 Found 1035 hits with Last Name = 'nencetti' and Initial = 's'
Found 1035 hits with Last Name = 'nencetti' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50294132
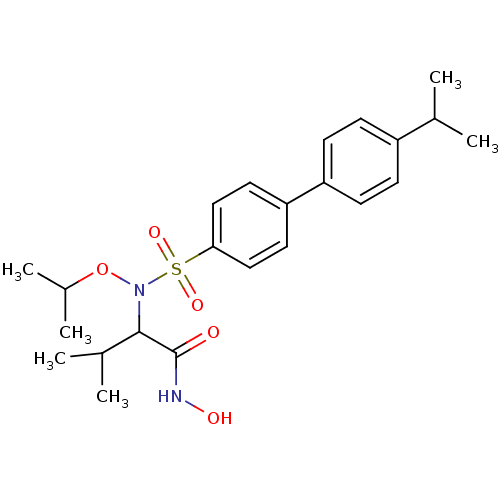
(CHEMBL561963 | N-hydroxy-2-(N-isopropoxy-4'-isopro...)Show SMILES CC(C)ON(C(C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(C)C Show InChI InChI=1S/C23H32N2O5S/c1-15(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)31(28,29)25(30-17(5)6)22(16(3)4)23(26)24-27/h7-17,22,27H,1-6H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50294132

(CHEMBL561963 | N-hydroxy-2-(N-isopropoxy-4'-isopro...)Show SMILES CC(C)ON(C(C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(C)C Show InChI InChI=1S/C23H32N2O5S/c1-15(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)31(28,29)25(30-17(5)6)22(16(3)4)23(26)24-27/h7-17,22,27H,1-6H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP13-mediated collagen degradation by SDS-PAGE |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50294122
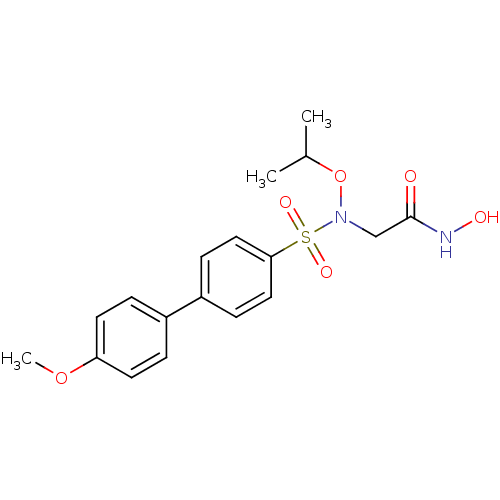
(CHEMBL560837 | N-Hydroxy-2-(N-isopropoxy-4'-methox...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N(CC(=O)NO)OC(C)C Show InChI InChI=1S/C18H22N2O6S/c1-13(2)26-20(12-18(21)19-22)27(23,24)17-10-6-15(7-11-17)14-4-8-16(25-3)9-5-14/h4-11,13,22H,12H2,1-3H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50366768

(CHEMBL611561)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NCCCCCCCCNc3ccc([N+]([O-])=O)c4[n-][o+]nc34)ncnc12 Show InChI InChI=1S/C26H34N10O7/c1-2-27-25(39)22-20(37)21(38)26(42-22)35-14-32-19-23(30-13-31-24(19)35)29-12-8-6-4-3-5-7-11-28-15-9-10-16(36(40)41)18-17(15)33-43-34-18/h9-10,13-14,20-22,26,28,37-38H,2-8,11-12H2,1H3,(H,27,39)(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human Adenosine A3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 3023-6 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4K0H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human Adenosine A3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 3023-6 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4K0H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 catalytic domain pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50161331
