 Found 163 hits with Last Name = 'voss' and Initial = 's'
Found 163 hits with Last Name = 'voss' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nonstructural protein 3
(Zika virus) | BDBM50212337

(CHEMBL5267698)Show InChI InChI=1S/C14H8ClNO4/c15-7-3-4-8-9(6-7)16-14(19)13(18)11(12(8)17)10-2-1-5-20-10/h1-6,17H,(H,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50212337

(CHEMBL5267698)Show InChI InChI=1S/C14H8ClNO4/c15-7-3-4-8-9(6-7)16-14(19)13(18)11(12(8)17)10-2-1-5-20-10/h1-6,17H,(H,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50030459
(CHEMBL3344321)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)Cc1ccc(Cl)c(Cl)c1)C(=O)NC[C@H]1CC[C@@H](CC1)NC(N)=N |r,wU:34.38,9.9,wD:31.31,5.4,(16.97,-13.05,;16.9,-11.52,;15.54,-10.81,;15.47,-9.27,;14.11,-8.56,;14.04,-7.02,;12.68,-6.3,;11.38,-7.13,;11.44,-8.67,;10.01,-6.42,;9.94,-4.88,;11.24,-4.05,;11.18,-2.52,;12.48,-1.69,;12.42,-.15,;8.71,-7.24,;7.38,-6.48,;7.37,-4.94,;6.05,-7.25,;4.72,-6.48,;4.71,-4.94,;3.38,-4.17,;2.05,-4.94,;.72,-4.17,;2.05,-6.48,;.72,-7.25,;3.38,-7.25,;15.34,-6.19,;15.28,-4.65,;16.7,-6.9,;18.02,-6.09,;19.38,-6.82,;19.42,-8.36,;20.78,-9.09,;22.1,-8.27,;22.05,-6.73,;20.68,-6,;23.46,-9,;24.77,-8.18,;24.72,-6.65,;26.13,-8.91,)| Show InChI InChI=1S/C28H46Cl2N8O3/c29-21-12-9-19(15-22(21)30)16-25(39)37-24(6-2-4-14-32)27(41)38-23(5-1-3-13-31)26(40)35-17-18-7-10-20(11-8-18)36-28(33)34/h9,12,15,18,20,23-24H,1-8,10-11,13-14,16-17,31-32H2,(H,35,40)(H,37,39)(H,38,41)(H4,33,34,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nonstructural protein 3
(Zika virus) | BDBM13142
((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)N)C(=O)N1CCC[C@H]1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C20H30N4O3/c1-13(2)17(23-18(25)14(3)21)20(27)24-11-7-10-16(24)19(26)22-12-15-8-5-4-6-9-15/h4-6,8-9,13-14,16-17H,7,10-12,21H2,1-3H3,(H,22,26)(H,23,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus NS2B-NS3 protease |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126965
BindingDB Entry DOI: 10.7270/Q27M0CGT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50212336
(CHEMBL5282745)Show SMILES COc1cccnc1-c1c(O)c(=O)[nH]c2cc(Cl)ccc2c1=O |(12.82,-1.04,;13.08,-2.56,;14.52,-3.11,;15.71,-2.14,;17.17,-2.69,;17.41,-4.22,;16.21,-5.18,;14.76,-4.64,;13.57,-5.61,;13.91,-7.1,;15.42,-7.43,;12.91,-8.36,;13.59,-9.75,;11.44,-8.36,;10.48,-7.17,;9.09,-7.69,;7.94,-6.73,;6.49,-7.27,;8.2,-5.25,;9.6,-4.74,;10.75,-5.7,;12.15,-4.95,;12.12,-3.4,)| Show InChI InChI=1S/C16H11ClN2O4/c1-23-11-3-2-6-18-13(11)12-14(20)9-5-4-8(17)7-10(9)19-16(22)15(12)21/h2-7,21H,1H3,(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM32580
