 Found 224 hits with Last Name = 'davies' and Initial = 'sj'
Found 224 hits with Last Name = 'davies' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236619

(US9388136, Table 1, Compound 11:Cyclopentyl (2S)-4...)Show SMILES NCC[C@H](NCCCOc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC1CCCC1 |r,wD:3.3,(8.82,-2.93,;8.82,-1.39,;7.48,-.62,;7.48,.92,;6.15,1.69,;4.82,.92,;3.48,1.69,;2.15,.92,;.82,1.69,;-.52,.92,;-1.85,1.69,;-3.19,.92,;-4.52,1.69,;-3.19,-.62,;-1.85,-1.39,;-1.85,-2.93,;-.52,-.62,;-4.52,-1.39,;-5.85,-.62,;-5.85,.92,;-7.19,-1.39,;-7.19,-2.93,;-5.85,-3.7,;-4.52,-2.93,;-3.19,-3.7,;-8.52,-.62,;-8.52,.92,;-9.85,-1.39,;-11.19,-.62,;-12.52,-1.39,;-12.52,-2.93,;-13.86,-3.7,;-11.19,-3.7,;-9.85,-2.93,;-8.52,-3.7,;8.82,1.69,;8.82,3.23,;10.15,.92,;11.49,1.69,;12.95,1.21,;13.86,2.46,;12.95,3.7,;11.49,3.23,)| Show InChI InChI=1S/C30H32F4N4O5/c31-17-6-7-20(22(32)14-17)28(40)21-8-9-26(39)38(29(21)36)27-23(33)15-19(16-24(27)34)42-13-3-12-37-25(10-11-35)30(41)43-18-4-1-2-5-18/h6-9,14-16,18,25,37H,1-5,10-13,35-36H2/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236615
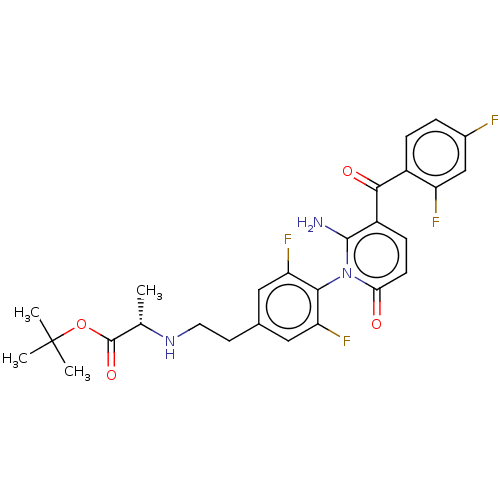
(US9388136, Table 1, Compound 1:tert-Butyl N-(2-{4-...)Show SMILES C[C@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC(C)(C)C |r,wD:1.0,(6.67,-.38,;6.67,1.15,;5.33,1.93,;4,1.15,;2.67,1.93,;1.33,1.15,;,1.93,;-1.33,1.15,;-2.67,1.93,;-1.33,-.38,;,-1.15,;,-2.69,;1.33,-.38,;-2.67,-1.15,;-4,-.38,;-4,1.15,;-5.33,-1.15,;-5.33,-2.69,;-4,-3.47,;-2.67,-2.69,;-1.33,-3.47,;-6.67,-.38,;-6.67,1.15,;-8,-1.15,;-9.34,-.38,;-10.67,-1.15,;-10.67,-2.69,;-12,-3.47,;-9.34,-3.47,;-8,-2.69,;-6.67,-3.47,;8,1.93,;8,3.47,;9.34,1.15,;10.67,1.93,;12,1.15,;10.67,3.47,;11.07,.44,)| Show InChI InChI=1S/C27H27F4N3O4/c1-14(26(37)38-27(2,3)4)33-10-9-15-11-20(30)23(21(31)12-15)34-22(35)8-7-18(25(34)32)24(36)17-6-5-16(28)13-19(17)29/h5-8,11-14,33H,9-10,32H2,1-4H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236623
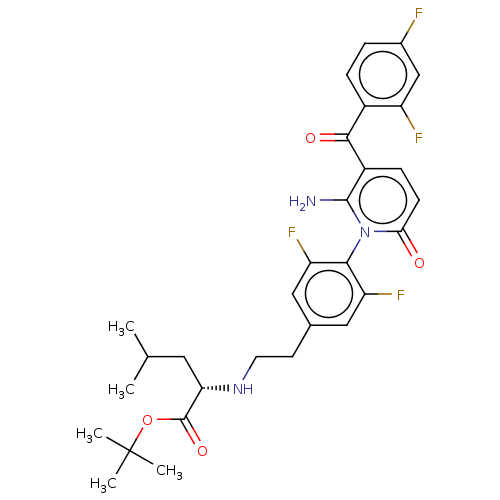
(US9388136, Table 1, Compound 15:tert-butyl N-(2- {...)Show SMILES CC(C)C[C@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC(C)(C)C |r,wD:4.4,(6,-2.69,;6,-1.15,;4.67,-.38,;7.34,-.38,;7.34,1.15,;6,1.93,;4.67,1.15,;3.33,1.93,;2,1.15,;.67,1.93,;-.67,1.15,;-2,1.93,;-.67,-.38,;.67,-1.15,;.67,-2.69,;2,-.38,;-2,-1.15,;-3.33,-.38,;-3.33,1.15,;-4.67,-1.15,;-4.67,-2.69,;-3.33,-3.47,;-2,-2.69,;-.67,-3.47,;-6,-.38,;-6,1.15,;-7.34,-1.15,;-7.34,-2.69,;-8.67,-3.47,;-10,-2.69,;-11.34,-3.47,;-10,-1.15,;-8.67,-.38,;-8.67,1.15,;8.67,1.93,;8.67,3.47,;10,1.15,;10,-.38,;11.34,-1.15,;8.67,-1.15,;10,-1.93,)| Show InChI InChI=1S/C30H33F4N3O4/c1-16(2)12-24(29(40)41-30(3,4)5)36-11-10-17-13-22(33)26(23(34)14-17)37-25(38)9-8-20(28(37)35)27(39)19-7-6-18(31)15-21(19)32/h6-9,13-16,24,36H,10-12,35H2,1-5H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236625