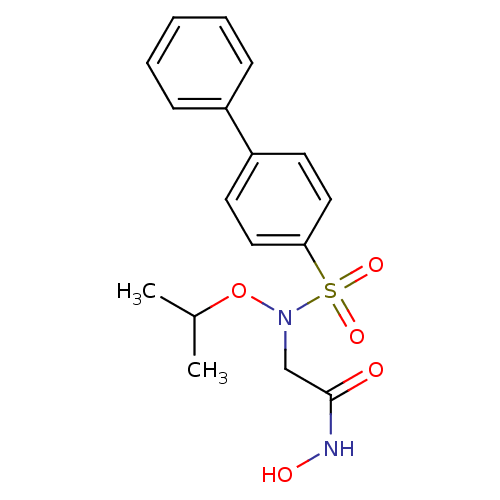
(2-[(Biphenyl-4-sulfonyl)-isopropoxy-amino]-N-hydro...)Show SMILES CC(C)ON(CC(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C17H20N2O5S/c1-13(2)24-19(12-17(20)18-21)25(22,23)16-10-8-15(9-11-16)14-6-4-3-5-7-14/h3-11,13,21H,12H2,1-2H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Affinity for A2a receptor by displacement of [3H]-CGS- 21680 from human striatum |
Bioorg Med Chem Lett 11: 3023-6 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4K0H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50399079
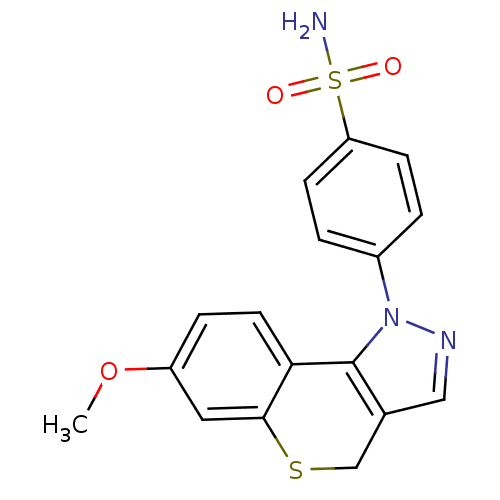
(CHEMBL2179308)Show SMILES COc1ccc2-c3c(CSc2c1)cnn3-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15N3O3S2/c1-23-13-4-7-15-16(8-13)24-10-11-9-19-20(17(11)15)12-2-5-14(6-3-12)25(18,21)22/h2-9H,10H2,1H3,(H2,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA2 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 catalytic domain pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA2 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50366767
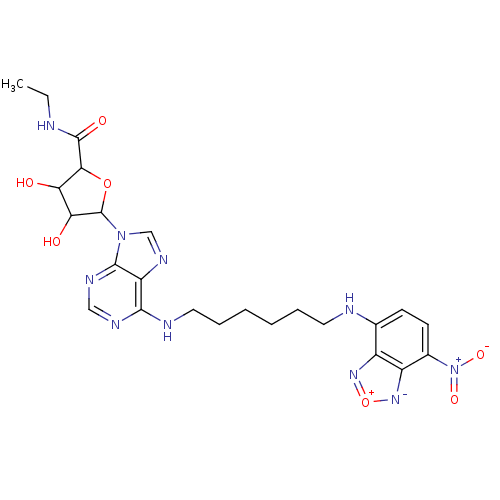
(CHEMBL611273)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NCCCCCCNc3ccc([N+]([O-])=O)c4[n-][o+]nc34)ncnc12 Show InChI InChI=1S/C24H30N10O7/c1-2-25-23(37)20-18(35)19(36)24(40-20)33-12-30-17-21(28-11-29-22(17)33)27-10-6-4-3-5-9-26-13-7-8-14(34(38)39)16-15(13)31-41-32-16/h7-8,11-12,18-20,24,26,35-36H,2-6,9-10H2,1H3,(H,25,37)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human Adenosine A3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 3023-6 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4K0H |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50294124
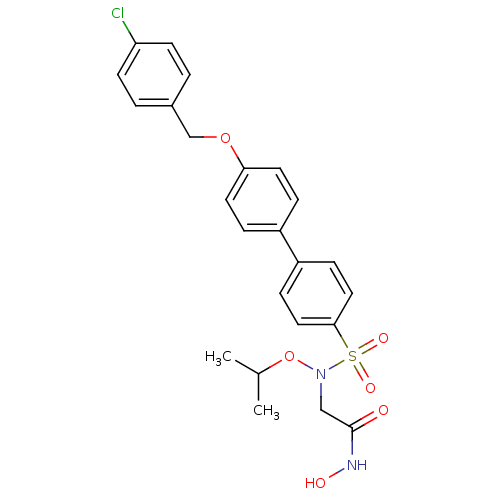
