

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427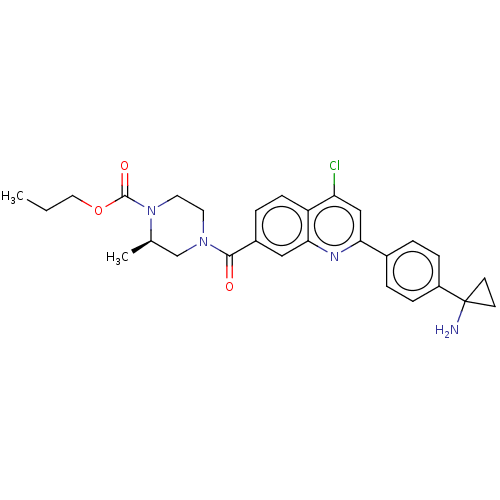 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433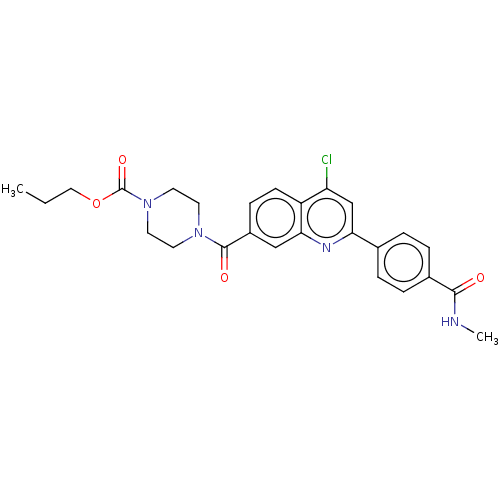 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434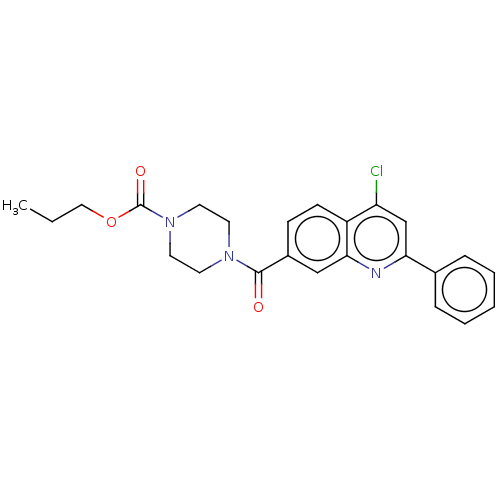 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50526157 (CHEMBL4550526) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50526161 (CHEMBL4464421) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26023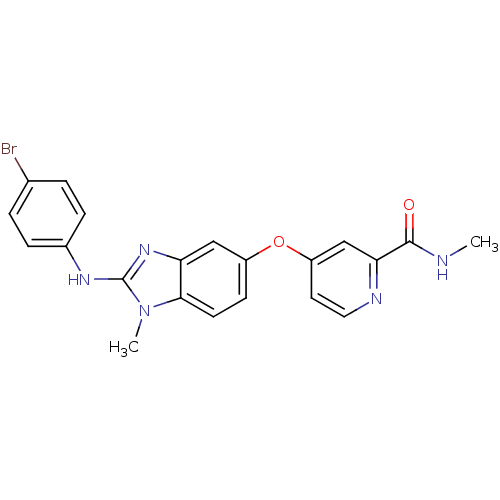 (4-({2-[(4-bromophenyl)(methyl)amino]-1H-1,3-benzod...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26034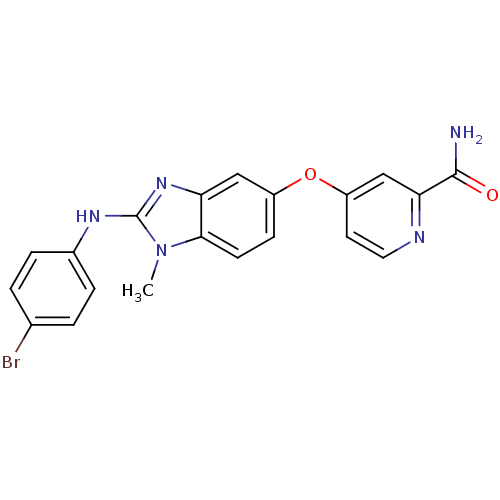 (4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50526161 (CHEMBL4464421) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50526157 (CHEMBL4550526) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM26023 (4-({2-[(4-bromophenyl)(methyl)amino]-1H-1,3-benzod...