(CS-3)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CC[C@H](C)[C@H]1C(=O)Nc1ccccc1-c1ncccn1 |r| Show InChI InChI=1S/C28H38N6O3/c1-18-14-17-34(28(37)23(20-10-5-4-6-11-20)33-26(35)19(2)29-3)24(18)27(36)32-22-13-8-7-12-21(22)25-30-15-9-16-31-25/h7-9,12-13,15-16,18-20,23-24,29H,4-6,10-11,14,17H2,1-3H3,(H,32,36)(H,33,35)/t18-,19-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus NS2B-NS3 protease |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126965
BindingDB Entry DOI: 10.7270/Q27M0CGT |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM50450046
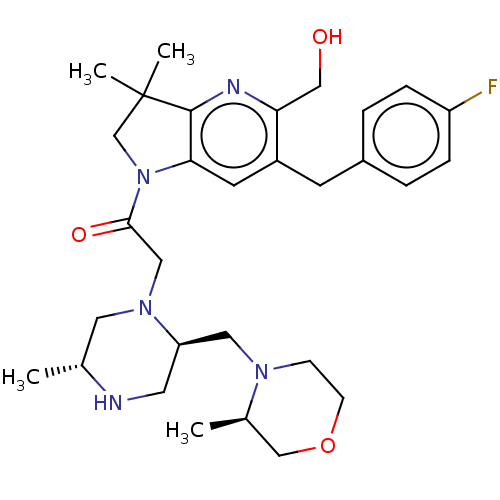
(CHEMBL4173974)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2CCOC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-20-14-35(25(13-32-20)15-34-9-10-39-18-21(34)2)16-28(38)36-19-30(3,4)29-27(36)12-23(26(17-37)33-29)11-22-5-7-24(31)8-6-22/h5-8,12,20-21,25,32,37H,9-11,13-19H2,1-4H3/t20-,21-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus NS2B-NS3 protease |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126965
BindingDB Entry DOI: 10.7270/Q27M0CGT |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303176
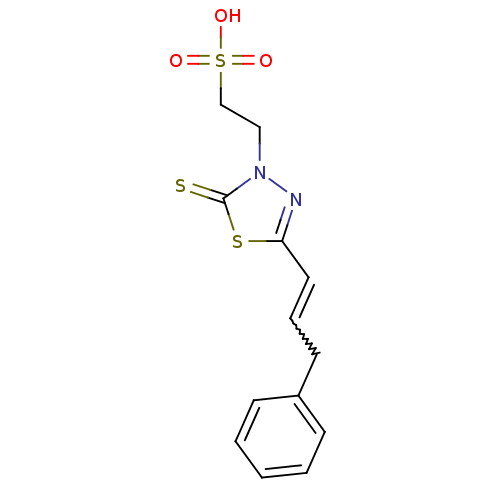
(2-((Z)-4-oxo-5-((E)-3-phenylallylidene)-2-thioxoth...)Show SMILES OS(=O)(=O)CCn1nc(C=CCc2ccccc2)sc1=S |w:10.10| Show InChI InChI=1S/C13H14N2O3S3/c16-21(17,18)10-9-15-13(19)20-12(14-15)8-4-7-11-5-2-1-3-6-11/h1-6,8H,7,9-10H2,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 809 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303177
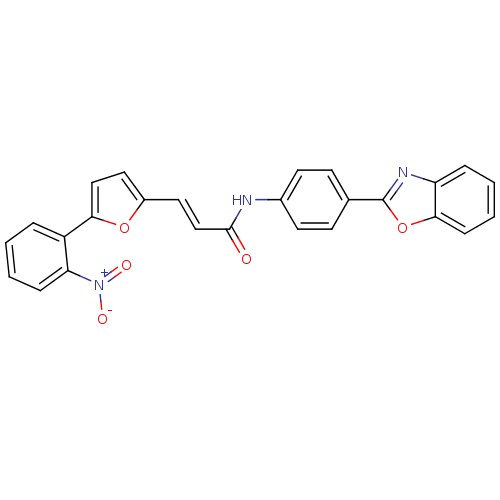
(CHEMBL565369 | N-(4-(benzo[d]oxazol-2-yl)phenyl)-3...)Show SMILES [O-][N+](=O)c1ccccc1-c1ccc(\C=C\C(=O)Nc2ccc(cc2)-c2nc3ccccc3o2)o1 Show InChI InChI=1S/C26H17N3O5/c30-25(16-14-19-13-15-23(33-19)20-5-1-3-7-22(20)29(31)32)27-18-11-9-17(10-12-18)26-28-21-6-2-4-8-24(21)34-26/h1-16H,(H,27,30)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303178
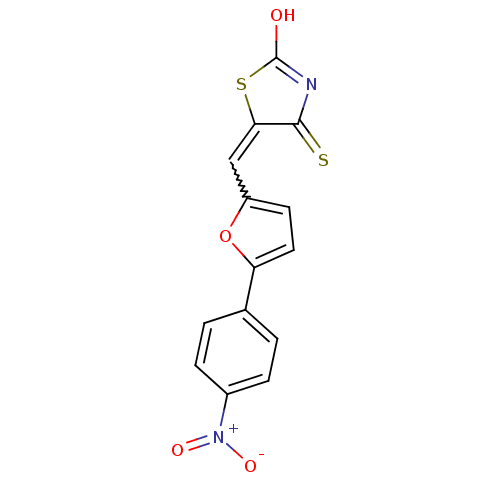
(5-((5-(4-nitrophenyl)furan-2-yl)methylene)-4-thiox...)Show SMILES OC1=NC(=S)C(S1)=Cc1ccc(o1)-c1ccc(cc1)[N+]([O-])=O |w:7.8,t:1| Show InChI InChI=1S/C14H8N2O4S2/c17-14-15-13(21)12(22-14)7-10-5-6-11(20-10)8-1-3-9(4-2-8)16(18)19/h1-7H,(H,15,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303179
(2-(4-oxo-5-(3-phenylallylidene)-2-thioxothiazolidi...)Show SMILES OC(=O)C(Cc1ccccc1)N1C(=S)S\C(=C/C=C/c2ccccc2)C1=O Show InChI InChI=1S/C21H17NO3S2/c23-19-18(13-7-12-15-8-3-1-4-9-15)27-21(26)22(19)17(20(24)25)14-16-10-5-2-6-11-16/h1-13,17H,14H2,(H,24,25)/b12-7+,18-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM11994