(2-(4'-(4-Chlorobenzyloxy)-N-isopropoxybiphenyl-4-y...)Show SMILES CC(C)ON(CC(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C24H25ClN2O6S/c1-17(2)33-27(15-24(28)26-29)34(30,31)23-13-7-20(8-14-23)19-5-11-22(12-6-19)32-16-18-3-9-21(25)10-4-18/h3-14,17,29H,15-16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50366766
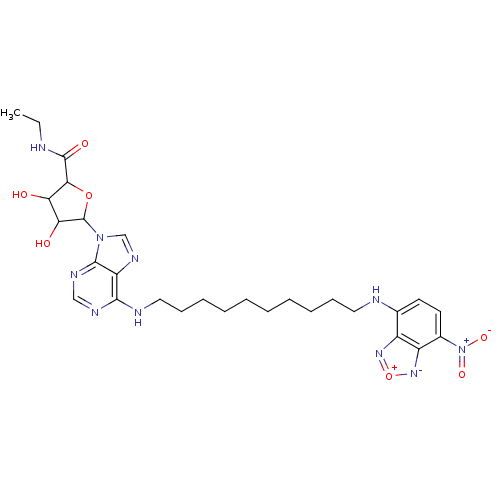
(CHEMBL611562)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NCCCCCCCCCCNc3ccc([N+]([O-])=O)c4[n-][o+]nc34)ncnc12 Show InChI InChI=1S/C28H38N10O7/c1-2-29-27(41)24-22(39)23(40)28(44-24)37-16-34-21-25(32-15-33-26(21)37)31-14-10-8-6-4-3-5-7-9-13-30-17-11-12-18(38(42)43)20-19(17)35-45-36-20/h11-12,15-16,22-24,28,30,39-40H,2-10,13-14H2,1H3,(H,29,41)(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human Adenosine A3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 3023-6 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4K0H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50399078
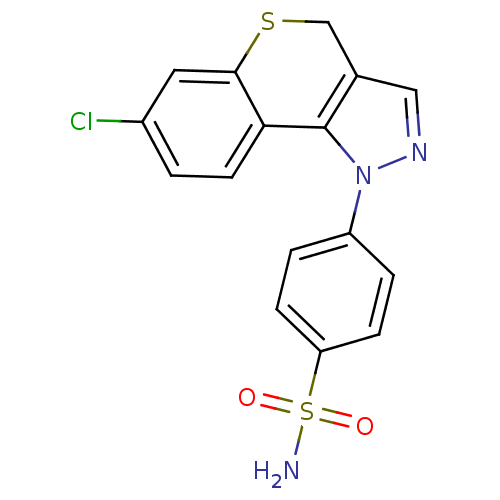
(CHEMBL2179307)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1ncc2CSc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C16H12ClN3O2S2/c17-11-1-6-14-15(7-11)23-9-10-8-19-20(16(10)14)12-2-4-13(5-3-12)24(18,21)22/h1-8H,9H2,(H2,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA2 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50294131
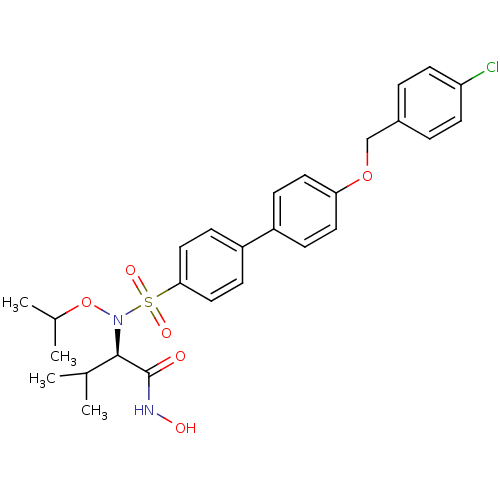
((R)-2-(4'-(4-Chlorobenzyloxy)-N-isopropoxybiphenyl...)Show SMILES CC(C)ON([C@H](C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccc(Cl)cc2)cc1 |r| Show InChI InChI=1S/C27H31ClN2O6S/c1-18(2)26(27(31)29-32)30(36-19(3)4)37(33,34)25-15-9-22(10-16-25)21-7-13-24(14-8-21)35-17-20-5-11-23(28)12-6-20/h5-16,18-19,26,32H,17H2,1-4H3,(H,29,31)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP13-mediated collagen degradation by SDS-PAGE |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA2 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50399075