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547958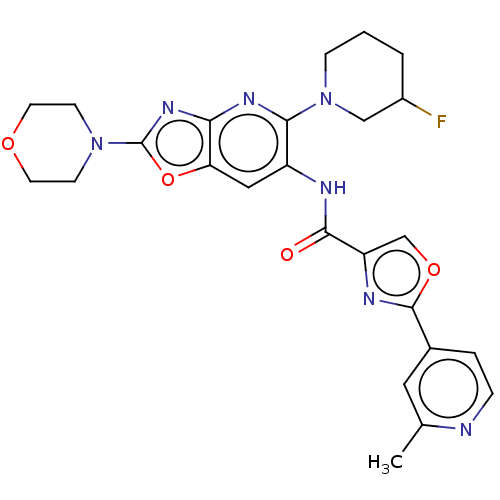 (CHEMBL4789918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50526156 (CHEMBL1630208 | WR301861) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50526161 (CHEMBL4464421) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50526161 (CHEMBL4464421) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547947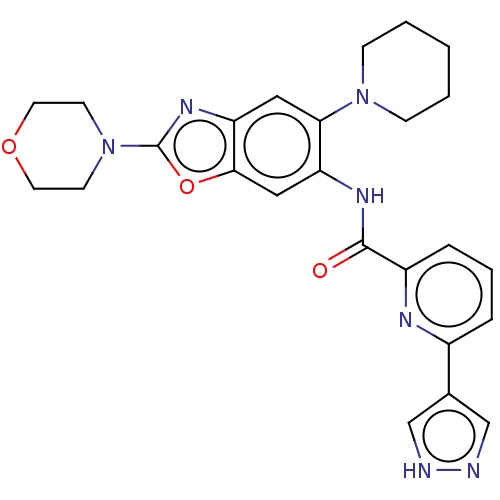 (CHEMBL4760206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547949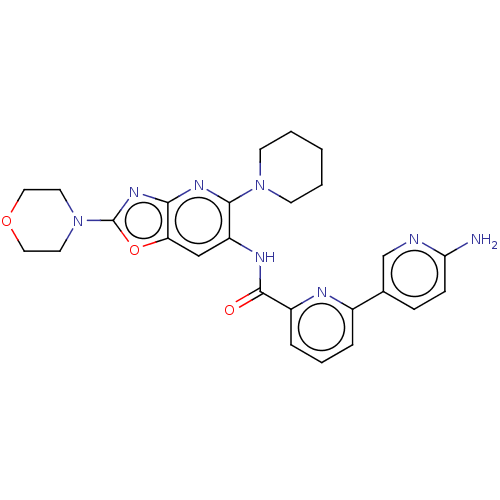 (CHEMBL4743091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50526156 (CHEMBL1630208 | WR301861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547959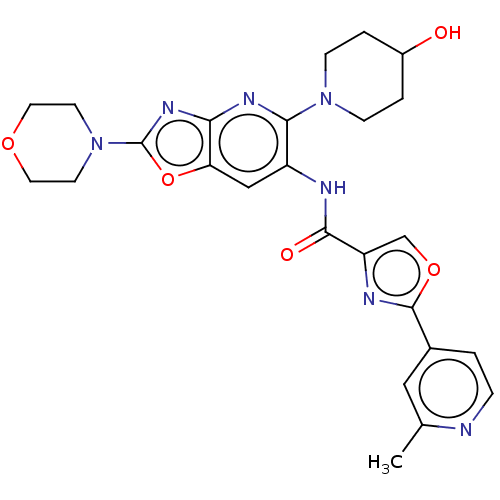 (CHEMBL4750630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547962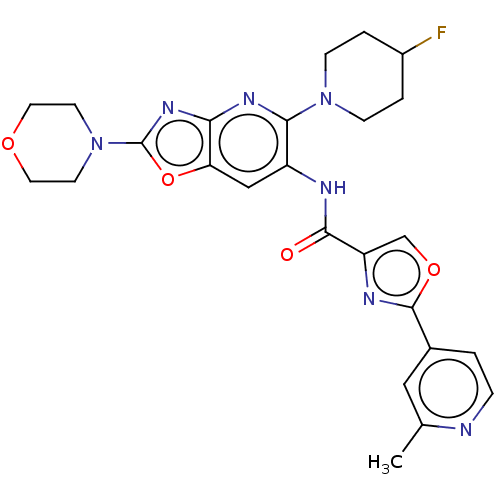 (CHEMBL4784665) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547955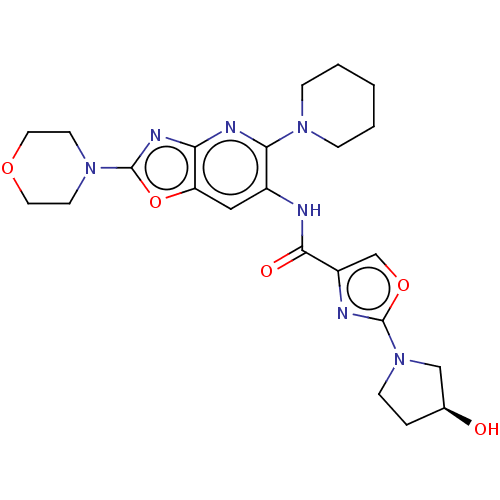 (CHEMBL4796558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50526156 (CHEMBL1630208 | WR301861) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50526156 (CHEMBL1630208 | WR301861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26028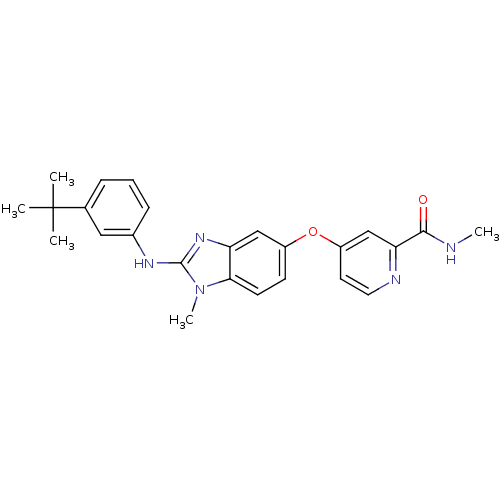 (4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26037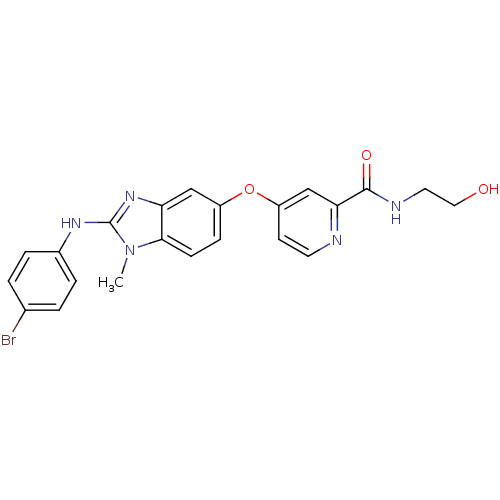 (4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26022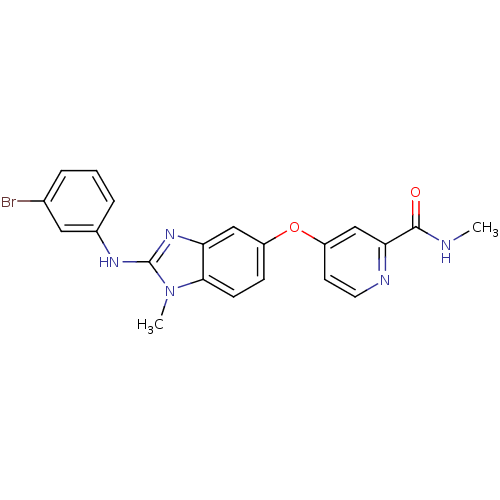 (4-({2-[(3-bromophenyl)(methyl)amino]-1H-1,3-benzod...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50526157 (CHEMBL4550526) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547954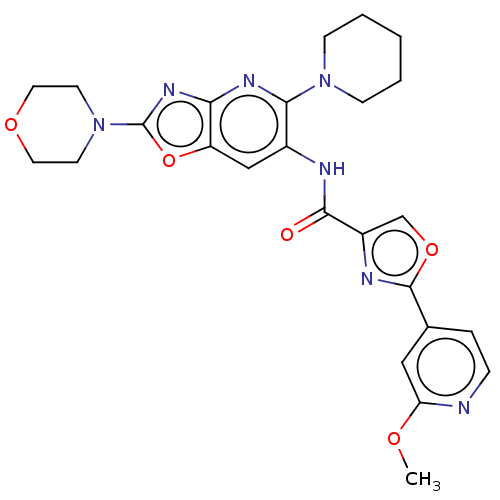 (CHEMBL4763990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547953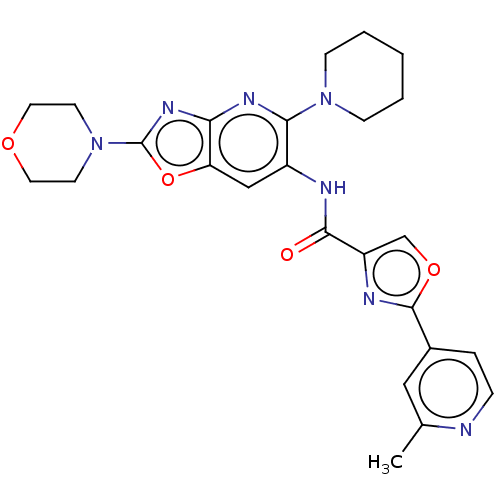 (CHEMBL4790506) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50526157 (CHEMBL4550526) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay | Eur J Med Chem 166: 369-380 (2019) Article DOI: 10.1016/j.ejmech.2019.01.077 BindingDB Entry DOI: 10.7270/Q2K93BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26025 (N-methyl-4-[(2-{methyl[3-(trifluoromethyl)phenyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547961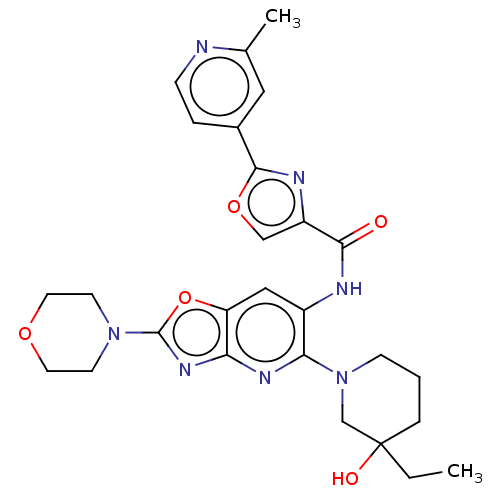 (CHEMBL4777409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM26038 (4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547946 (CHEMBL4757687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26017 (4-({2-[(3-tert-butylphenyl)amino]-1H-1,3-benzodiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM26025 (N-methyl-4-[(2-{methyl[3-(trifluoromethyl)phenyl]a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375523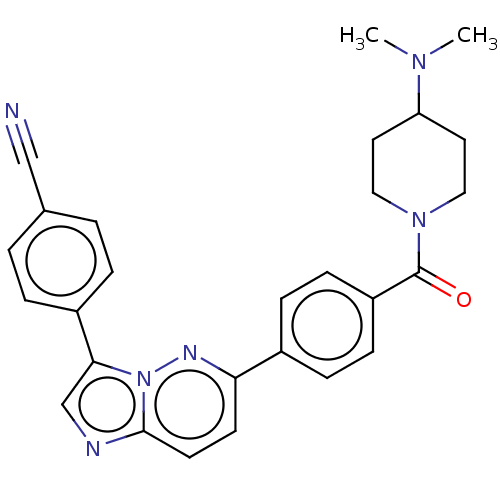 