(2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...)Show InChI InChI=1S/C14H15NO2S/c1-10-3-4-11(2)15(10)12-5-7-13(8-6-12)18-9-14(16)17/h3-8H,9H2,1-2H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303180
(CHEMBL577311 | ethyl 5-(4-bromophenyl)-4,6-dioxo-2...)Show SMILES CCOC(=O)c1n[nH]c2C(=O)N(C(=O)c12)c1ccc(Br)cc1 Show InChI InChI=1S/C14H10BrN3O4/c1-2-22-14(21)11-9-10(16-17-11)13(20)18(12(9)19)8-5-3-7(15)4-6-8/h3-6H,2H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303181

(1-(dibenzo[b,d]furan-3-yl)-3-(naphthalen-1-yl)thio...)Show InChI InChI=1S/C23H16N2OS/c27-23(25-20-10-5-7-15-6-1-2-8-17(15)20)24-16-12-13-19-18-9-3-4-11-21(18)26-22(19)14-16/h1-14H,(H2,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50212339
(CHEMBL4173093)Show SMILES COc1ccc(cc1)-c1c(O)c(=O)[nH]c2cc(Cl)ccc2c1=O Show InChI InChI=1S/C17H12ClNO4/c1-23-11-5-2-9(3-6-11)14-15(20)12-7-4-10(18)8-13(12)19-17(22)16(14)21/h2-8,21H,1H3,(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303182
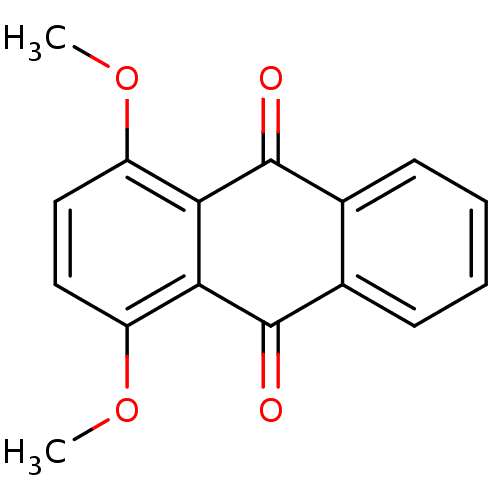
(1,4-dimethoxyanthracene-9,10-dione | CHEMBL570408 ...)Show InChI InChI=1S/C16H12O4/c1-19-11-7-8-12(20-2)14-13(11)15(17)9-5-3-4-6-10(9)16(14)18/h3-8H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM28851
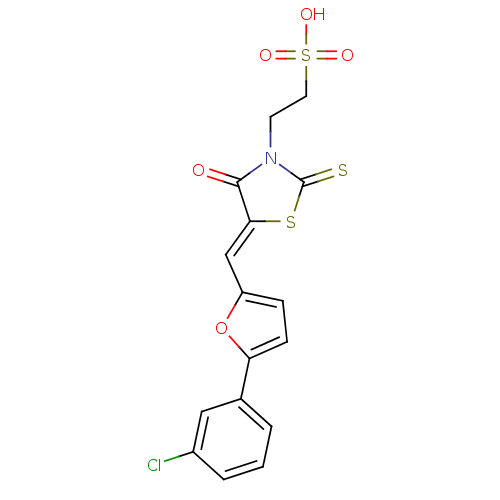
(2-[(5Z)-5-{[5-(3-chlorophenyl)furan-2-yl]methylide...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2ccc(o2)-c2cccc(Cl)c2)C1=O Show InChI InChI=1S/C16H12ClNO5S3/c17-11-3-1-2-10(8-11)13-5-4-12(23-13)9-14-15(19)18(16(24)25-14)6-7-26(20,21)22/h1-5,8-9H,6-7H2,(H,20,21,22)/b14-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303184
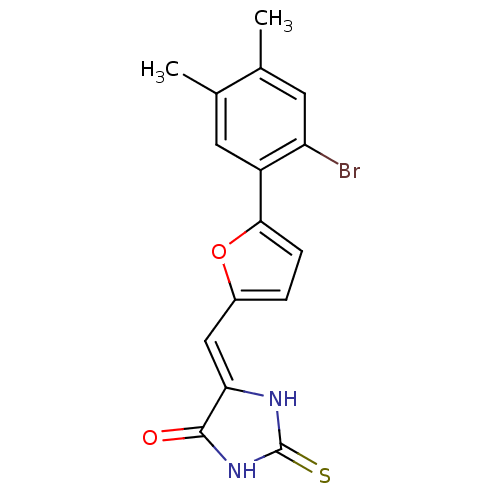
(5-((5-(2-bromo-4,5-dimethylphenyl)furan-2-yl)methy...)Show SMILES Cc1cc(Br)c(cc1C)-c1ccc(\C=C2/NC(=S)NC2=O)o1 Show InChI InChI=1S/C16H13BrN2O2S/c1-8-5-11(12(17)6-9(8)2)14-4-3-10(21-14)7-13-15(20)19-16(22)18-13/h3-7H,1-2H3,(H2,18,19,20,22)/b13-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303185
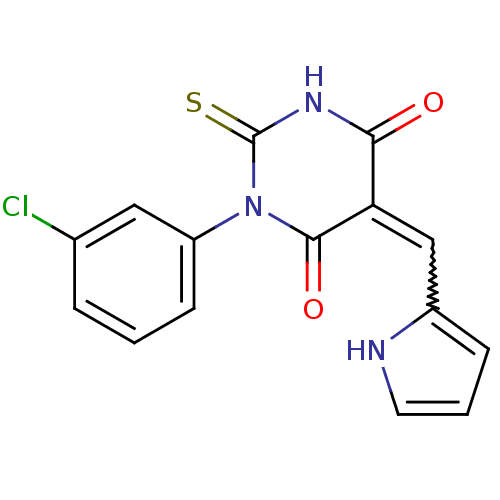
((E/Z)-5-((1H-pyrrol-2-yl)methylene)-1-(3-chlorophe...)Show SMILES Clc1cccc(c1)N1C(=S)NC(=O)C(=Cc2ccc[nH]2)C1=O |w:14.15| Show InChI InChI=1S/C15H10ClN3O2S/c16-9-3-1-5-11(7-9)19-14(21)12(13(20)18-15(19)22)8-10-4-2-6-17-10/h1-8,17H,(H,18,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM15186
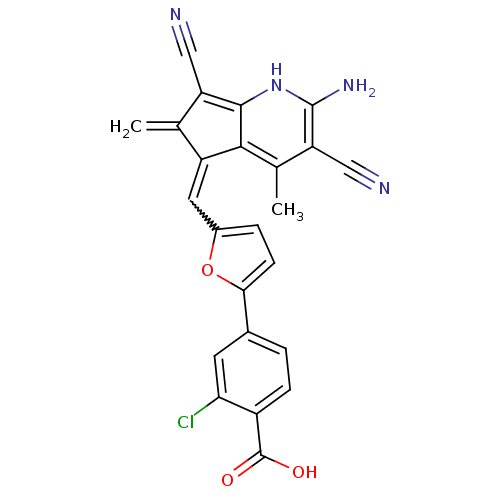
(4-(5-{[(5E)-2-amino-3,7-dicyano-4,6-dimethyl-5H-cy...)Show SMILES Cc1c(C#N)c(N)[nH]c2c(C#N)c(=C)c(=Cc3ccc(o3)-c3ccc(C(O)=O)c(Cl)c3)c12 |w:15.15| Show InChI InChI=1S/C24H15ClN4O3/c1-11-16(21-12(2)18(10-27)23(28)29-22(21)17(11)9-26)8-14-4-6-20(32-14)13-3-5-15(24(30)31)19(25)7-13/h3-8,29H,1,28H2,2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303186
