

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50075473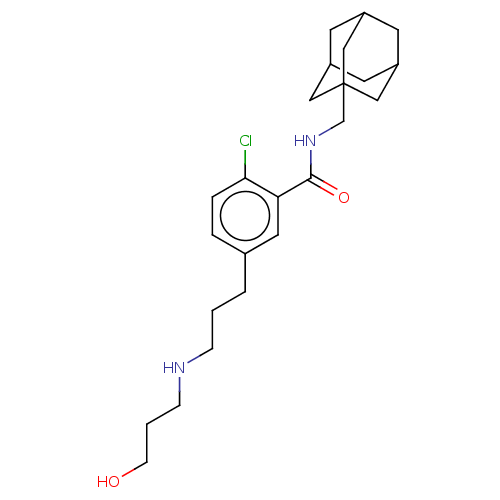 (CHEMBL3415305) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370300 (CHEMBL4165149) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370300 (CHEMBL4165149) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake measured after 2 hrs by fluo... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50075473 (CHEMBL3415305) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake measured after 2 hrs by fluo... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM144315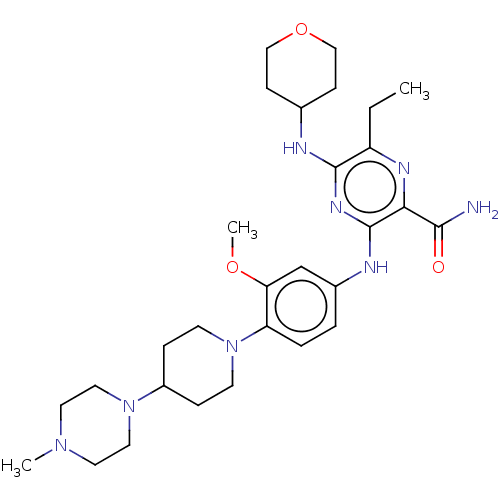 (Gilteritinib | US11512074, Example T-9 | US8969336...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 expressed in human SEMK2 cells assessed as reduction in FLT3 phosphorylation incubated for 1 hr by immunoblotting analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50564363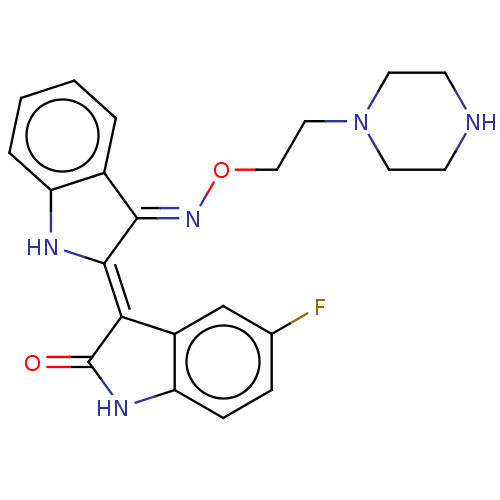 (CHEMBL4791998) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant in presence of ATP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536405 (CHEMBL4521847) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536370 (CHEMBL4582650) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake measured after 2 hrs by fluo... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50564363 (CHEMBL4791998) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human FLT3 expressed in baculovirus expression system using peptide substrate incubated for 30 mins in presence ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536370 (CHEMBL4582650) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 autophosphorylation in human RS4-11 cells preincubated for 2 hrs followed by FLT3 ligand addition and measured after 15 