(US9388136, Table 1, Compound 17:cyclopentyl N-(2- ...)Show SMILES CC(C)C[C@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC1CCCC1 |r,wD:4.4,(4.82,-2.93,;4.82,-1.39,;3.48,-.62,;6.15,-.62,;6.15,.92,;4.82,1.69,;3.48,.92,;2.15,1.69,;.82,.92,;-.52,1.69,;-1.85,.92,;-3.19,1.69,;-1.85,-.62,;-.52,-1.39,;-.52,-2.93,;.82,-.62,;-3.19,-1.39,;-4.52,-.62,;-4.52,.92,;-5.85,-1.39,;-5.85,-2.93,;-4.52,-3.7,;-3.19,-2.93,;-1.85,-3.7,;-7.19,-.62,;-7.19,.92,;-8.52,-1.39,;-9.85,-.62,;-11.19,-1.39,;-11.19,-2.93,;-12.52,-3.7,;-9.85,-3.7,;-8.52,-2.93,;-7.19,-3.7,;7.48,1.69,;7.48,3.23,;8.82,.92,;10.15,1.69,;11.62,1.21,;12.52,2.46,;11.62,3.7,;10.15,3.23,)| Show InChI InChI=1S/C31H33F4N3O4/c1-17(2)13-26(31(41)42-20-5-3-4-6-20)37-12-11-18-14-24(34)28(25(35)15-18)38-27(39)10-9-22(30(38)36)29(40)21-8-7-19(32)16-23(21)33/h7-10,14-17,20,26,37H,3-6,11-13,36H2,1-2H3/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236628
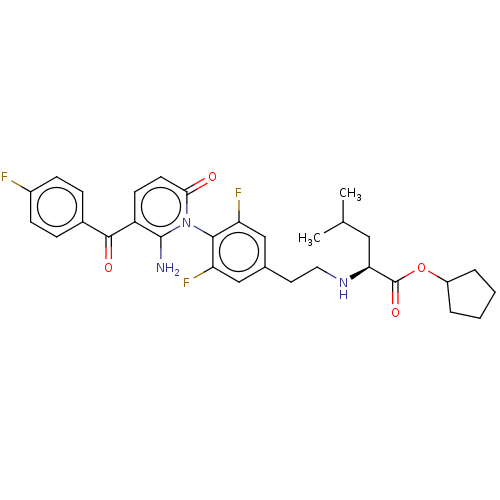
(US9388136, Table 1, Compound 20:Cyclopentyl N- (2-...)Show SMILES CC(C)C[C@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1)C(=O)OC1CCCC1 |r,wD:4.4,(4.82,-2.93,;4.82,-1.39,;3.48,-.62,;6.15,-.62,;6.15,.92,;4.82,1.69,;3.48,.92,;2.15,1.69,;.82,.92,;-.52,1.69,;-1.85,.92,;-3.19,1.69,;-1.85,-.62,;-.52,-1.39,;-.52,-2.93,;.82,-.62,;-3.19,-1.39,;-4.52,-.62,;-4.52,.92,;-5.85,-1.39,;-5.85,-2.93,;-4.52,-3.7,;-3.19,-2.93,;-1.85,-3.7,;-7.19,-.62,;-7.19,.92,;-8.52,-1.39,;-9.85,-.62,;-11.19,-1.39,;-11.19,-2.93,;-12.52,-3.7,;-9.85,-3.7,;-8.52,-2.93,;7.48,1.69,;7.48,3.23,;8.82,.92,;10.15,1.69,;11.62,1.21,;12.52,2.46,;11.62,3.7,;10.15,3.23,)| Show InChI InChI=1S/C31H34F3N3O4/c1-18(2)15-26(31(40)41-22-5-3-4-6-22)36-14-13-19-16-24(33)28(25(34)17-19)37-27(38)12-11-23(30(37)35)29(39)20-7-9-21(32)10-8-20/h7-12,16-18,22,26,36H,3-6,13-15,35H2,1-2H3/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236629
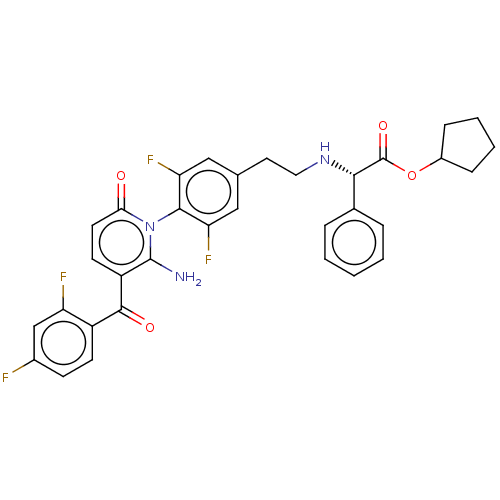
(US9388136, Table 1, Compound 21:Cyclopentyl (2S)- ...)Show SMILES Nc1c(ccc(=O)n1-c1c(F)cc(CCN[C@H](C(=O)OC2CCCC2)c2ccccc2)cc1F)C(=O)c1ccc(F)cc1F |r,wD:16.16,(-4.52,.92,;-4.52,-.62,;-5.85,-1.39,;-5.85,-2.93,;-4.52,-3.7,;-3.19,-2.93,;-1.85,-3.7,;-3.19,-1.39,;-1.85,-.62,;-1.85,.92,;-3.19,1.69,;-.52,1.69,;.82,.92,;2.15,1.69,;3.48,.92,;4.82,1.69,;6.15,.92,;7.48,1.69,;7.48,3.23,;8.82,.92,;10.15,1.69,;11.62,1.21,;12.52,2.46,;11.62,3.7,;10.15,3.23,;6.15,-.62,;4.82,-1.39,;4.82,-2.93,;6.15,-3.7,;7.48,-2.93,;7.48,-1.39,;.82,-.62,;-.52,-1.39,;-.52,-2.93,;-7.19,-.62,;-7.19,.92,;-8.52,-1.39,;-8.52,-2.93,;-9.85,-3.7,;-11.19,-2.93,;-12.52,-3.7,;-11.19,-1.39,;-9.85,-.62,;-9.85,.92,)| Show InChI InChI=1S/C33H29F4N3O4/c34-21-10-11-23(25(35)18-21)31(42)24-12-13-28(41)40(32(24)38)30-26(36)16-19(17-27(30)37)14-15-39-29(20-6-2-1-3-7-20)33(43)44-22-8-4-5-9-22/h1-3,6-7,10-13,16-18,22,29,39H,4-5,8-9,14-15,38H2/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236630