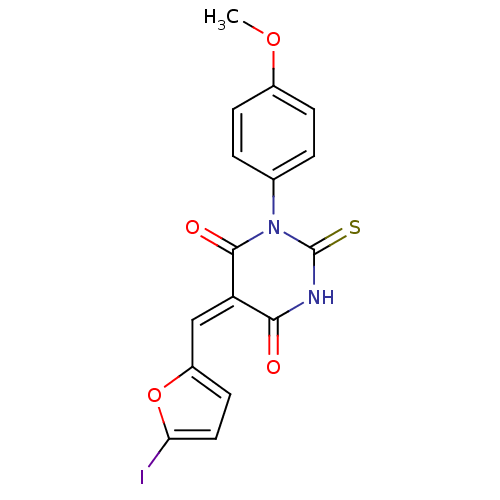
((E/Z)-5-((5-iodofuran-2-yl)methylene)-1-(4-methoxy...)Show SMILES COc1ccc(cc1)N1C(=S)NC(=O)\C(=C/c2ccc(I)o2)C1=O Show InChI InChI=1S/C16H11IN2O4S/c1-22-10-4-2-9(3-5-10)19-15(21)12(14(20)18-16(19)24)8-11-6-7-13(17)23-11/h2-8H,1H3,(H,18,20,24)/b12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50212340
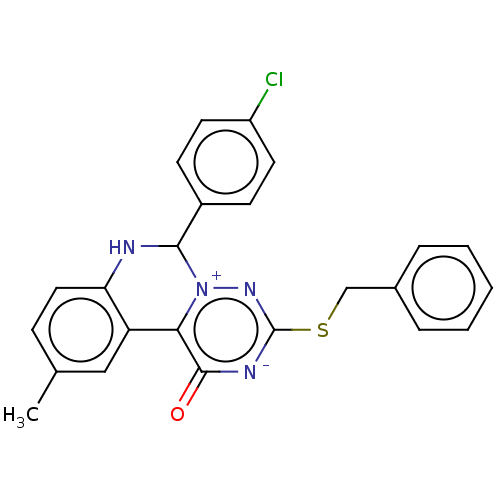
(CHEMBL5276951)Show InChI InChI=1S/C15H9ClN2O3/c16-9-3-4-10-11(6-9)18-15(21)14(20)12(13(10)19)8-2-1-5-17-7-8/h1-7,19H,(H,18,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303187
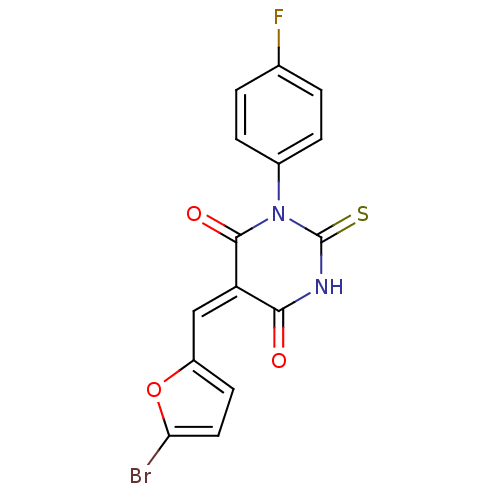
(5-((5-bromofuran-2-yl)methylene)-1-(4-fluorophenyl...)Show SMILES Fc1ccc(cc1)N1C(=S)NC(=O)\C(=C/c2ccc(Br)o2)C1=O Show InChI InChI=1S/C15H8BrFN2O3S/c16-12-6-5-10(22-12)7-11-13(20)18-15(23)19(14(11)21)9-3-1-8(17)2-4-9/h1-7H,(H,18,20,23)/b11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM11985

(4-(2,5-dimethyl-1H-pyrrol-1-yl)-2-hydroxybenzoic a...)Show InChI InChI=1S/C13H13NO3/c1-8-3-4-9(2)14(8)10-5-6-11(13(16)17)12(15)7-10/h3-7,15H,1-2H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303188
(4-chloro-N-(2,3,5-trichloro-4-oxocyclohexa-2,5-die...)Show SMILES ClC1=C\C(=N/S(=O)(=O)c2ccc(Cl)cc2)C(Cl)=C(Cl)C1=O |t:1,17| Show InChI InChI=1S/C12H5Cl4NO3S/c13-6-1-3-7(4-2-6)21(19,20)17-9-5-8(14)12(18)11(16)10(9)15/h1-5H/b17-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM50393505
(CHEMBL2158051)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(CC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1)C(=O)CC(C)C |r| Show InChI InChI=1S/C32H43N5O4/c1-21(2)19-28(38)36-18-17-25-15-16-27(37(25)32(41)26(20-36)34-30(39)22(3)33-4)31(40)35-29(23-11-7-5-8-12-23)24-13-9-6-10-14-24/h5-14,21-22,25-27,29,33H,15-20H2,1-4H3,(H,34,39)(H,35,40)/t22-,25+,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Zika virus NS2B-NS3 protease (1 to 187 residues) using Boc-Gly-Arg-Arg-AMC as substrate |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126965
BindingDB Entry DOI: 10.7270/Q27M0CGT |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303190
(3-(5-((2-(5-nitropyridin-2-yl)hydrazono)methyl)fur...)Show SMILES OC(=O)c1cccc(c1)-c1ccc(CN=Nc2ccc(cn2)[N+]([O-])=O)o1 |w:15.16| Show InChI InChI=1S/C17H12N4O5/c22-17(23)12-3-1-2-11(8-12)15-6-5-14(26-15)10-19-20-16-7-4-13(9-18-16)21(24)25/h1-9H,10H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303189
(5,5-dihydroxy-4H-pyrido[3,2,1-jk]carbazole-4,6(5H)...)Show InChI InChI=1S/C15H9NO4/c17-13-10-6-3-5-9-8-4-1-2-7-11(8)16(12(9)10)14(18)15(13,19)20/h1-7,19-20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303191