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50564363 (CHEMBL4791998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human RET in presence of ATP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386567 (CHEMBL2048437) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM185149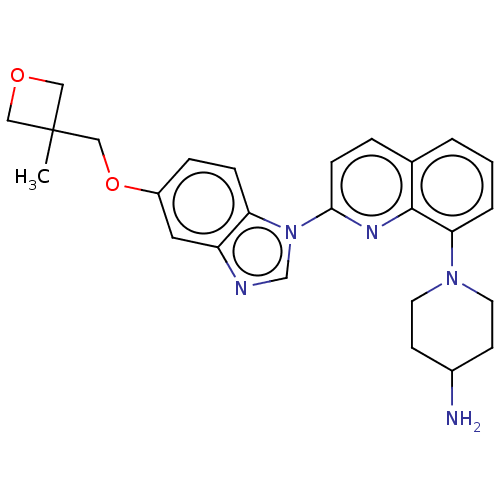 (1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536393 (CHEMBL4561852) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536393 (CHEMBL4561852) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake measured after 2 hrs by fluo... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370240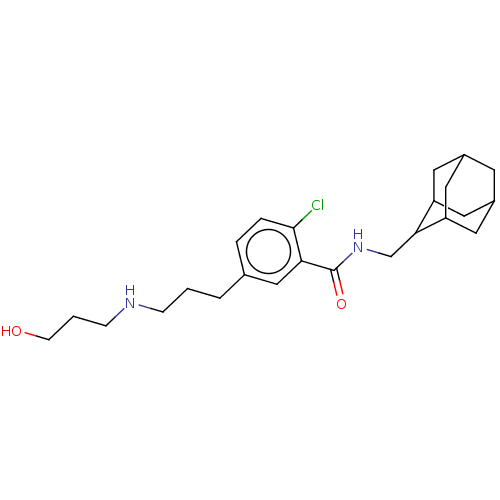 (CHEMBL4171354) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536383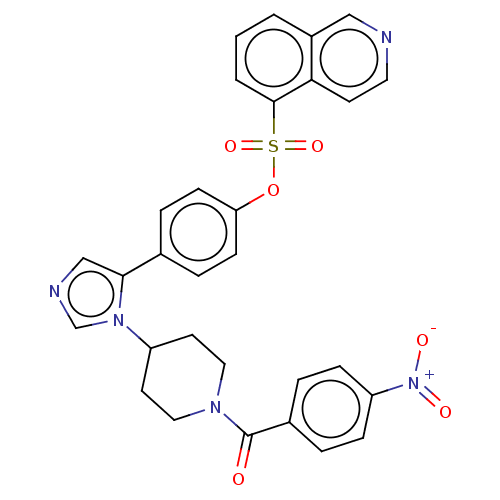 (CHEMBL4584526) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake measured after 2 hrs by fluo... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317169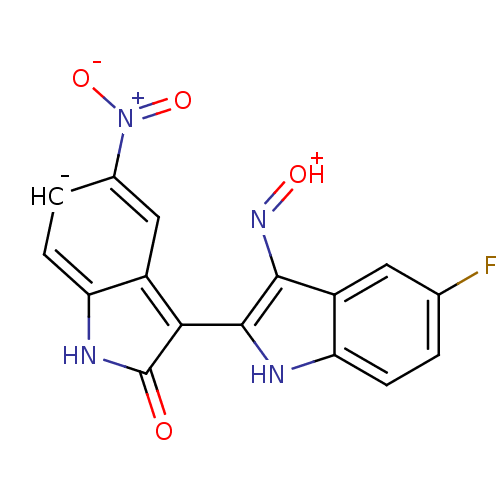 ((2'Z,3'E)-5-Nitro-5'-fluoro-indirubin-3'-oxime | C...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317171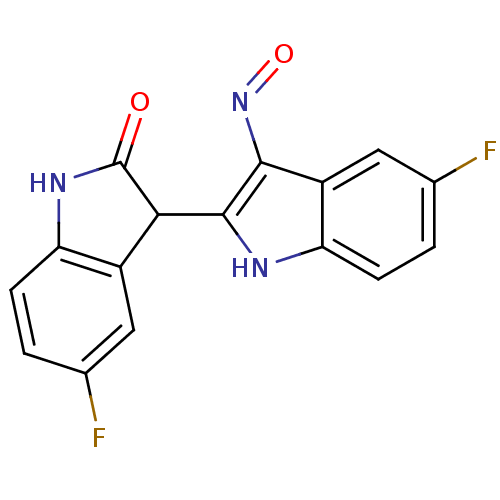 ((2'Z,3'E)-5-Fluoro-5'-fluoro-indirubin-3'-oxime | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536367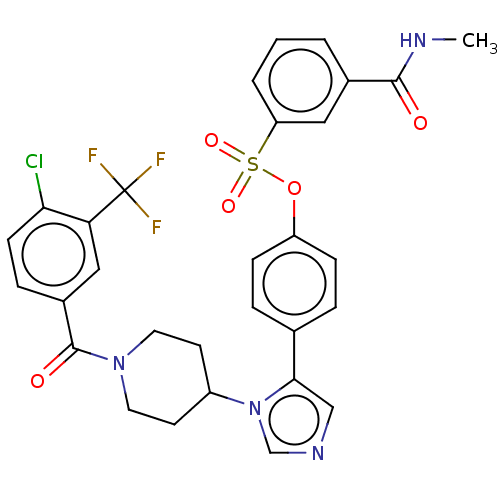 (CHEMBL4560935) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317161 ((2'Z,3'E)-5-Nitro-5'-hydroxy-indirubin-3'-oxime | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50564363 (CHEMBL4791998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TrkB in presence of ATP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370240 (CHEMBL4171354) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake after 2 hrs by fluorescence ... | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50564373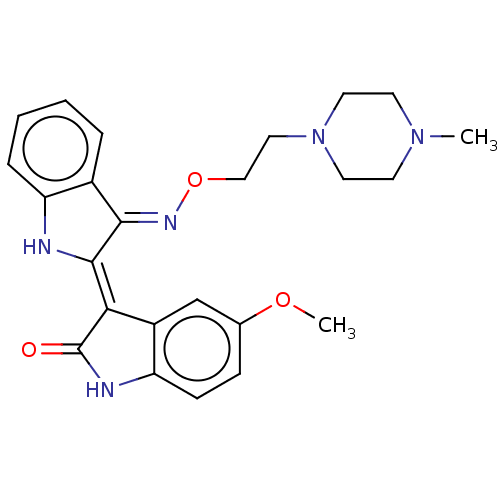 (CHEMBL4093032) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human FLT3 expressed in baculovirus expression system using peptide substrate incubated for 30 mins in presence ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370300 (CHEMBL4165149) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317163 ((2'Z,3'E)-5-Fluoro-5'-hydroxy-indirubin-3'-oxime |...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50564368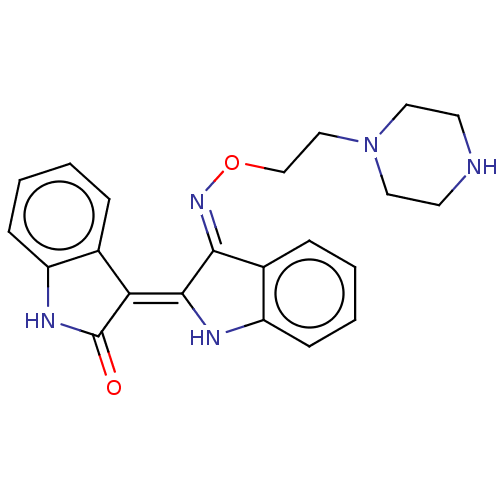 (CHEMBL4781746) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human FLT3 expressed in baculovirus expression system using peptide substrate incubated for 30 mins in presence ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50564376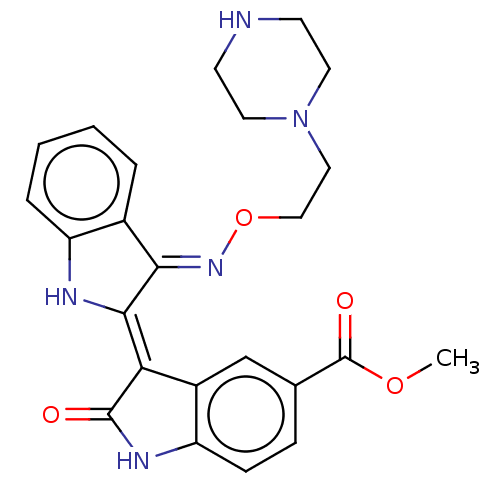 (CHEMBL4781229 | US11370779, Compound 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLT3 using peptide substrate in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50308060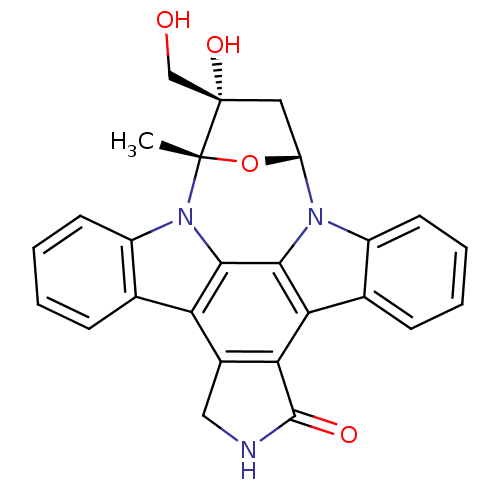 (16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human FLT3 expressed in baculovirus expression system using peptide substrate incubated for 30 mins in presence ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370186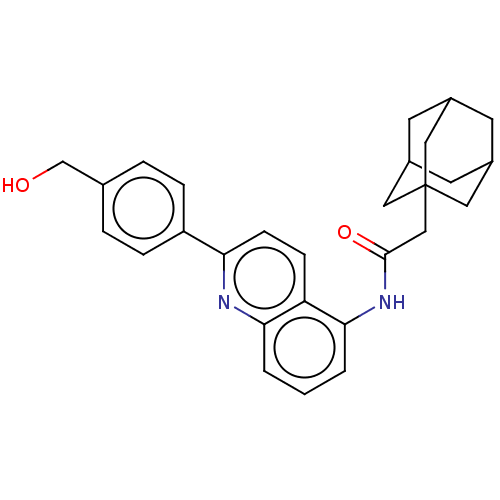 (CHEMBL4159914) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake after 2 hrs by fluorescence ... | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50564363 (CHEMBL4791998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MER in presence of ATP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370240 (CHEMBL4171354) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at P2X7 in LPS/IFN gamma differentiated human THP-1 cells assessed as inhibition of