(CHEMBL2179304)Show SMILES Cc1ccc2-c3c(CSc2n1)cnn3-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N4O2S2/c1-10-2-7-14-15-11(9-23-16(14)19-10)8-18-20(15)12-3-5-13(6-4-12)24(17,21)22/h2-8H,9H2,1H3,(H2,17,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA2 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50036835
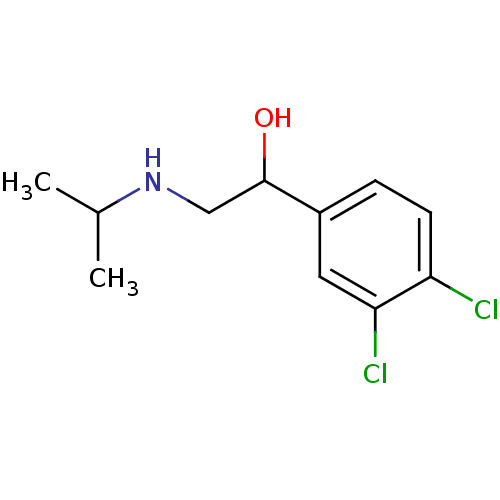
(1-(3,4-Dichloro-phenyl)-2-isopropylamino-ethanol |...)Show InChI InChI=1S/C11H15Cl2NO/c1-7(2)14-6-11(15)8-3-4-9(12)10(13)5-8/h3-5,7,11,14-15H,6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Pisa
Curated by ChEMBL
| Assay Description
Binding affinity against beta1-adrenergic receptor in rat brain |
J Med Chem 37: 1518-25 (1994)
BindingDB Entry DOI: 10.7270/Q2V69HM4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50366765

(CHEMBL611552)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NCCNc3ccc([N+]([O-])=O)c4[n-][o+]nc34)ncnc12 Show InChI InChI=1S/C20H22N10O7/c1-2-21-19(33)16-14(31)15(32)20(36-16)29-8-26-13-17(24-7-25-18(13)29)23-6-5-22-9-3-4-10(30(34)35)12-11(9)27-37-28-12/h3-4,7-8,14-16,20,22,31-32H,2,5-6H2,1H3,(H,21,33)(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human Adenosine A3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 3023-6 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4K0H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50399079

(CHEMBL2179308)Show SMILES COc1ccc2-c3c(CSc2c1)cnn3-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15N3O3S2/c1-23-13-4-7-15-16(8-13)24-10-11-9-19-20(17(11)15)12-2-5-14(6-3-12)25(18,21)22/h2-9H,10H2,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA1 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50399076
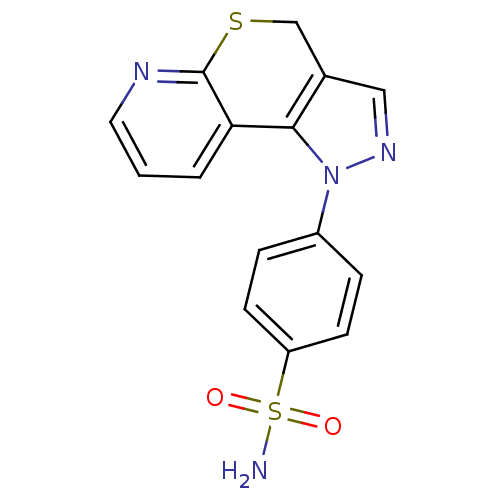
(CHEMBL2179305)Show InChI InChI=1S/C15H12N4O2S2/c16-23(20,21)12-5-3-11(4-6-12)19-14-10(8-18-19)9-22-15-13(14)2-1-7-17-15/h1-8H,9H2,(H2,16,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA2 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50429815
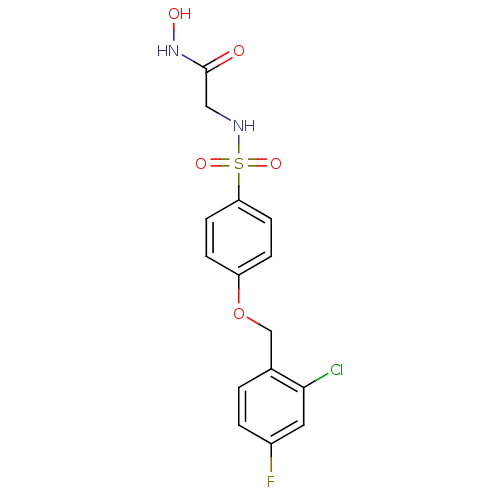