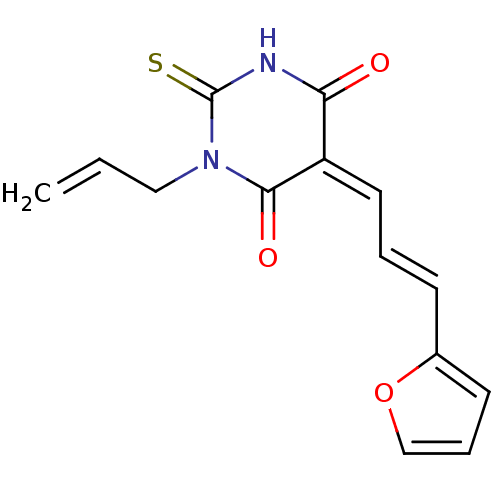
((E/Z)-1-allyl-5-(3-(furan-2-yl)allylidene)-2-thiox...)Show InChI InChI=1S/C14H12N2O3S/c1-2-8-16-13(18)11(12(17)15-14(16)20)7-3-5-10-6-4-9-19-10/h2-7,9H,1,8H2,(H,15,17,20)/b5-3+,11-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303192
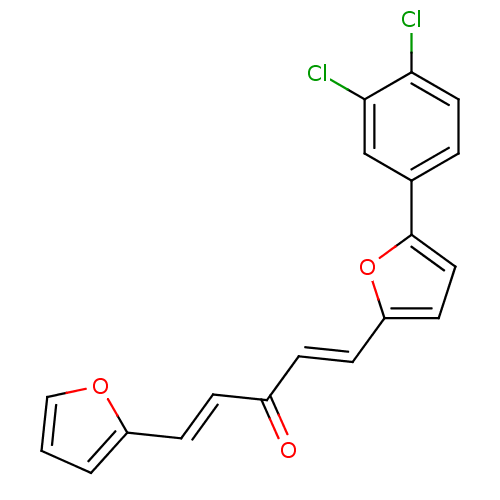
(1-(5-(3,4-dichlorophenyl)furan-2-yl)-5-(furan-2-yl...)Show SMILES Clc1ccc(cc1Cl)-c1ccc(\C=C\C(=O)\C=C\c2ccco2)o1 Show InChI InChI=1S/C19H12Cl2O3/c20-17-9-3-13(12-18(17)21)19-10-8-16(24-19)7-5-14(22)4-6-15-2-1-11-23-15/h1-12H/b6-4+,7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303193
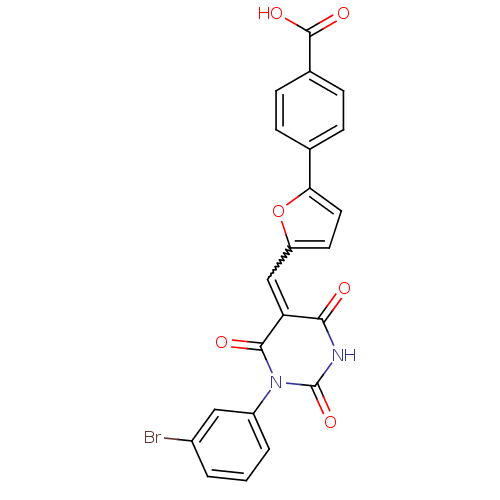
((E/Z)-4-(5-((1-(3-bromophenyl)-2,4,6-trioxotetrahy...)Show SMILES OC(=O)c1ccc(cc1)-c1ccc(C=C2C(=O)NC(=O)N(C2=O)c2cccc(Br)c2)o1 |w:13.13| Show InChI InChI=1S/C22H13BrN2O6/c23-14-2-1-3-15(10-14)25-20(27)17(19(26)24-22(25)30)11-16-8-9-18(31-16)12-4-6-13(7-5-12)21(28)29/h1-11H,(H,28,29)(H,24,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM50467780
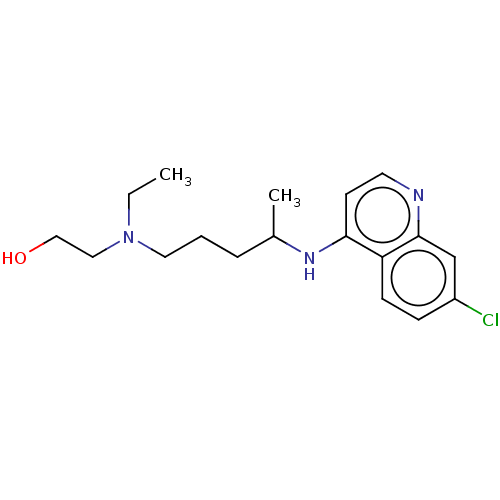
(CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...)Show InChI InChI=1S/C18H26ClN3O/c1-3-22(11-12-23)10-4-5-14(2)21-17-8-9-20-18-13-15(19)6-7-16(17)18/h6-9,13-14,23H,3-5,10-12H2,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 9.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus NS2B-NS3 protease |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126965
BindingDB Entry DOI: 10.7270/Q27M0CGT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50026808
(CHEMBL2440341)Show SMILES CCCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(\C=C(/C#N)C(=O)NC2CC2)cc1)C(N)=O |r| Show InChI InChI=1S/C32H48N10O5/c1-2-3-7-24(27(35)43)40-30(46)25(8-4-5-16-33)42-31(47)26(9-6-17-38-32(36)37)41-28(44)21-12-10-20(11-13-21)18-22(19-34)29(45)39-23-14-15-23/h10-13,18,23-26H,2-9,14-17,33H2,1H3,(H2,35,43)(H,39,45)(H,40,46)(H,41,44)(H,42,47)(H4,36,37,38)/b22-18+/t24-,25-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50030459

(CHEMBL3344321)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)Cc1ccc(Cl)c(Cl)c1)C(=O)NC[C@H]1CC[C@@H](CC1)NC(N)=N |r,wU:34.38,9.9,wD:31.31,5.4,(16.97,-13.05,;16.9,-11.52,;15.54,-10.81,;15.47,-9.27,;14.11,-8.56,;14.04,-7.02,;12.68,-6.3,;11.38,-7.13,;11.44,-8.67,;10.01,-6.42,;9.94,-4.88,;11.24,-4.05,;11.18,-2.52,;12.48,-1.69,;12.42,-.15,;8.71,-7.24,;7.38,-6.48,;7.37,-4.94,;6.05,-7.25,;4.72,-6.48,;4.71,-4.94,;3.38,-4.17,;2.05,-4.94,;.72,-4.17,;2.05,-6.48,;.72,-7.25,;3.38,-7.25,;15.34,-6.19,;15.28,-4.65,;16.7,-6.9,;18.02,-6.09,;19.38,-6.82,;19.42,-8.36,;20.78,-9.09,;22.1,-8.27,;22.05,-6.73,;20.68,-6,;23.46,-9,;24.77,-8.18,;24.72,-6.65,;26.13,-8.91,)| Show InChI InChI=1S/C28H46Cl2N8O3/c29-21-12-9-19(15-22(21)30)16-25(39)37-24(6-2-4-14-32)27(41)38-23(5-1-3-13-31)26(40)35-17-18-7-10-20(11-8-18)36-28(33)34/h9,12,15,18,20,23-24H,1-8,10-11,13-14,16-17,31-32H2,(H,35,40)(H,37,39)(H,38,41)(H4,33,34,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50212341