BzATP-induced IL-1beta release preincubated f... | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50564364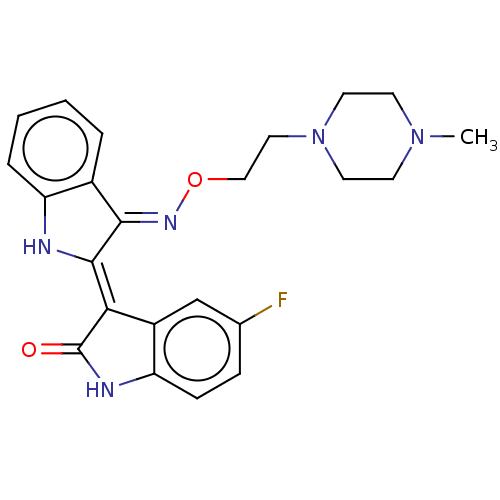 (CHEMBL4779016) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human FLT3 expressed in baculovirus expression system using peptide substrate incubated for 30 mins in presence ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50352039 (CHEMBL1823817) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370305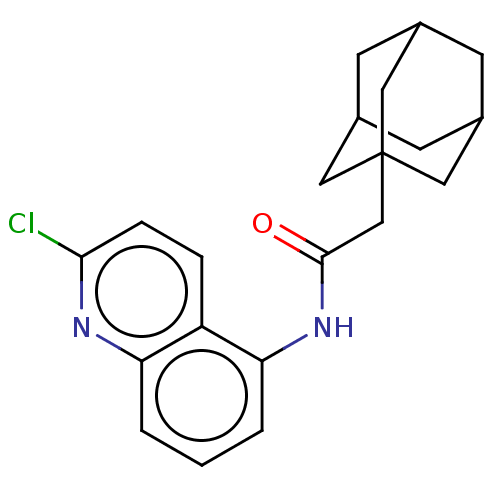 (CHEMBL4175875) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake after 2 hrs by fluorescence ... | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50352039 (CHEMBL1823817) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50564363 (CHEMBL4791998) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FMS in presence of ATP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536366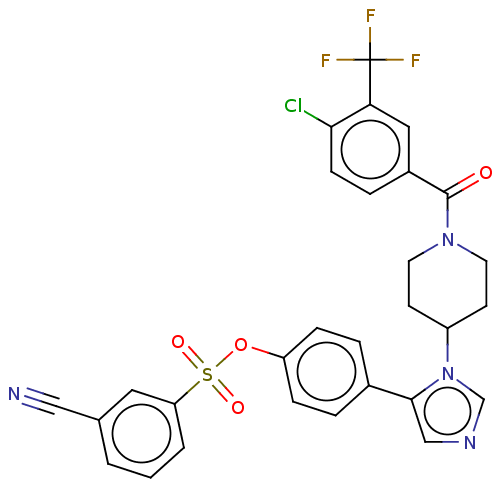 (CHEMBL4529050) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536406 (CHEMBL4573595) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in LPS/IFN-gamma-differentiated human THP-1 cells assessed as suppression of BzATP-stimulated IL-1beta rel... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386567 (CHEMBL2048437) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50423773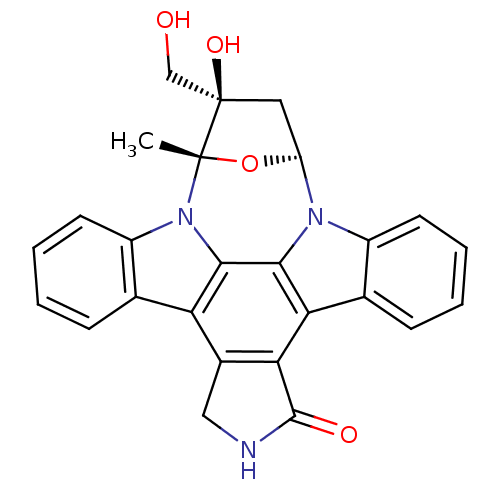 (A-1544750 | CEP-701 | KT-5555 | LESTAURTINIB | SP9...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of FLT3 by HTRF assay | Bioorg Med Chem Lett 20: 2033-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.039 BindingDB Entry DOI: 10.7270/Q29W0GF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50320578 (CHEMBL1165545 | N-[(1S,2R)-1-Benzyl-2-hydroxy-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of BACE1 | Eur J Med Chem 45: 2578-90 (2010) Article DOI: 10.1016/j.ejmech.2010.02.046 BindingDB Entry DOI: 10.7270/Q23778WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317162 ((2'Z,3'E)-5-Chloro-5'-hydroxy-indirubin-3'-oxime |...