(CHEMBL2337698)Show SMILES ONC(=O)CNS(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C15H14ClFN2O5S/c16-14-7-11(17)2-1-10(14)9-24-12-3-5-13(6-4-12)25(22,23)18-8-15(20)19-21/h1-7,18,21H,8-9H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ADAMTS5 using bovine nasal cartilage aggrecan as substrate assessed as inhibition of 1772-AGEG neopeptide formation i... |
Eur J Med Chem 62: 379-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.058
BindingDB Entry DOI: 10.7270/Q20C4X4W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA5B pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50204371

(2-{isopropoxy[(4-methoxyphenyl)sulfonyl]amino}-N-h...)Show InChI InChI=1S/C12H18N2O6S/c1-9(2)20-14(8-12(15)13-16)21(17,18)11-6-4-10(19-3)5-7-11/h4-7,9,16H,8H2,1-3H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA5B pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA6 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50204384
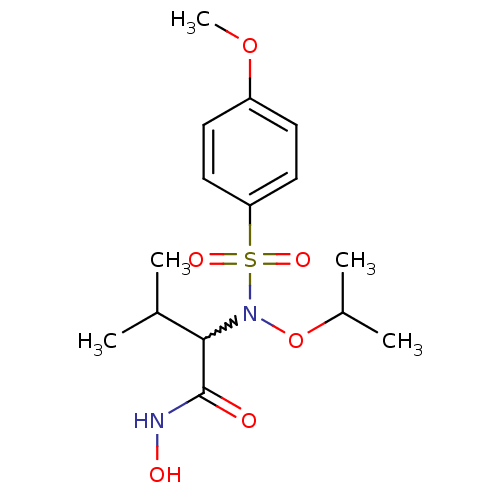
(CHEMBL442057 | N-hydroxy-2-[N-isopropoxy(4-methoxy...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(OC(C)C)C(C(C)C)C(=O)NO |w:16.16| Show InChI InChI=1S/C15H24N2O6S/c1-10(2)14(15(18)16-19)17(23-11(3)4)24(20,21)13-8-6-12(22-5)7-9-13/h6-11,14,19H,1-5H3,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50204376
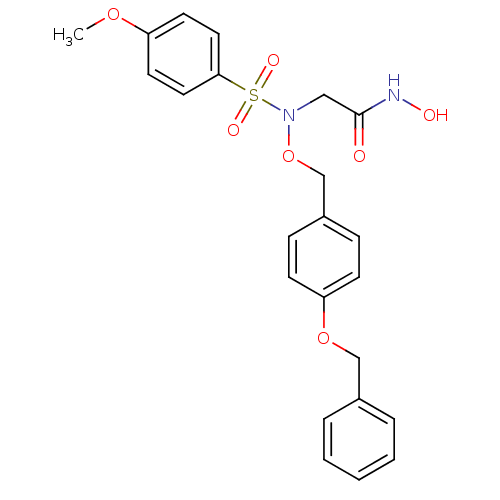
(2-{benzyloxybenzyloxy[(4-methoxyphenyl)sulfonyl]am...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(=O)NO)OCc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C23H24N2O7S/c1-30-20-11-13-22(14-12-20)33(28,29)25(15-23(26)24-27)32-17-19-7-9-21(10-8-19)31-16-18-5-3-2-4-6-18/h2-14,27H,15-17H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50204380
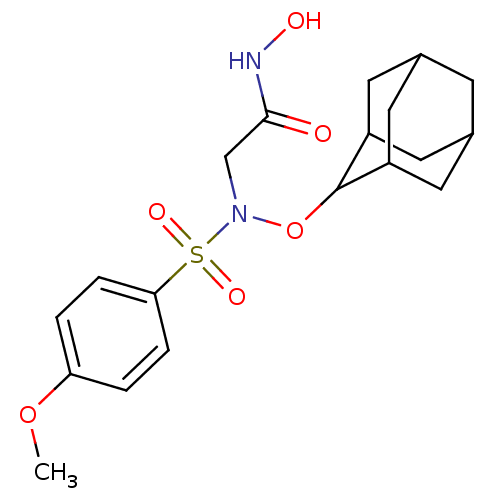
(2-{adamantyloxy[(4-methoxyphenyl)sulfonyl]-amino}-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(=O)NO)OC1C2CC3CC(C2)CC1C3 |TLB:17:18:20:24.22.23,17:18:20.21.27:24.23.25,THB:22:21:18:24.23.25,22:23:20.21.27:18,25:23:20:27.26.18,25:26:20:24.22.23,(33.1,-3.11,;31.77,-2.34,;30.44,-3.12,;30.44,-4.67,;29.1,-5.44,;27.77,-4.67,;27.77,-3.13,;29.1,-2.36,;26.44,-5.44,;25.09,-6.2,;25.68,-4.1,;27.2,-6.78,;26.42,-8.11,;24.88,-8.1,;24.12,-6.76,;24.1,-9.43,;22.56,-9.42,;28.74,-6.79,;29.5,-8.12,;29.51,-9.61,;28.31,-10.88,;29.81,-10.46,;31.22,-11.03,;32.23,-9.75,;30.84,-10.1,;32.24,-8.22,;30.85,-7.64,;29.8,-8.88,)| Show InChI InChI=1S/C19H26N2O6S/c1-26-16-2-4-17(5-3-16)28(24,25)21(11-18(22)20-23)27-19-14-7-12-6-13(9-14)10-15(19)8-12/h2-5,12-15,19,23H,6-11H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50204377
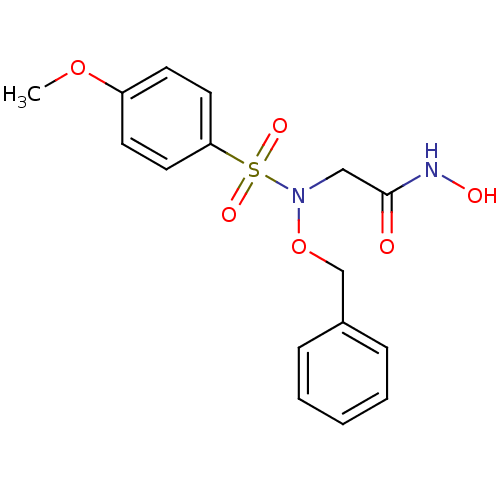
(2-{benzyloxy[(4-methoxyphenyl)sulfonyl]amino}-N-hy...)Show InChI InChI=1S/C16H18N2O6S/c1-23-14-7-9-15(10-8-14)25(21,22)18(11-16(19)17-20)24-12-13-5-3-2-4-6-13/h2-10,20H,11-12H2,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50204373
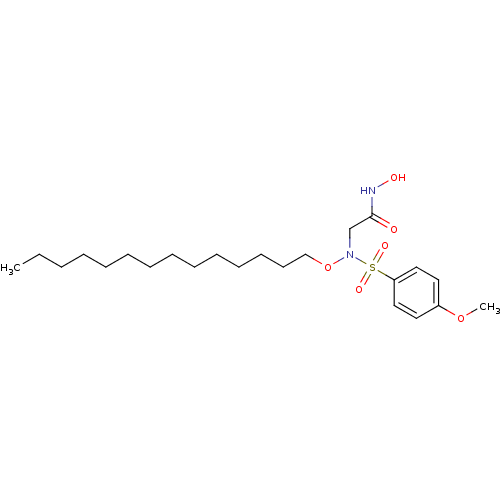
(2-{n-tetradecyloxy[(4-methoxyphenyl)sulfonyl]amino...)Show SMILES CCCCCCCCCCCCCCON(CC(=O)NO)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C23H40N2O6S/c1-3-4-5-6-7-8-9-10-11-12-13-14-19-31-25(20-23(26)24-27)32(28,29)22-17-15-21(30-2)16-18-22/h15-18,27H,3-14,19-20H2,1-2H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 15: 2298-311 (2007)
Article DOI: 10.1016/j.bmc.2007.01.023
BindingDB Entry DOI: 10.7270/Q2VX0HBC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA13 catalytic domain pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA14 catalytic domain pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Beta-carbonic anhydrase 1
(Mycobacterium tuberculosis) | BDBM50399075

(CHEMBL2179304)Show SMILES Cc1ccc2-c3c(CSc2n1)cnn3-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N4O2S2/c1-10-2-7-14-15-11(9-23-16(14)19-10)8-18-20(15)12-3-5-13(6-4-12)24(17,21)22/h2-8H,9H2,1H3,(H2,17,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Beta-carbonic anhydrase 1
(Mycobacterium tuberculosis) | BDBM50399076

(CHEMBL2179305)Show InChI InChI=1S/C15H12N4O2S2/c16-23(20,21)12-5-3-11(4-6-12)19-14-10(8-18-19)9-22-15-13(14)2-1-7-17-15/h1-8H,9H2,(H2,16,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data