(CHEMBL5287718)Show InChI InChI=1S/C16H10ClNO3/c17-10-6-7-11-12(8-10)18-16(21)15(20)13(14(11)19)9-4-2-1-3-5-9/h1-8,19H,(H,18,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50030459

(CHEMBL3344321)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)Cc1ccc(Cl)c(Cl)c1)C(=O)NC[C@H]1CC[C@@H](CC1)NC(N)=N |r,wU:34.38,9.9,wD:31.31,5.4,(16.97,-13.05,;16.9,-11.52,;15.54,-10.81,;15.47,-9.27,;14.11,-8.56,;14.04,-7.02,;12.68,-6.3,;11.38,-7.13,;11.44,-8.67,;10.01,-6.42,;9.94,-4.88,;11.24,-4.05,;11.18,-2.52,;12.48,-1.69,;12.42,-.15,;8.71,-7.24,;7.38,-6.48,;7.37,-4.94,;6.05,-7.25,;4.72,-6.48,;4.71,-4.94,;3.38,-4.17,;2.05,-4.94,;.72,-4.17,;2.05,-6.48,;.72,-7.25,;3.38,-7.25,;15.34,-6.19,;15.28,-4.65,;16.7,-6.9,;18.02,-6.09,;19.38,-6.82,;19.42,-8.36,;20.78,-9.09,;22.1,-8.27,;22.05,-6.73,;20.68,-6,;23.46,-9,;24.77,-8.18,;24.72,-6.65,;26.13,-8.91,)| Show InChI InChI=1S/C28H46Cl2N8O3/c29-21-12-9-19(15-22(21)30)16-25(39)37-24(6-2-4-14-32)27(41)38-23(5-1-3-13-31)26(40)35-17-18-7-10-20(11-8-18)36-28(33)34/h9,12,15,18,20,23-24H,1-8,10-11,13-14,16-17,31-32H2,(H,35,40)(H,37,39)(H,38,41)(H4,33,34,36)/t18-,20-,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM28879

(2-[(5Z)-5-[(3-{4-[(4-chlorophenyl)methoxy]phenyl}-...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccc(Cl)cc3)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H22ClN3O5S3/c29-22-10-6-19(7-11-22)18-37-24-12-8-20(9-13-24)26-21(17-32(30-26)23-4-2-1-3-5-23)16-25-27(33)31(28(38)39-25)14-15-40(34,35)36/h1-13,16-17H,14-15,18H2,(H,34,35,36)/b25-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303173
(2-(5-((3-(4-(2-chlorobenzyloxy)phenyl)-1-phenyl-1H...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccccc3Cl)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H22ClN3O5S3/c29-24-9-5-4-6-20(24)18-37-23-12-10-19(11-13-23)26-21(17-32(30-26)22-7-2-1-3-8-22)16-25-27(33)31(28(38)39-25)14-15-40(34,35)36/h1-13,16-17H,14-15,18H2,(H,34,35,36)/b25-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303174

(2-(5-((3-(4-(2-fluorobenzyloxy)phenyl)-1-phenyl-1H...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccccc3F)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H22FN3O5S3/c29-24-9-5-4-6-20(24)18-37-23-12-10-19(11-13-23)26-21(17-32(30-26)22-7-2-1-3-8-22)16-25-27(33)31(28(38)39-25)14-15-40(34,35)36/h1-13,16-17H,14-15,18H2,(H,34,35,36)/b25-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303175

