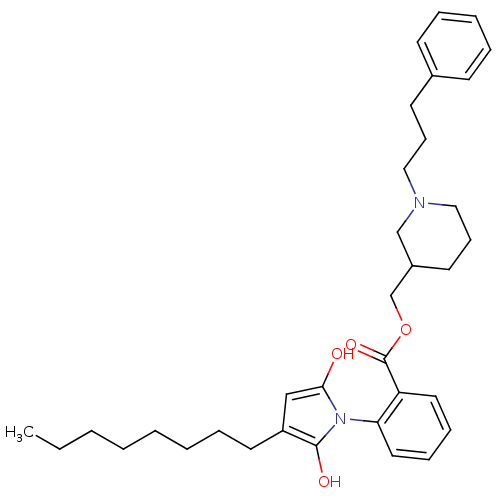 (CHEMBL125960) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50451974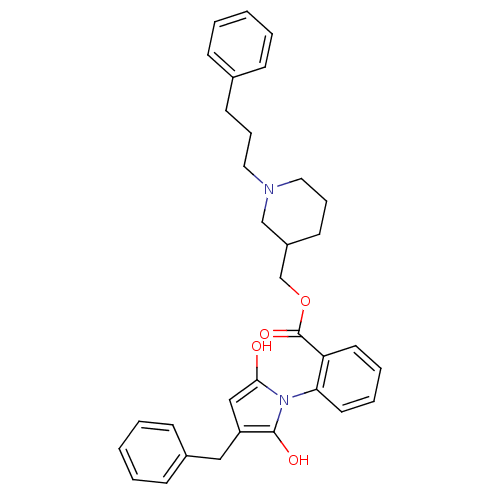 (CHEMBL340342) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50149051 (2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-benzoic ac...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50451977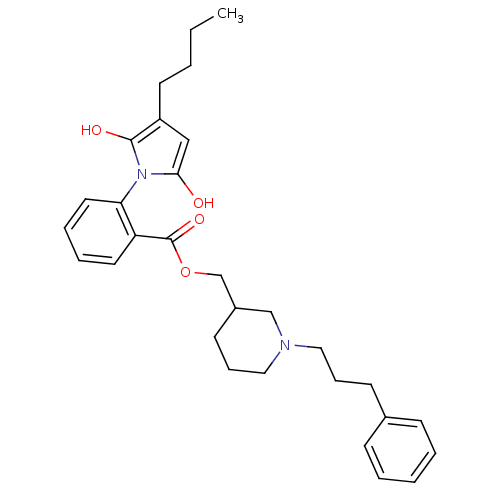 (CHEMBL122505) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50419792 (CHEMBL1950609) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Antagonist activity at human nAChR alpha4/beta2 receptor expressed in HEK ts201 cells assessed as calcium accumulation by fluorescence analysis | Bioorg Med Chem Lett 22: 1797-813 (2012) Article DOI: 10.1016/j.bmcl.2011.11.051 BindingDB Entry DOI: 10.7270/Q22Z16S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50149040 (Biphenyl-2-carboxylic acid 1-(3-phenyl-propyl)-pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50451973 (CHEMBL332769) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50166940 (NP-031112 | NP-12 | Tideglusib | US20230414581, Co...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GSK-3beta incubated with substrate in presence of ATP measured by serial dilution assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116179 BindingDB Entry DOI: 10.7270/Q2H70KMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367943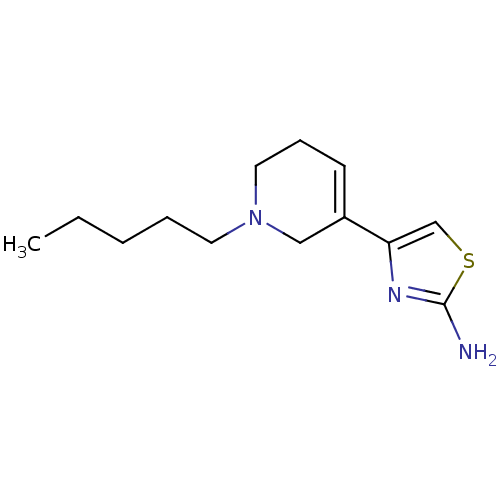 (CHEMBL1202201) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]N-propylnorapomorphine binding to Dopamine receptor D2 of rat striatal membranes | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50366799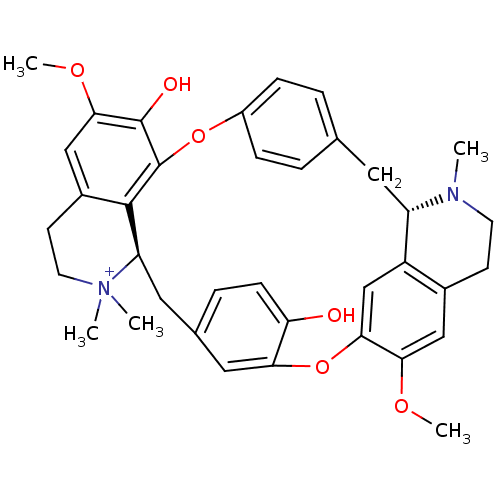 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367940 (CHEMBL1203007) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367944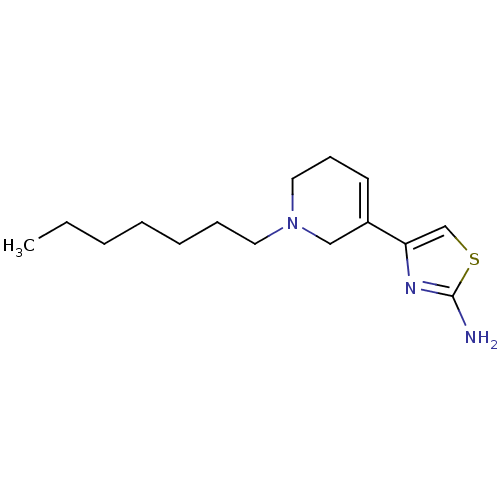 (CHEMBL1203004) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50419827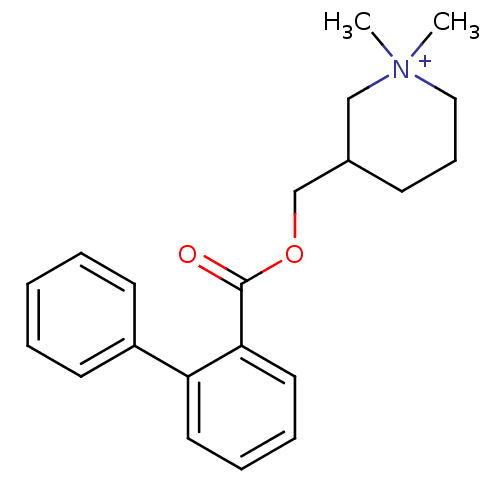 (CHEMBL1950506) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Antagonist activity at human nAChR alpha4/beta2 receptor expressed in HEK ts201 cells assessed as calcium accumulation by fluorescence analysis | Bioorg Med Chem Lett 22: 1797-813 (2012) Article DOI: 10.1016/j.bmcl.2011.11.051 BindingDB Entry DOI: 10.7270/Q22Z16S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50451978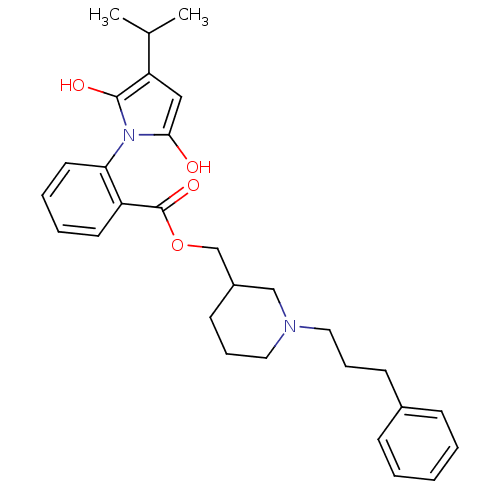 (CHEMBL334123) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50366779 (METHYLLYCACONITINE) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367939 (CHEMBL1203002) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding for Dopamine receptor D2 in rat striatal membranes. | J Med Chem 33: 311-7 (1990) BindingDB Entry DOI: 10.7270/Q28S4QHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50419851 (CHEMBL1950510) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Antagonist activity at human nAChR alpha4/beta2 receptor expressed in HEK ts201 cells assessed as calcium accumulation by fluorescence analysis | Bioorg Med Chem Lett 22: 1797-813 (2012) Article DOI: 10.1016/j.bmcl.2011.11.051 BindingDB Entry DOI: 10.7270/Q22Z16S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 148 total ) | Next | Last >> |