(US9388136, Table 1, Compound 22:tert-butyl (2S)- [...)Show SMILES CC(C)(C)OC(=O)[C@@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)c1ccccc1 |r,wD:7.7,(10.99,3.41,;10.59,1.92,;10.99,.44,;12.08,1.53,;9.26,1.15,;7.93,1.93,;7.93,3.47,;6.59,1.15,;5.26,1.93,;3.92,1.15,;2.59,1.93,;1.26,1.15,;-.08,1.93,;-1.41,1.15,;-2.74,1.93,;-1.41,-.38,;-.08,-1.15,;-.08,-2.69,;1.26,-.38,;-2.74,-1.15,;-4.08,-.38,;-4.08,1.15,;-5.41,-1.15,;-5.41,-2.69,;-4.08,-3.47,;-2.74,-2.69,;-1.41,-3.47,;-6.75,-.38,;-6.75,1.15,;-8.08,-1.15,;-8.08,-2.69,;-9.41,-3.47,;-10.75,-2.69,;-12.08,-3.47,;-10.75,-1.15,;-9.41,-.38,;-9.41,1.15,;6.59,-.38,;5.26,-1.15,;5.26,-2.69,;6.59,-3.47,;7.93,-2.69,;7.93,-1.15,)| Show InChI InChI=1S/C32H29F4N3O4/c1-32(2,3)43-31(42)27(19-7-5-4-6-8-19)38-14-13-18-15-24(35)28(25(36)16-18)39-26(40)12-11-22(30(39)37)29(41)21-10-9-20(33)17-23(21)34/h4-12,15-17,27,38H,13-14,37H2,1-3H3/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236631