(2-(5-((3-(4-(benzyloxy)phenyl)-1-phenyl-1H-pyrazol...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccccc3)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H23N3O5S3/c32-27-25(38-28(37)30(27)15-16-39(33,34)35)17-22-18-31(23-9-5-2-6-10-23)29-26(22)21-11-13-24(14-12-21)36-19-20-7-3-1-4-8-20/h1-14,17-18H,15-16,19H2,(H,33,34,35)/b25-17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM26218
((3S,6S,10aS)-N-(diphenylmethyl)-6-[(2S)-2-(methyla...)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CCCC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-19(29-2)26(33)30-23-16-10-9-15-22-17-18-24(32(22)28(23)35)27(34)31-25(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,19,22-25,29H,9-10,15-18H2,1-2H3,(H,30,33)(H,31,34)/t19-,22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus NS2B-NS3 protease |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126965
BindingDB Entry DOI: 10.7270/Q27M0CGT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM35491
(Benzoic acid 5-amino-1-(4-methoxy-benzenesulfonyl)...)Show SMILES COc1ccc(cc1)S(=O)(=O)n1nc(OC(=O)c2ccccc2)cc1N Show InChI InChI=1S/C17H15N3O5S/c1-24-13-7-9-14(10-8-13)26(22,23)20-15(18)11-16(19-20)25-17(21)12-5-3-2-4-6-12/h2-11H,18H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50567466
(CHEMBL4862682)Show SMILES NCc1ccc(cc1)-c1ncc(OCC2CCNCC2)nc1-c1ccc(cc1)-c1cn[nH]c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50212335
(CHEMBL5275144)Show InChI InChI=1S/C14H8ClNO3S/c15-7-3-4-8-9(6-7)16-14(19)13(18)11(12(8)17)10-2-1-5-20-10/h1-6,17H,(H,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM28884
(2-[(5Z)-4-oxo-5-({1-phenyl-3-[4-(piperidine-1-sulf...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(cc2)S(=O)(=O)N2CCCCC2)-c2ccccc2)C1=O Show InChI InChI=1S/C26H26N4O6S4/c31-25-23(38-26(37)29(25)15-16-39(32,33)34)17-20-18-30(21-7-3-1-4-8-21)27-24(20)19-9-11-22(12-10-19)40(35,36)28-13-5-2-6-14-28/h1,3-4,7-12,17-18H,2,5-6,13-16H2,(H,32,33,34)/b23-17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant vaccina H1-related phosphatase |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50303173

(2-(5-((3-(4-(2-chlorobenzyloxy)phenyl)-1-phenyl-1H...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccccc3Cl)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H22ClN3O5S3/c29-24-9-5-4-6-20(24)18-37-23-12-10-19(11-13-23)26-21(17-32(30-26)22-7-2-1-3-8-22)16-25-27(33)31(28(38)39-25)14-15-40(34,35)36/h1-13,16-17H,14-15,18H2,(H,34,35,36)/b25-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CD45 |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM50542237
