

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191171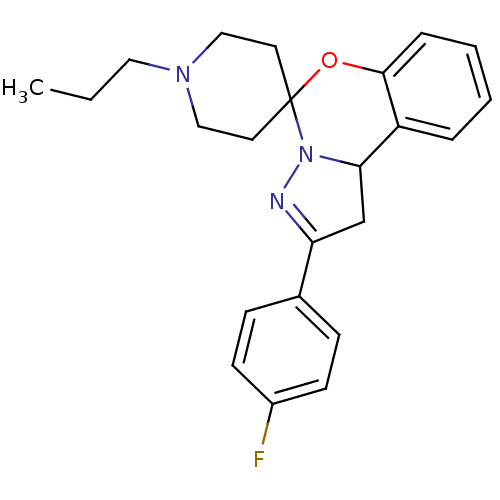 (2-(4-flurophenyl)-1,10b-dihydro-benzo[e]pyrazolo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191182 (1-propyl-4'-(pyridin-4-yl)-8'-oxa-5',6'-diazaspiro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191179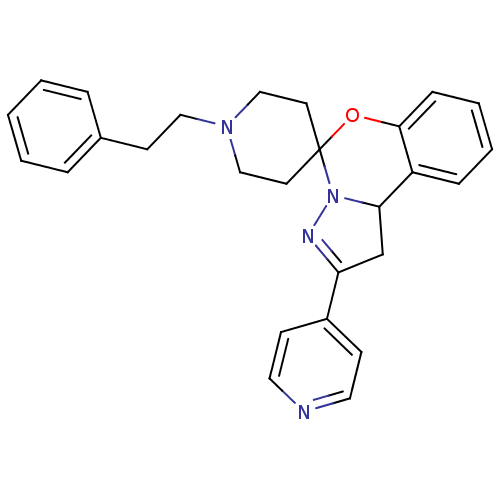 (1-(2-phenylethyl)-4'-(pyridin-4-yl)-8'-oxa-5',6'-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191173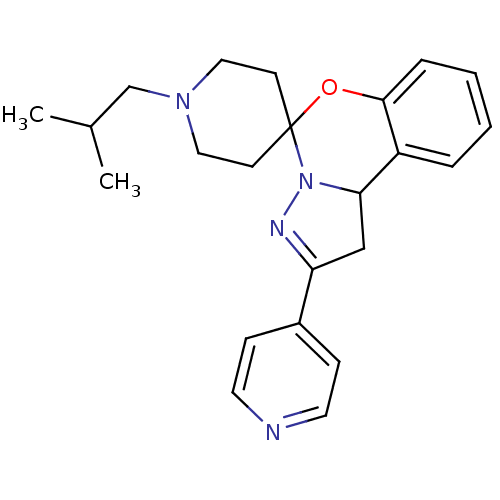 (1-(2-methylpropyl)-4'-(pyridin-4-yl)-8'-oxa-5',6'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191181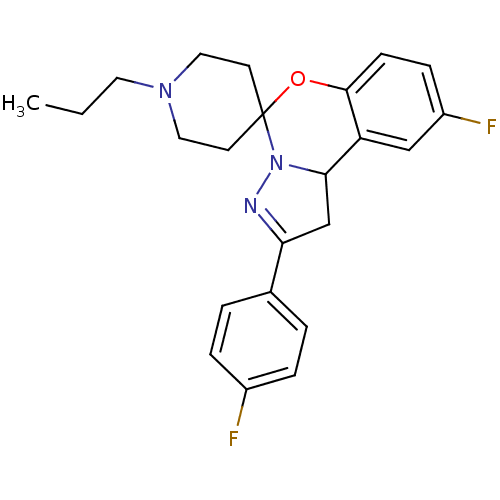 (12'-fluoro-4'-(4-fluorophenyl)-1-propyl-8'-oxa-5',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191208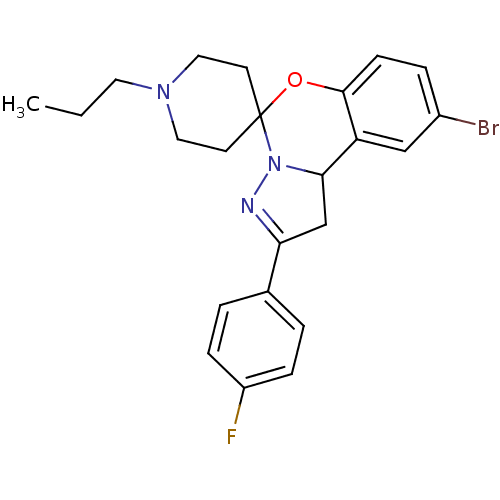 (12'-bromo-4'-(4-fluorophenyl)-1-propyl-8'-oxa-5',6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191194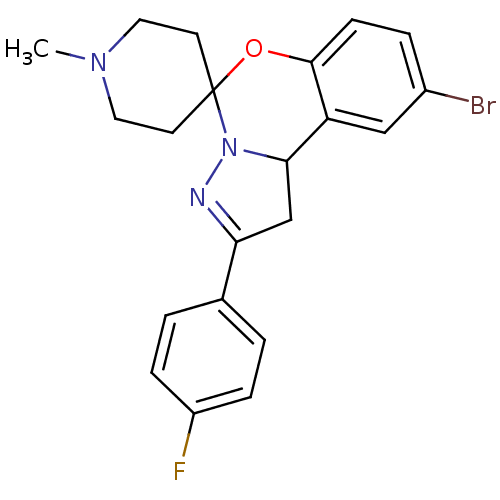 (12'-bromo-4'-(4-fluorophenyl)-1-methyl-8'-oxa-5',6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191191 (12'-chloro-4'-(4-fluorophenyl)-1-propyl-8'-oxa-5',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191196 (1-ethyl-4'-(pyridin-4-yl)-8'-oxa-5',6'-diazaspiro[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191209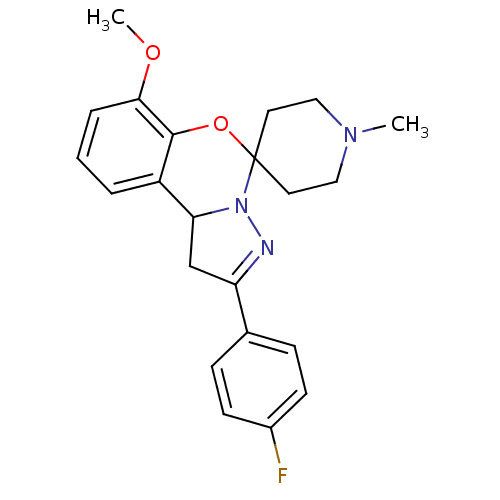 (4'-(4-fluorophenyl)-10'-methoxy-1-methyl-8'-oxa-5'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191188 (1-methyl-4'-phenyl-8'-oxa-5',6'-diazaspiro[piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM240726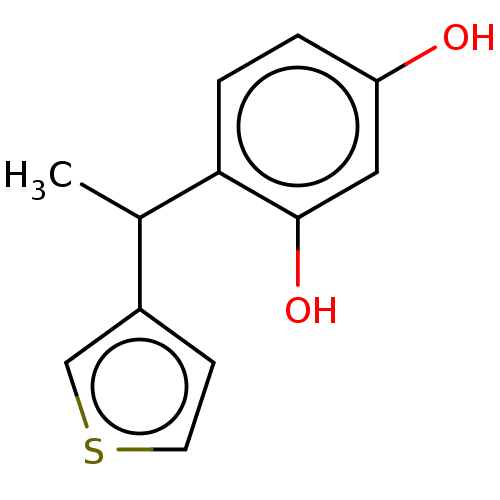 (US9422261, Compound 4) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM240723 (US9422261, Compound 1) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191178 (12'-bromo-1-methyl-4'-phenyl-8'-oxa-5',6'-diazaspi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191212 (1-methyl-4'-(pyridin-4-yl)-8'-oxa-5',6'-diazaspiro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191187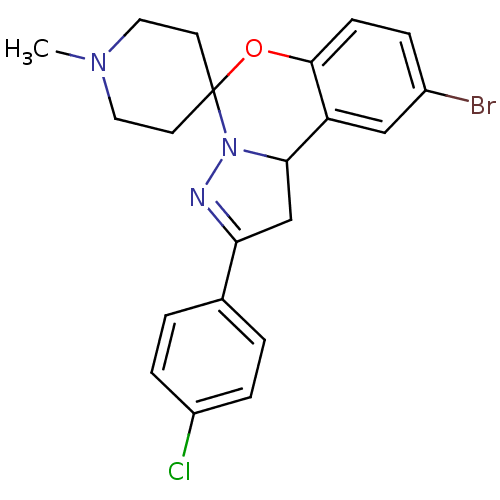 (12'-bromo-4'-(4-chlorophenyl)-1-methyl-8'-oxa-5',6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM240731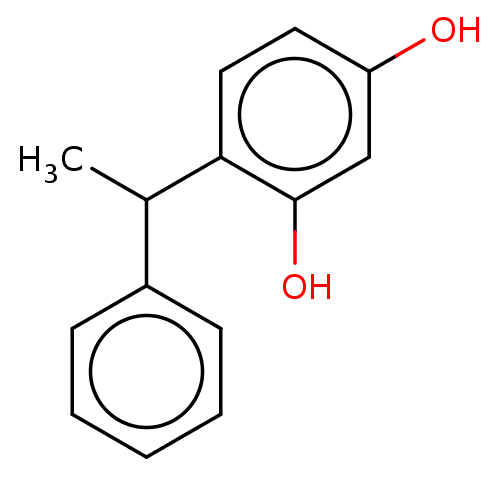 (US9422261, 4-(1- phenylethyl) benzene-1,3- diol) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191180 (4'-(4-fluorophenyl)-12'-methyl-1-propyl-8'-oxa-5',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191175 (4'-(4-chlorophenyl)-1-(propan-2-yl)-8'-oxa-5',6'-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191211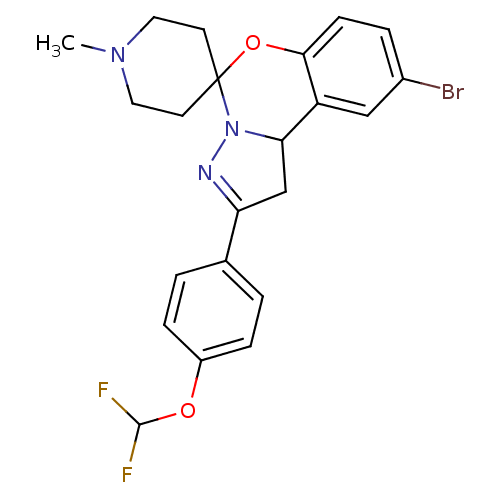 (12'-bromo-4'-[4-(difluoromethoxy)phenyl]-1-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191172 (12'-bromo-4'-(4-methoxyphenyl)-1-propyl-8'-oxa-5',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191185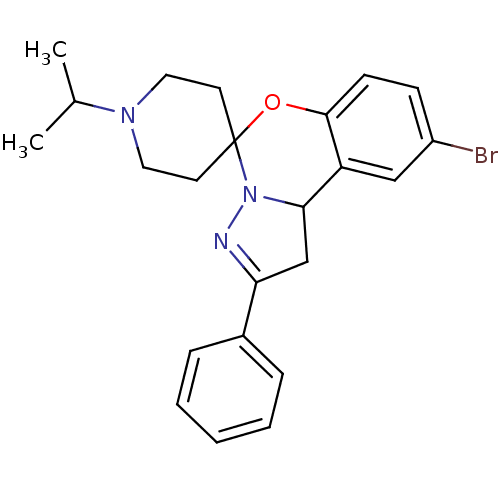 (12'-bromo-4'-phenyl-1-(propan-2-yl)-8'-oxa-5',6'-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM240725 (US9422261, Compound 3) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191204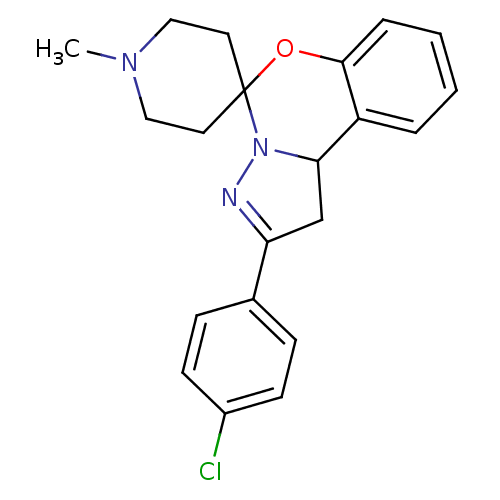 (4'-(4-chlorophenyl)-1-methyl-8'-oxa-5',6'-diazaspi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191206 (1-benzyl-4'-(4-chlorophenyl)-8'-oxa-5',6'-diazaspi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM240724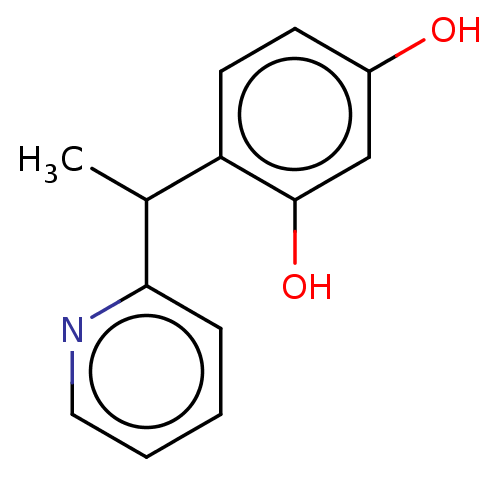 (US9422261, Compound 2) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191210 (4'-(4-bromophenyl)-1-methyl-8'-oxa-5',6'-diazaspir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191199 (12'-bromo-4'-[2-(difluoromethoxy)phenyl]-1-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191176 (4'-(2-chlorophenyl)-1-propyl-8'-oxa-5',6'-diazaspi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191192 (12'-bromo-4'-(3,4-dichlorophenyl)-1-methyl-8'-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 542 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191186 (4'-(2-chlorophenyl)-1-methyl-8'-oxa-5',6'-diazaspi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 753 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191195 (12'-bromo-4'-(4-bromophenyl)-1-propyl-8'-oxa-5',6'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 791 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191207 (12'-bromo-4'-(2-chlorophenyl)-1-methyl-8'-oxa-5',6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 856 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM50248158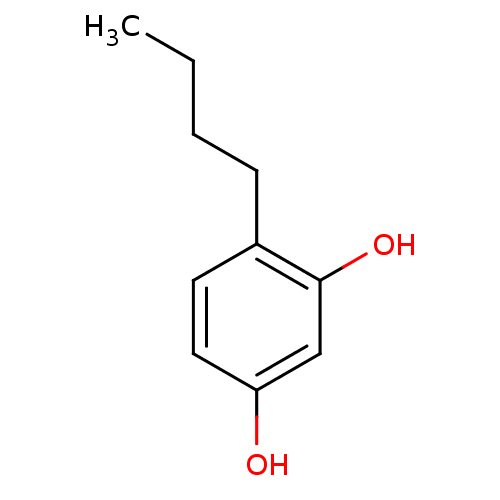 (CHEMBL450195 | N-butylresorcinol | US8993596, 4-Bu...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191203 (4'-(4-bromophenyl)-10'-methoxy-1-methyl-8'-oxa-5',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191193 (4'-(4-chlorophenyl)-10'-methoxy-1-methyl-8'-oxa-5'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM240727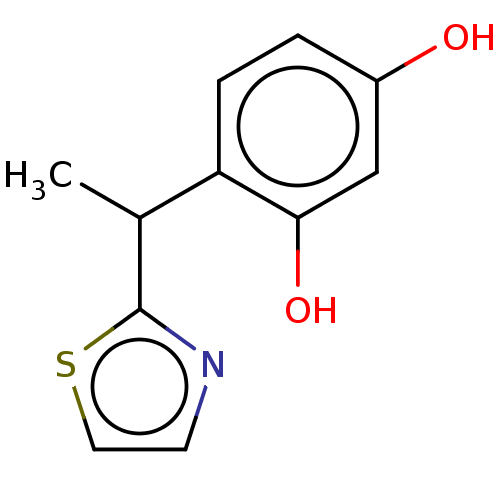 (US9422261, Compound 5) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188974 (5-chloro-3-(phenethylamino)-2-(pyridin-2-yl)indoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM240729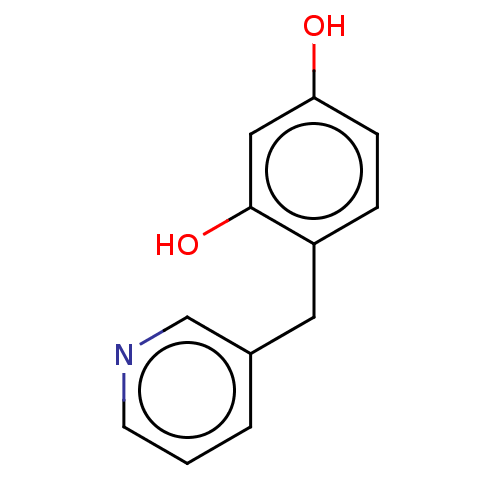 (US9422261, Unigen resorcinol derivative | US968291...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 6.8 | n/a |
PIERRE FABRE DERMO-COSMETIQUE US Patent | Assay Description Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phos... | US Patent US9422261 (2016) BindingDB Entry DOI: 10.7270/Q2PG1QMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188968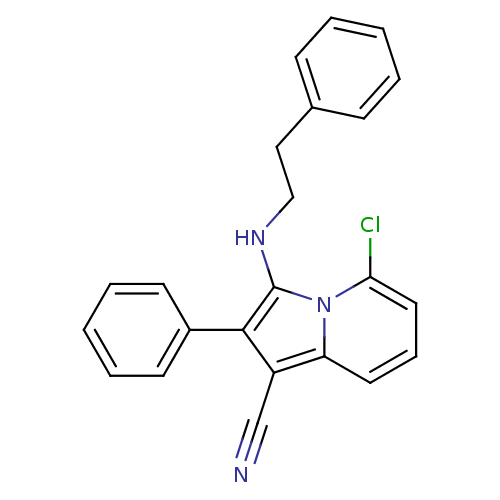 (5-chloro-3-(phenethylamino)-2-phenylindolizine-1-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188970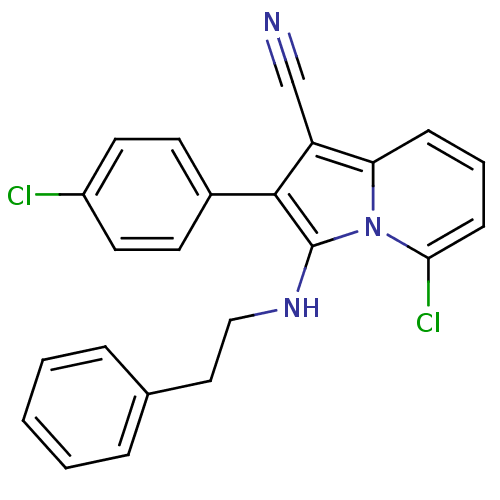 (5-chloro-2-(4-chlorophenyl)-3-(phenethylamino)indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50189004 (5-chloro-2-phenethyl-3-(phenethylamino)indolizine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188994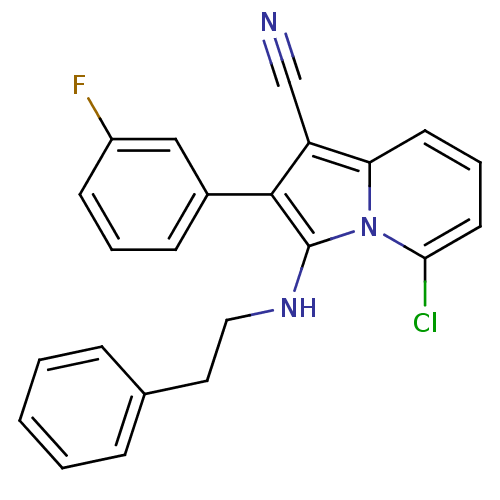 (5-chloro-2-(3-fluorophenyl)-3-(phenethylamino)indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188998 (5-chloro-2-(3-methoxyphenyl)-3-(phenethylamino)ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188969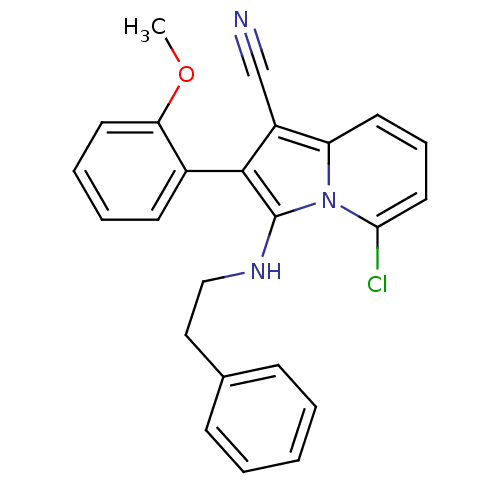 (5-chloro-2-(2-methoxyphenyl)-3-(phenethylamino)ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188971 (5-chloro-2-(4-(methylthio)phenyl)-3-(phenethylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188985 (5-chloro-2-(3-chlorophenyl)-3-(phenethylamino)indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188979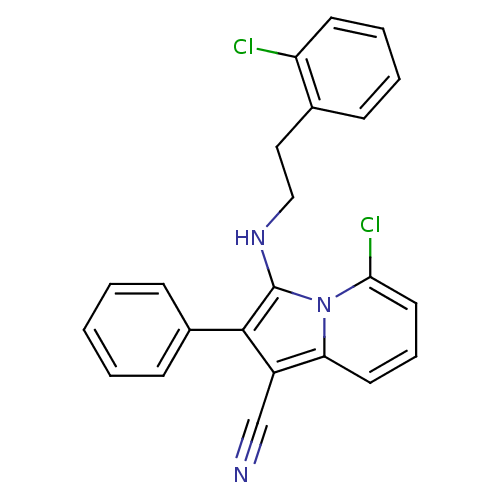 (3-(2-chlorophenethylamino)-5-chloro-2-phenylindoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188967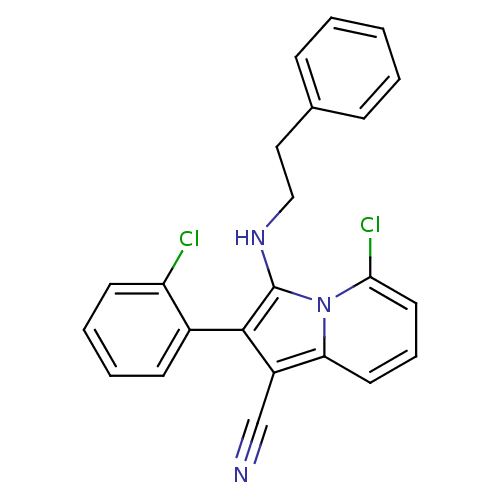 (5-chloro-2-(2-chlorophenyl)-3-(phenethylamino)indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropilin-1 (Homo sapiens (Human)) | BDBM50188990 (5-chloro-2-(2-fluorophenyl)-3-(phenethylamino)indo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie Curated by ChEMBL | Assay Description Inhibition of VEGF165-NRP1 interaction by ELISA | Bioorg Med Chem Lett 16: 3998-4001 (2006) Article DOI: 10.1016/j.bmcl.2006.05.014 BindingDB Entry DOI: 10.7270/Q20Z72WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 92 total ) | Next | Last >> |