(US9388136, Table 1, Compound 23:Cyclopentyl N- (2-...)Show SMILES CC(C)(C)OC[C@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC1CCCC1 |r,wD:6.6,(5.94,-2.93,;7.48,-2.93,;8.82,-2.16,;8.82,-3.7,;7.48,-1.39,;6.15,-.62,;6.15,.92,;4.82,1.69,;3.48,.92,;2.15,1.69,;.82,.92,;-.52,1.69,;-1.85,.92,;-3.19,1.69,;-1.85,-.62,;-.52,-1.39,;-.52,-2.93,;.82,-.62,;-3.19,-1.39,;-4.52,-.62,;-4.52,.92,;-5.85,-1.39,;-5.85,-2.93,;-4.52,-3.7,;-3.19,-2.93,;-1.85,-3.7,;-7.19,-.62,;-7.19,.92,;-8.52,-1.39,;-8.52,-2.93,;-9.85,-3.7,;-11.19,-2.93,;-12.52,-3.7,;-11.19,-1.39,;-9.85,-.62,;-9.85,.92,;7.48,1.69,;7.48,3.23,;8.82,.92,;10.15,1.69,;11.62,1.21,;12.52,2.46,;11.62,3.7,;10.15,3.23,)| Show InChI InChI=1S/C32H35F4N3O5/c1-32(2,3)43-17-26(31(42)44-20-6-4-5-7-20)38-13-12-18-14-24(35)28(25(36)15-18)39-27(40)11-10-22(30(39)37)29(41)21-9-8-19(33)16-23(21)34/h8-11,14-16,20,26,38H,4-7,12-13,17,37H2,1-3H3/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236632
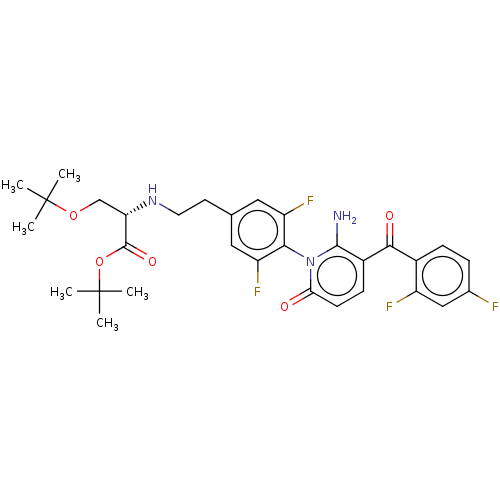
(US9388136, Table 1, Compound 24:tert-butyl N-(2- {...)Show SMILES CC(C)(C)OC[C@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC(C)(C)C |r,wD:6.6,(3.85,-1.64,;5.18,-2.41,;3.85,-3.18,;5.95,-3.75,;5.18,-.87,;6.51,-.1,;6.51,1.44,;5.18,2.21,;3.85,1.44,;2.51,2.21,;1.18,1.44,;-.15,2.21,;-1.49,1.44,;-2.82,2.21,;-1.49,-.1,;-.15,-.87,;-.15,-2.41,;1.18,-.1,;-2.82,-.87,;-4.15,-.1,;-4.15,1.44,;-5.49,-.87,;-5.49,-2.41,;-4.15,-3.18,;-2.82,-2.41,;-1.49,-3.18,;-6.82,-.1,;-6.82,1.44,;-8.16,-.87,;-8.16,-2.41,;-9.49,-3.18,;-10.82,-2.41,;-12.16,-3.18,;-10.82,-.87,;-9.49,-.1,;-9.49,1.44,;7.85,2.21,;7.85,3.75,;9.18,1.44,;10.67,1.84,;11.07,3.32,;11.76,.75,;12.16,2.23,)| Show InChI InChI=1S/C31H35F4N3O5/c1-30(2,3)42-16-24(29(41)43-31(4,5)6)37-12-11-17-13-22(34)26(23(35)14-17)38-25(39)10-9-20(28(38)36)27(40)19-8-7-18(32)15-21(19)33/h7-10,13-15,24,37H,11-12,16,36H2,1-6H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236618
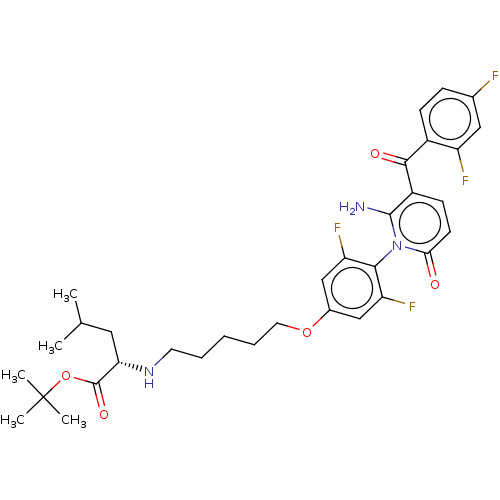
(US9388136, Table 1, Compound 5:tert-Butyl N-(5- {4...)Show SMILES CC(C)C[C@H](NCCCCCOc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC(C)(C)C |r,wD:4.4,(6.67,-.39,;8,-1.15,;8,-2.69,;9.34,-.39,;9.34,1.15,;8,1.92,;6.67,1.15,;5.33,1.92,;4,1.15,;2.67,1.92,;1.33,1.15,;,1.92,;-1.33,1.15,;-2.67,1.92,;-4,1.15,;-5.33,1.92,;-4,-.39,;-2.67,-1.15,;-2.67,-2.69,;-1.33,-.39,;-5.33,-1.15,;-6.67,-.39,;-6.67,1.15,;-8,-1.15,;-8,-2.69,;-6.67,-3.46,;-5.33,-2.69,;-4,-3.46,;-9.34,-.39,;-9.34,1.15,;-10.67,-1.15,;-12,-.39,;-13.34,-1.15,;-13.34,-2.69,;-14.67,-3.46,;-12,-3.46,;-10.67,-2.69,;-9.34,-3.46,;10.67,1.92,;10.67,3.46,;12,1.15,;13.34,1.92,;14.67,1.15,;13.34,3.46,;13.34,.39,)| Show InChI InChI=1S/C33H39F4N3O5/c1-19(2)15-27(32(43)45-33(3,4)5)39-13-7-6-8-14-44-21-17-25(36)29(26(37)18-21)40-28(41)12-11-23(31(40)38)30(42)22-10-9-20(34)16-24(22)35/h9-12,16-19,27,39H,6-8,13-15,38H2,1-5H3/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236620