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50564363 (CHEMBL4791998) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MELK in presence of ATP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50564375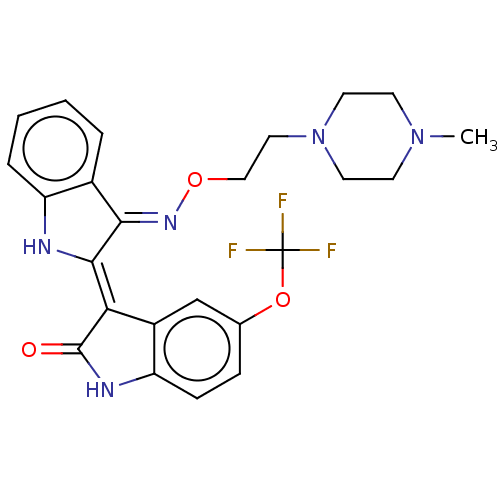 (CHEMBL4795997) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant His-tagged human FLT3 expressed in baculovirus expression system using peptide substrate incubated for 30 mins in presence ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370309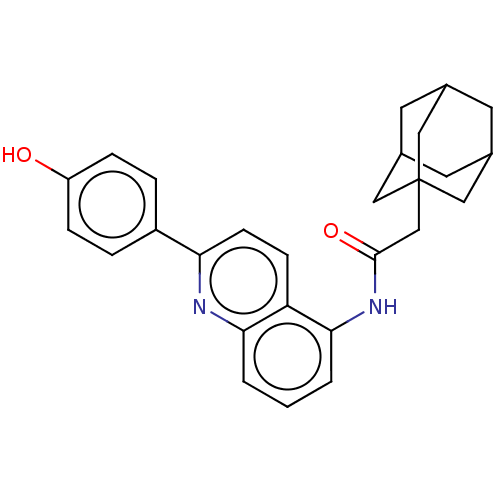 (CHEMBL4175330) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at P2X7 in LPS/IFN gamma differentiated human THP-1 cells assessed as inhibition of BzATP-induced IL-1beta release preincubated f... | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50564363 (CHEMBL4791998) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TrkA in presence of ATP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112205 BindingDB Entry DOI: 10.7270/Q2VM4H1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50536402 (CHEMBL4594110) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced EtBr uptake measured after 2 hrs by fluo... | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50370305 (CHEMBL4175875) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at P2X7 in LPS/IFN gamma differentiated human THP-1 cells assessed as inhibition of BzATP-induced IL-1beta release preincubated f... | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1065 total ) | Next | Last >> |