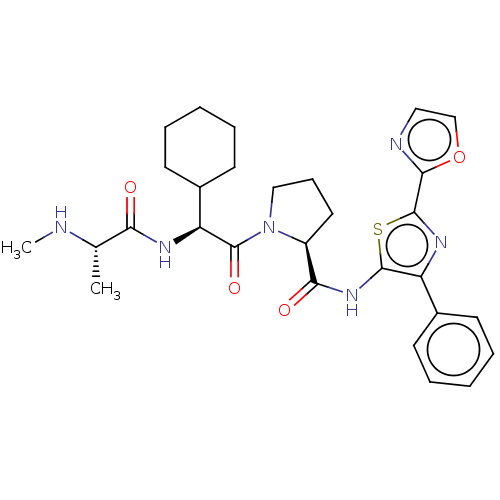
(Cudc 427 | Cudc-427 | Gdc-0917)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)Nc1sc(nc1-c1ccccc1)-c1ncco1 Show InChI InChI=1S/C29H36N6O4S/c1-18(30-2)24(36)32-23(20-12-7-4-8-13-20)29(38)35-16-9-14-21(35)25(37)34-27-22(19-10-5-3-6-11-19)33-28(40-27)26-31-15-17-39-26/h3,5-6,10-11,15,17-18,20-21,23,30H,4,7-9,12-14,16H2,1-2H3,(H,32,36)(H,34,37)/t18-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus NS2B-NS3 protease |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126965
BindingDB Entry DOI: 10.7270/Q27M0CGT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50303174

(2-(5-((3-(4-(2-fluorobenzyloxy)phenyl)-1-phenyl-1H...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccccc3F)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H22FN3O5S3/c29-24-9-5-4-6-20(24)18-37-23-12-10-19(11-13-23)26-21(17-32(30-26)22-7-2-1-3-8-22)16-25-27(33)31(28(38)39-25)14-15-40(34,35)36/h1-13,16-17H,14-15,18H2,(H,34,35,36)/b25-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(West Nile virus) | BDBM50218663
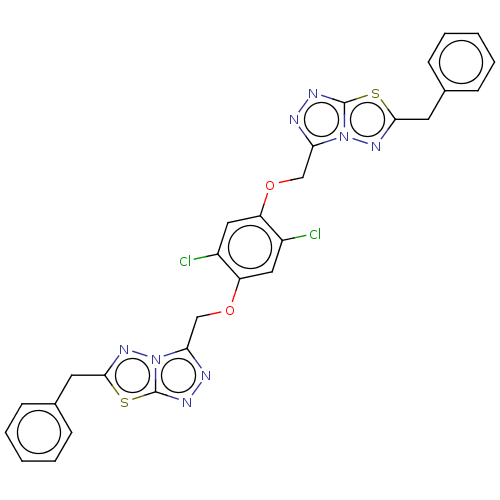
(CHEMBL5269219)Show SMILES [H][C@@]12CCCN(N1)C(=O)[C@H](Cc1cccc(O)c1)NC(=O)[C@@H](NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)CCCCC[C@H](OC2=O)C(C)CCCCC[C@H](C)[C@@H](O)C[C@@H]1O[C@@]2(NC(=O)[C@@H](CC)C[C@@H]2C)[C@@H](C)[C@@H](O)[C@H]1C)C(C)C Show InChI InChI=1S/C60H99N5O13/c1-11-43-30-37(6)60(63-55(43)72)41(10)53(70)40(9)51(78-60)33-49(69)35(4)20-14-12-15-21-36(5)50-26-17-13-16-25-48(68)39(8)54(71)45(28-27-38(7)66)56(73)62-52(34(2)3)57(74)61-47(32-42-22-18-23-44(67)31-42)58(75)65-29-19-24-46(64-65)59(76)77-50/h18,22-23,31,34-37,39-41,43,45-54,64,67-71H,11-17,19-21,24-30,32-33H2,1-10H3,(H,61,74)(H,62,73)(H,63,72)/t35-,36?,37-,39-,40-,41-,43-,45+,46-,47-,48-,49-,50-,51-,52-,53-,54+,60+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Bioorg Med Chem 18: 1434-40 (2010)
Article DOI: 10.1016/j.bmc.2010.01.015 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM28879

(2-[(5Z)-5-[(3-{4-[(4-chlorophenyl)methoxy]phenyl}-...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccc(Cl)cc3)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H22ClN3O5S3/c29-22-10-6-19(7-11-22)18-37-24-12-8-20(9-13-24)26-21(17-32(30-26)23-4-2-1-3-5-23)16-25-27(33)31(28(38)39-25)14-15-40(34,35)36/h1-13,16-17H,14-15,18H2,(H,34,35,36)/b25-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
J Med Chem 52: 6716-23 (2009)
Article DOI: 10.1021/jm901016k
BindingDB Entry DOI: 10.7270/Q2VX0GK3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data