(US9388136, Table 1, Compound 12:Cyclopentyl N-(5-{...)Show SMILES CC(C)C[C@H](NCCCCCOc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC1CCCC1 |r,wD:4.4,(7.48,-2.93,;7.48,-1.39,;6.15,-.62,;8.82,-.62,;8.82,.92,;7.48,1.69,;6.15,.92,;4.82,1.69,;3.48,.92,;2.15,1.69,;.82,.92,;-.52,1.69,;-1.85,.92,;-3.19,1.69,;-4.52,.92,;-5.85,1.69,;-4.52,-.62,;-3.19,-1.39,;-3.19,-2.93,;-1.85,-.62,;-5.85,-1.39,;-7.19,-.62,;-7.19,.92,;-8.52,-1.39,;-8.52,-2.93,;-7.19,-3.7,;-5.85,-2.93,;-4.52,-3.7,;-9.85,-.62,;-9.85,.92,;-11.19,-1.39,;-12.52,-.62,;-13.85,-1.39,;-13.85,-2.93,;-15.19,-3.7,;-12.52,-3.7,;-11.19,-2.93,;-9.85,-3.7,;10.15,1.69,;10.15,3.23,;11.49,.92,;12.82,1.69,;14.28,1.21,;15.19,2.46,;14.28,3.7,;12.82,3.23,)| Show InChI InChI=1S/C34H39F4N3O5/c1-20(2)16-29(34(44)46-22-8-4-5-9-22)40-14-6-3-7-15-45-23-18-27(37)31(28(38)19-23)41-30(42)13-12-25(33(41)39)32(43)24-11-10-21(35)17-26(24)36/h10-13,17-20,22,29,40H,3-9,14-16,39H2,1-2H3/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236624
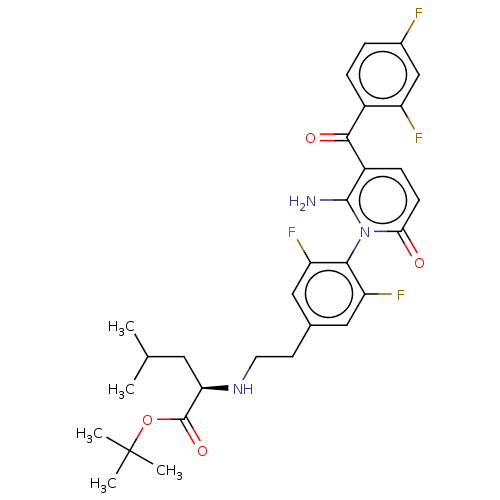
(US9388136, Table 1, Compound 16:tert-butyl N-(2- {...)Show SMILES CC(C)C[C@@H](NCCc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC(C)(C)C |r,wU:4.4,(5.23,-2.69,;5.23,-1.15,;3.9,-.38,;6.57,-.38,;6.57,1.15,;5.23,1.93,;3.9,1.15,;2.56,1.93,;1.23,1.15,;-.1,1.93,;-1.44,1.15,;-2.77,1.93,;-1.44,-.38,;-.1,-1.15,;-.1,-2.69,;1.23,-.38,;-2.77,-1.15,;-4.1,-.38,;-4.1,1.15,;-5.44,-1.15,;-5.44,-2.69,;-4.1,-3.47,;-2.77,-2.69,;-1.44,-3.47,;-6.77,-.38,;-6.77,1.15,;-8.11,-1.15,;-8.11,-2.69,;-9.44,-3.47,;-10.77,-2.69,;-12.11,-3.47,;-10.77,-1.15,;-9.44,-.38,;-9.44,1.15,;7.9,1.93,;7.9,3.47,;9.23,1.15,;10.57,1.93,;12.11,1.93,;11.34,3.26,;11.34,.59,)| Show InChI InChI=1S/C30H33F4N3O4/c1-16(2)12-24(29(40)41-30(3,4)5)36-11-10-17-13-22(33)26(23(34)14-17)37-25(38)9-8-20(28(37)35)27(39)19-7-6-18(31)15-21(19)32/h6-9,13-16,24,36H,10-12,35H2,1-5H3/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Coll-3 MMP-13 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081866

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C23H35N3O7S/c1-25(34(30,31)19-9-7-18(32-2)8-10-19)16-21(22(27)24-29)20(15-17-5-3-4-6-17)23(28)26-11-13-33-14-12-26/h7-10,17,20-21,29H,3-6,11-16H2,1-2H3,(H,24,27)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081869
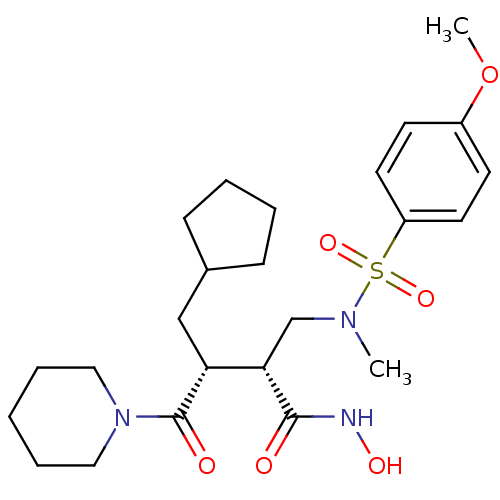
((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C24H37N3O6S/c1-26(34(31,32)20-12-10-19(33-2)11-13-20)17-22(23(28)25-30)21(16-18-8-4-5-9-18)24(29)27-14-6-3-7-15-27/h10-13,18,21-22,30H,3-9,14-17H2,1-2H3,(H,25,28)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081852

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C27H37N3O5S/c1-29(36(34,35)25-15-9-13-21-12-5-6-14-22(21)25)19-24(26(31)28-33)23(18-20-10-3-4-11-20)27(32)30-16-7-2-8-17-30/h5-6,9,12-15,20,23-24,33H,2-4,7-8,10-11,16-19H2,1H3,(H,28,31)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081859

((2R,3R)-2-((4-chloro-N-methylphenylsulfonamido)met...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H34ClN3O5S/c1-26(33(31,32)19-11-9-18(24)10-12-19)16-21(22(28)25-30)20(15-17-7-3-4-8-17)23(29)27-13-5-2-6-14-27/h9-12,17,20-21,30H,2-8,13-16H2,1H3,(H,25,28)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM236619