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26026 (N-methyl-4-[(2-{methyl[4-(trifluoromethyl)phenyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547963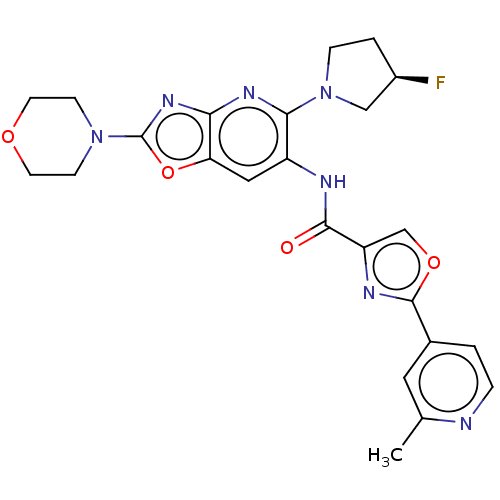 (CHEMBL4757349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26029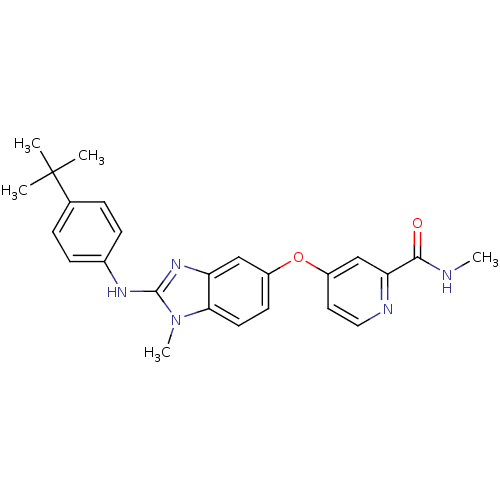 (4-({2-[(4-tert-butylphenyl)(methyl)amino]-1H-1,3-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26021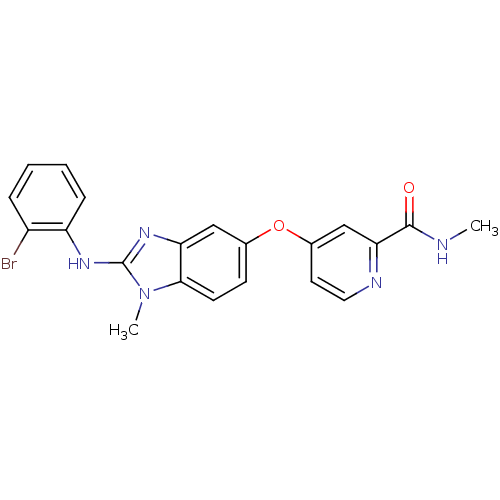 (4-({2-[(2-bromophenyl)(methyl)amino]-1H-1,3-benzod...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547948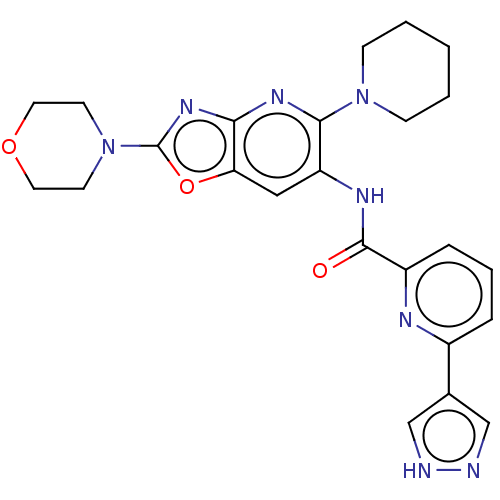 (CHEMBL4742305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM26032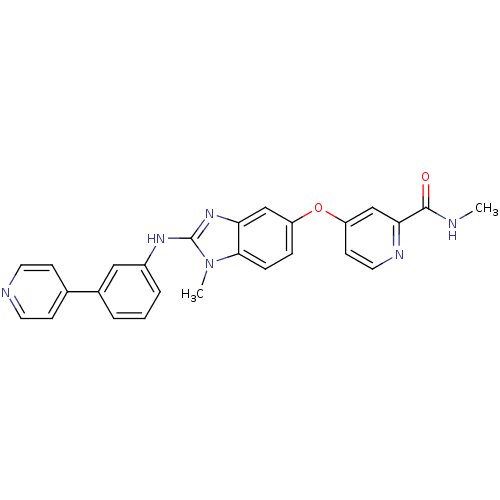 (N-methyl-4-[(2-{methyl[3-(pyridin-4-yl)phenyl]amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103282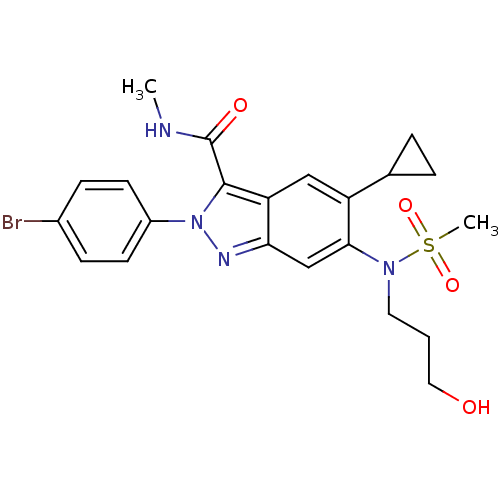 (US8546389, 4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375523 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM26037 (4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50547950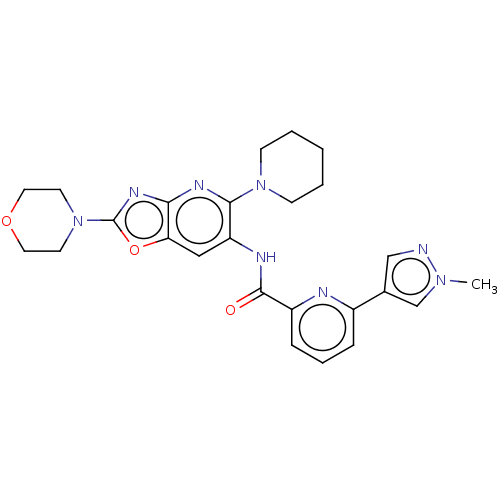 (CHEMBL4748475) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length N-terminal His6-tagged human IRAK4 expressed in baculovirus infected sf9 cells using biotinylated histone H3 as... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00255 BindingDB Entry DOI: 10.7270/Q28G8QB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM26016 (N-methyl-4-[(2-{[4-(trifluoromethyl)phenyl]amino}-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... | J Med Chem 51: 7049-52 (2008) Article DOI: 10.1021/jm801050k BindingDB Entry DOI: 10.7270/Q2B56H1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 622 total ) | Next | Last >> |