(US9388136, Table 1, Compound 11:Cyclopentyl (2S)-4...)Show SMILES NCC[C@H](NCCCOc1cc(F)c(c(F)c1)-n1c(N)c(ccc1=O)C(=O)c1ccc(F)cc1F)C(=O)OC1CCCC1 |r,wD:3.3,(8.82,-2.93,;8.82,-1.39,;7.48,-.62,;7.48,.92,;6.15,1.69,;4.82,.92,;3.48,1.69,;2.15,.92,;.82,1.69,;-.52,.92,;-1.85,1.69,;-3.19,.92,;-4.52,1.69,;-3.19,-.62,;-1.85,-1.39,;-1.85,-2.93,;-.52,-.62,;-4.52,-1.39,;-5.85,-.62,;-5.85,.92,;-7.19,-1.39,;-7.19,-2.93,;-5.85,-3.7,;-4.52,-2.93,;-3.19,-3.7,;-8.52,-.62,;-8.52,.92,;-9.85,-1.39,;-11.19,-.62,;-12.52,-1.39,;-12.52,-2.93,;-13.86,-3.7,;-11.19,-3.7,;-9.85,-2.93,;-8.52,-3.7,;8.82,1.69,;8.82,3.23,;10.15,.92,;11.49,1.69,;12.95,1.21,;13.86,2.46,;12.95,3.7,;11.49,3.23,)| Show InChI InChI=1S/C30H32F4N4O5/c31-17-6-7-20(22(32)14-17)28(40)21-8-9-26(39)38(29(21)36)27-23(33)15-19(16-24(27)34)42-13-3-12-37-25(10-11-35)30(41)43-18-4-1-2-5-18/h6-9,14-16,18,25,37H,1-5,10-13,35-36H2/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Chroma Therapeutics Ltd
US Patent
| Assay Description
The ability of compounds to inhibit p38 MAP a Kinase activity was measured in an assay performed by Upstate (Dundee UK). In a final reaction volume o... |
US Patent US9388136 (2016)
BindingDB Entry DOI: 10.7270/Q2M61J5H |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081871

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CC(C)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C20H37N3O5S/c1-15(2)29(27,28)22(3)14-18(19(24)21-26)17(13-16-9-5-6-10-16)20(25)23-11-7-4-8-12-23/h15-18,26H,4-14H2,1-3H3,(H,21,24)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081860

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCOCC1)C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C17H31N3O6S/c1-19(27(2,24)25)12-15(16(21)18-23)14(11-13-5-3-4-6-13)17(22)20-7-9-26-10-8-20/h13-15,23H,3-12H2,1-2H3,(H,18,21)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50131342
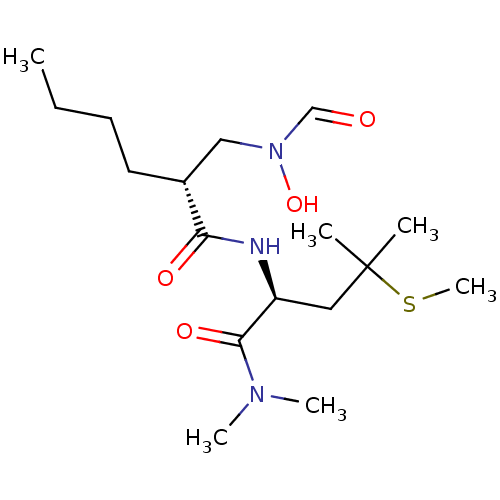
((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@@H](CC(C)(C)SC)C(=O)N(C)C Show InChI InChI=1S/C17H33N3O4S/c1-7-8-9-13(11-20(24)12-21)15(22)18-14(16(23)19(4)5)10-17(2,3)25-6/h12-14,24H,7-11H2,1-6H3,(H,18,22)/t13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081864

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H33N3O5S/c1-20(27(2,25)26)13-16(17(22)19-24)15(12-14-8-4-5-9-14)18(23)21-10-6-3-7-11-21/h14-16,24H,3-13H2,1-2H3,(H,19,22)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081865

((2R,3R)-2-((1-(dimethylamino)-N-methylnaphthalene-...)Show SMILES CC(C)C[C@H]([C@H](CN(C)S(=O)(=O)c1cccc2c(cccc12)N(C)C)C(=O)NO)C(=O)N1CCCCC1 Show InChI InChI=1S/C27H40N4O5S/c1-19(2)17-22(27(33)31-15-7-6-8-16-31)23(26(32)28-34)18-30(5)37(35,36)25-14-10-11-20-21(25)12-9-13-24(20)29(3)4/h9-14,19,22-23,34H,6-8,15-18H2,1-5H3,(H,28,32)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081867

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CCS(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C19H35N3O5S/c1-3-28(26,27)21(2)14-17(18(23)20-25)16(13-15-9-5-6-10-15)19(24)22-11-7-4-8-12-22/h15-17,25H,3-14H2,1-2H3,(H,20,23)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081872

((2R,3R)-3-(cyclopentylmethyl)-2-{[(dimethylsulfamo...)Show SMILES CN(C)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C19H36N4O5S/c1-21(2)29(27,28)22(3)14-17(18(24)20-26)16(13-15-9-5-6-10-15)19(25)23-11-7-4-8-12-23/h15-17,26H,4-14H2,1-3H3,(H,20,24)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081852

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C27H37N3O5S/c1-29(36(34,35)25-15-9-13-21-12-5-6-14-22(21)25)19-24(26(31)28-33)23(18-20-10-3-4-11-20)27(32)30-16-7-2-8-17-30/h5-6,9,12-15,20,23-24,33H,2-4,7-8,10-11,16-19H2,1H3,(H,28,31)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Gel-A MMP-2 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131303
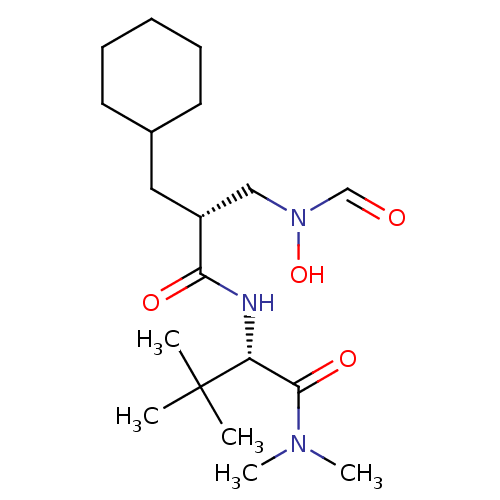
((S)-2-[(R)-2-Cyclohexylmethyl-3-(formyl-hydroxy-am...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@H](CC1CCCCC1)CN(O)C=O)C(C)(C)C Show InChI InChI=1S/C19H35N3O4/c1-19(2,3)16(18(25)21(4)5)20-17(24)15(12-22(26)13-23)11-14-9-7-6-8-10-14/h13-16,26H,6-12H2,1-5H3,(H,20,24)/t15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Escherichia coli) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Strom-1 MMP-3 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Antibacterial activity of the compound against E. coli Peptide deformylase. Ni |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Coll-3 MMP-13 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50081852

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C27H37N3O5S/c1-29(36(34,35)25-15-9-13-21-12-5-6-14-22(21)25)19-24(26(31)28-33)23(18-20-10-3-4-11-20)27(32)30-16-7-2-8-17-30/h5-6,9,12-15,20,23-24,33H,2-4,7-8,10-11,16-19H2,1H3,(H,28,31)/t23-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Coll-3 MMP-13 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131290
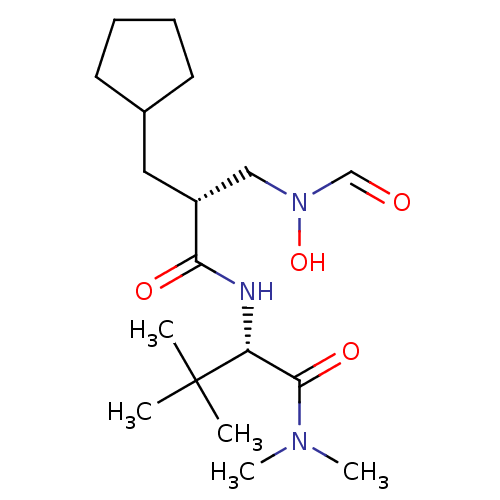
((S)-2-[(R)-2-Cyclopentylmethyl-3-(formyl-hydroxy-a...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@H](CC1CCCC1)CN(O)C=O)C(C)(C)C Show InChI InChI=1S/C18H33N3O4/c1-18(2,3)15(17(24)20(4)5)19-16(23)14(11-21(25)12-22)10-13-8-6-7-9-13/h12-15,25H,6-11H2,1-5H3,(H,19,23)/t14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50221045
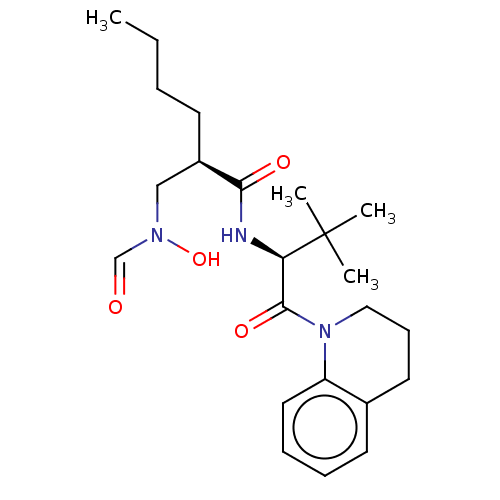
(CHEMBL3706628)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N1CCCc2ccccc12)C(C)(C)C Show InChI InChI=1S/C23H35N3O4/c1-5-6-10-18(15-25(30)16-27)21(28)24-20(23(2,3)4)22(29)26-14-9-12-17-11-7-8-13-19(17)26/h7-8,11,13,16,18,20,30H,5-6,9-10,12,14-15H2,1-4H3,(H,24,28)/t18-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081871

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CC(C)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C20H37N3O5S/c1-15(2)29(27,28)22(3)14-18(19(24)21-26)17(13-16-9-5-6-10-16)20(25)23-11-7-4-8-12-23/h15-18,26H,4-14H2,1-3H3,(H,21,24)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081862

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-4-oxo-4-(p...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)C(F)(F)F Show InChI InChI=1S/C18H30F3N3O5S/c1-23(30(28,29)18(19,20)21)12-15(16(25)22-27)14(11-13-7-3-4-8-13)17(26)24-9-5-2-6-10-24/h13-15,27H,2-12H2,1H3,(H,22,25)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081859

((2R,3R)-2-((4-chloro-N-methylphenylsulfonamido)met...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H34ClN3O5S/c1-26(33(31,32)19-11-9-18(24)10-12-19)16-21(22(28)25-30)20(15-17-7-3-4-8-17)23(29)27-13-5-2-6-14-27/h9-12,17,20-21,30H,2-8,13-16H2,1H3,(H,25,28)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50131324
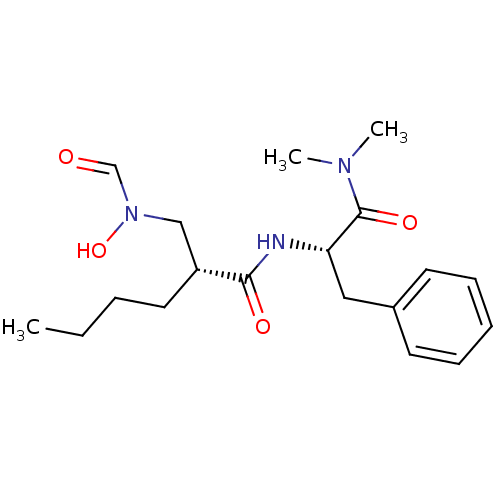
((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(C)C Show InChI InChI=1S/C19H29N3O4/c1-4-5-11-16(13-22(26)14-23)18(24)20-17(19(25)21(2)3)12-15-9-7-6-8-10-15/h6-10,14,16-17,26H,4-5,11-13H2,1-3H3,(H,20,24)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50131311
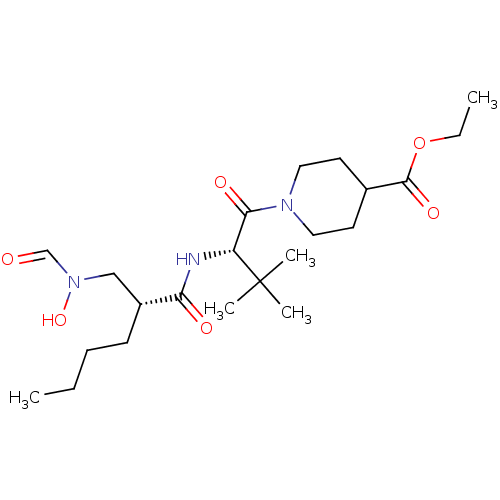
(1-((S)-2-{(R)-2-[(Formyl-hydroxy-amino)-methyl]-he...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N1CCC(CC1)C(=O)OCC)C(C)(C)C Show InChI InChI=1S/C22H39N3O6/c1-6-8-9-17(14-25(30)15-26)19(27)23-18(22(3,4)5)20(28)24-12-10-16(11-13-24)21(29)31-7-2/h15-18,30H,6-14H2,1-5H3,(H,23,27)/t17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50131315

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N1CCC(CC1)C(C)=O)C(C)(C)C Show InChI InChI=1S/C21H37N3O5/c1-6-7-8-17(13-24(29)14-25)19(27)22-18(21(3,4)5)20(28)23-11-9-16(10-12-23)15(2)26/h14,16-18,29H,6-13H2,1-5H3,(H,22,27)/t17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50131343
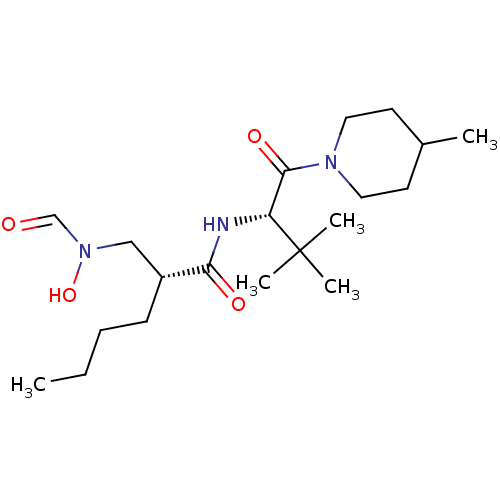
((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N1CCC(C)CC1)C(C)(C)C Show InChI InChI=1S/C20H37N3O4/c1-6-7-8-16(13-23(27)14-24)18(25)21-17(20(3,4)5)19(26)22-11-9-15(2)10-12-22/h14-17,27H,6-13H2,1-5H3,(H,21,25)/t16-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50131314
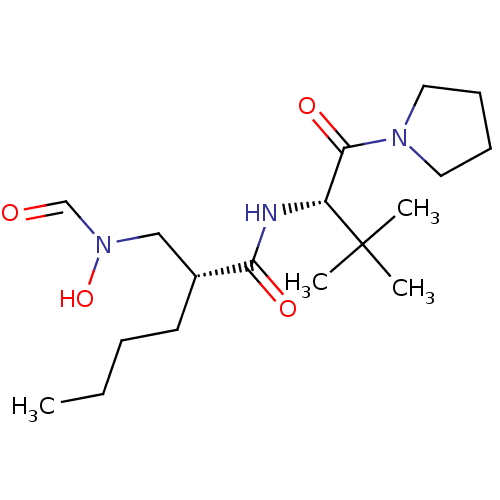
((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N1CCCC1)C(C)(C)C Show InChI InChI=1S/C18H33N3O4/c1-5-6-9-14(12-21(25)13-22)16(23)19-15(18(2,3)4)17(24)20-10-7-8-11-20/h13-15,25H,5-12H2,1-4H3,(H,19,23)/t14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition concentration against Escherichia coli peptide deformylase. |
Bioorg Med Chem Lett 13: 2715-8 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ336P |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081857
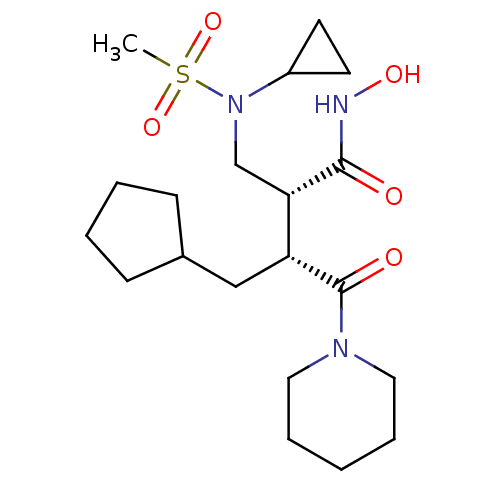
((2R,3R)-3-(cyclopentylmethyl)-2-((N-cyclopropylmet...)Show SMILES CS(=O)(=O)N(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)C1CC1 Show InChI InChI=1S/C20H35N3O5S/c1-29(27,28)23(16-9-10-16)14-18(19(24)21-26)17(13-15-7-3-4-8-15)20(25)22-11-5-2-6-12-22/h15-18,26H,2-14H2,1H3,(H,21,24)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data